Abstract
To ascertain the quantitative effect on the disease β-thalassemia of a low-magnesium (Mg) diet compared with a high-Mg diet and a standard-Mg diet, we studied the effect these diets had over a 4-week period on β-thalassemic (β thal) mice compared with normal C57BL/6 mice used as controls. The low-Mg diet consisted of 6 ± 2 mg Mg/kg body weight/d, the high-Mg diet 1,000 ± 20 mg Mg/kg body weight/d, and the standard-Mg diet 400 ± 20 mg Mg/kg body weight/d. β thal mice that were fed the low-Mg diet became more anemic, had reduced serum and erythrocyte Mg, and had decreased erythrocyte K. Their K-Cl cotransport increased, followed by commensurate cell dehydration. The high-Mg group showed a significant improvement of the anemia, increased serum and erythrocyte Mg, increased erythrocyte Mg, increased erythrocyte K, reduced K-Cl cotransport, and diminished cell dehydration. C57BL/6 control mice that received the low-Mg diet experienced anemia with erythrocyte dehydration, whereas the high-Mg diet had little effect on the hematologic parameters. β thal and C57BL/6 control mice that were fed a standard diet showed no changes. These results indicate that dietary Mg supplementation corrects hypomagnesemia and improves anemia in murine β thal and should be assessed in human β-thalassemia.
MAGNESIUM (Mg) is the second most abundant intracellular metal after potassium (K), and plays an essential role in the activity of many enzymes involved in cellular metabolism.1 Cell Mg is an important modulator in cell volume regulation and affects the activity of various membrane cation transport pathways such as the sodium (Na)/K pump, Na-K-chloride (Cl) cotransport, calcium (Ca) and K channels, and K-Cl cotransport. The physical properties of the erythrocyte membrane are also markedly affected by changes in cell Mg content.1 2
Mg deficiency spontaneously occurs in farm animals and in humans; it has been extensively studied in rats, where it is easily produced by dietary Mg restriction.3,4 With a Mg-deficient diet, rats develop hemolytic anemia characterized by changes in erythrocyte morphology and reduction in erythrocyte survival.3 4 The mechanism(s) by which Mg deficiency produces anemia remains poorly understood.
The anemia of human β-thalassemia is determined by a combination of different factors such as ineffective erythropoiesis, destruction of erythrocytes containing α chain inclusions, and reduced hemoglobin (Hb) content with hypochromic, microcytic erythrocytes.5,6 Although the molecular defects responsible for decreasing β-globin chain synthesis have been characterized in great detail, the pathophysiologic consequences of membrane damage imposed by the excess of α chains and the determinants of the reduced erythrocyte survival are less well characterized.5-9
Abnormalities of Mg metabolism have been described in β-thalassemia, and low serum Mg has been reported in children affected by the homozygous form of the disease.10,11 Subjects with heterozygous β-thalassemia and β-thalassemia intermedia showed an abnormally low erythrocyte Mg content when compared with normal controls and patients with hypochromic sideropenic anemia.11 12 There is no clear explanation for the abnormalities in Mg metabolism observed in β-thalassemia. There have also been no systematic studies on the possible role played by Mg deficiency in the anemia of β-thalassemia.
Mice homozygous for deletion of the β major gene (β thal) have clinical and biologic features similar to those observed in human β-thalassemia intermedia. This spontaneous murine model for β-thalassemia reproduces several erythrocyte abnormalities characteristic of the human disease.13-20 The anemia of this mouse model has been shown to improve following treatment with either hydroxyurea14 or recombinant human erythropoietin (rHuEPO),13 which also improve some hematologic parameters of human β-thalassemia intermedia. Additionally, clotrimazole may have a benefit when combined with either hydroxyurea or rHuEPO.20 Different mouse models for β-thalassemia obtained from deletion of both the b1 and b2 adult globin genes have been recently reported.21 22
We therefore hypothesized that regulation of dietary Mg intake would affect the course of some hematologic parameters in β thal mice. These results could point the way to experimentation with dietary Mg in human β-thalassemia.
The objective of this study was to evaluate the extent to which diets containing different amounts of Mg might affect the anemia of β thal mice. We show that Mg dietary supplementation leads to increased hematocrit (Hct), Hb, and cell K content and reduces mean corpuscular Hb content (MCHC) and K-Cl cotransport activity in β thal mouse erythrocytes.
MATERIALS AND METHODS
Drugs and chemicals.NaCl, KCl, ouabain, bumetanide, Tris, 3(N-morpholino)propanesulfonic acid (MOPS), choline chloride, and Acationox were purchased from Sigma Chemical Co (St Louis, MO). MgCl2 , dimethylsulfoxide, sulfamic acid, phthalate esters, and all other chemicals were purchased from Fisher Scientific Co (Fair Lawn, NJ). Microhematocrit tubes were purchased from Drummond Scientific Co (Bromall, PA). All solutions were prepared using double-distilled water.
Animals and experimental design.Mice homozygous for β thal (Hbbthal/thal) were obtained from breedings performed in the animal facility of Institute Nationale Scientifique et Recherche Médicale at Henri Mondor Hospital, Creteil, France. Animals between 4 and 6 months old, females weighing 25 to 28 g and males weighing 28 to 30 g, were selected for study. Because the original β thal mutation in DA 2J was back-crossed in C57BL/6 for more than 11 generations, normal C57BL/6 mice were selected as a control group.20
Both β thal and C57BL/6 control mice were divided into three groups of six animals each and placed on a diet with a known content of Mg. The low-Mg group received a diet that included 6 ± 2 mg Mg/kg body weight/d. This was achieved using mouse feed with a Mg content of 30 ± 10 mg/kg (Uar; Villemoisson, Epinay-s/Orge, France). The standard-Mg group received a diet that included 400 ± 20 mg Mg/kg body weight/d using a regular mouse feed with a Mg content of 1,900 ± 100 mg/kg. The high-Mg group received a diet that included 1,000 ± 20 mg Mg/kg body weight/d by supplementing the Mg contained in the regular mouse feed with an additional 600 mg Mg/kg body weight/d. This Mg supplement consisted of magnesium hydroxide dissolved in water and was administered by gavage. Normal daily Mg intake for humans is 418 ± 120 mg for males and 343 ± 94 mg for females.23 Measurements were made in six groups of mice at baseline and after 14 and 28 days of treatment. No changes in body weight were observed during treatment.
Hematologic Data in C57BL/6 Controls and β Thal Mice Maintained on a Standard-Mg Diet
| Parameter . | C57BL/6 . | β thal . |
|---|---|---|
| Hct (%) | 45.2 ± 0.5 | 32.1 ± 1.3* |
| Hb (g/dL) | 14.3 ± 0.4 | 9.90 ± 0.3* |
| MCHC (g/dL) | 29.1 ± 0.5 | 32.2 ± 0.8* |
| D50 | 1.090 ± 0.001 | 1.101 ± 0.001* |
| Reticulocytes (%) | 3.2 ± 0.2 | 28.6 ± 2.1* |
| Corrected reticulocyte count (%) | 2.3 ± 0.2 | 20.3 ± 1.5* |
| Serum Mg (mmol/L) | 2.7 ± 0.3 | 1.9 ± 0.2* |
| Cell Mg (mmol/kg Hb) | 9.05 ± 0.2 | 8.9 ± 0.3 |
| Cell Na (mmol/kg Hb) | 52.6 ± 2.7 | 51.5 ± 1.8 |
| Cell K (mmol/kg Hb) | 423.3 ± 19.5 | 287.4 ± 12.1* |
| Cell Na + K (mmol/kg Hb) | 472 ± 16 | 336.5 ± 7.4* |
| Parameter . | C57BL/6 . | β thal . |
|---|---|---|
| Hct (%) | 45.2 ± 0.5 | 32.1 ± 1.3* |
| Hb (g/dL) | 14.3 ± 0.4 | 9.90 ± 0.3* |
| MCHC (g/dL) | 29.1 ± 0.5 | 32.2 ± 0.8* |
| D50 | 1.090 ± 0.001 | 1.101 ± 0.001* |
| Reticulocytes (%) | 3.2 ± 0.2 | 28.6 ± 2.1* |
| Corrected reticulocyte count (%) | 2.3 ± 0.2 | 20.3 ± 1.5* |
| Serum Mg (mmol/L) | 2.7 ± 0.3 | 1.9 ± 0.2* |
| Cell Mg (mmol/kg Hb) | 9.05 ± 0.2 | 8.9 ± 0.3 |
| Cell Na (mmol/kg Hb) | 52.6 ± 2.7 | 51.5 ± 1.8 |
| Cell K (mmol/kg Hb) | 423.3 ± 19.5 | 287.4 ± 12.1* |
| Cell Na + K (mmol/kg Hb) | 472 ± 16 | 336.5 ± 7.4* |
Data are presented as the mean ± SD; n = 18 for both C57BL/6 and β thal groups. Corrected reticulocyte count based on baseline 45.2% Hct of C57BL/6 controls.
P < .005 v control mouse group.
Effect of Low-Mg Diet in C57BL6 Control Mice
| Parameter . | Baseline (n = 6) . | Day 14 (n = 6) . | Day 28 (n = 6) . |
|---|---|---|---|
| Hct (%) | 43.7 ± 0.8 | 43.4 ± 1.0 | 40 ± 2.2* |
| Hb (g/dL) | 14.2 ± 0.6 | 13.6 ± 0.4 | 11.2 ± 1.2* |
| MCHC (g/dL) | 30.4 ± 1.2 | 31.5 ± 0.8 | 32.0 ± 0.4† |
| D50 | 1.090 ± 0.001 | 1.094 ± 0.001* | 1.094 ± 0.002* |
| Reticulocytes (%) | 3.6 ± 0.4 | 6.1 ± 1.2* | 3.1 ± 0.4 |
| Corrected reticulocyte count (%) | 3.6 ± 0.4 | 6.1 ± 1.2* | 2.84 ± 0.4 |
| Serum Mg (mmol/L) | 2.7 ± 0.2 | 1.3 ± 0.1* | 1.4 ± 0.4* |
| Erythrocyte Mg (mmol/kg Hb) | 9.2 ± 0.4 | 7.2 ± 0.1* | 7.9 ± 0.1* |
| Cell Na (mmol/kg Hb) | 52.2 ± 2.1 | 55 ± 2.8 | 56.5 ± 2.4† |
| Cell K (mmol/kg Hb) | 430 ± 17 | 324 ± 12* | 375 ± 22.3* |
| Cell Na + K (mmol/kg Hb) | 483 ± 17.2 (6) | 379 ± 12 (6)* | 431 ± 20 (6)* |
| K-Cl cotransport (mmol/L cells⋅h) | 4.2 ± 0.5 | 5.7 ± 0.8* | 5.6 ± 1.3* |
| Parameter . | Baseline (n = 6) . | Day 14 (n = 6) . | Day 28 (n = 6) . |
|---|---|---|---|
| Hct (%) | 43.7 ± 0.8 | 43.4 ± 1.0 | 40 ± 2.2* |
| Hb (g/dL) | 14.2 ± 0.6 | 13.6 ± 0.4 | 11.2 ± 1.2* |
| MCHC (g/dL) | 30.4 ± 1.2 | 31.5 ± 0.8 | 32.0 ± 0.4† |
| D50 | 1.090 ± 0.001 | 1.094 ± 0.001* | 1.094 ± 0.002* |
| Reticulocytes (%) | 3.6 ± 0.4 | 6.1 ± 1.2* | 3.1 ± 0.4 |
| Corrected reticulocyte count (%) | 3.6 ± 0.4 | 6.1 ± 1.2* | 2.84 ± 0.4 |
| Serum Mg (mmol/L) | 2.7 ± 0.2 | 1.3 ± 0.1* | 1.4 ± 0.4* |
| Erythrocyte Mg (mmol/kg Hb) | 9.2 ± 0.4 | 7.2 ± 0.1* | 7.9 ± 0.1* |
| Cell Na (mmol/kg Hb) | 52.2 ± 2.1 | 55 ± 2.8 | 56.5 ± 2.4† |
| Cell K (mmol/kg Hb) | 430 ± 17 | 324 ± 12* | 375 ± 22.3* |
| Cell Na + K (mmol/kg Hb) | 483 ± 17.2 (6) | 379 ± 12 (6)* | 431 ± 20 (6)* |
| K-Cl cotransport (mmol/L cells⋅h) | 4.2 ± 0.5 | 5.7 ± 0.8* | 5.6 ± 1.3* |
Data are presented as the mean ± SD.
P < .005, † P < .05: v baseline.
The data obtained on C57BL/6 control animals at baseline and 14 days were also used for a simultaneous study on the effect of Mg in the SAD mouse, a transgenic model for sickle cell disease, which is being reported in a separate publication.2
At specific times, 200 μL blood was drawn from each animal and used for assays of K-Cl cotransport activity, erythrocyte phthalate density distribution curves, erythrocyte cation content, and other hematologic parameters.
Hematologic data and cation content.Blood was collected from ether-anesthetized mice by retro-orbital venipuncture into heparinized microhematocrit tubes. Hb concentration was determined by spectroscopic measurement of the cyanmet derivative. Hct was determined by centrifugation in a micro-Hct centrifuge. MCHC was calculated from the measured Hb and Hct values. Reticulocytes were counted on a Coulter EPICS Profile II (Coulter Electronics, Hialeah, FL) after thiazole orange staining during which 2.5 μL whole blood was incubated with 0.1 mg thiazole orange in 1 mL filtered phosphate-buffered saline (PBS) buffer for 30 minutes. The fluorescence of 50,000 erythrocytes was collected with logarithmic amplification.24 Corrected reticulocyte counts were calculated using the Finch reticulocyte index.25
Density distribution curves and median density of erythrocytes (D50 ) were obtained according to the method used by Danon and Marikovsky,26 using phthalate esters in micro-Hct tubes after washing the cells three times with PBS solution (300 mOsm) at 25°C in 2-mL tubes. The remaining cells were washed four additional times with mouse choline washing solution (170 mmol/L choline, 1 mmol/L MgCl2 , Tris-MOPS, pH 7.4, at 4°C, 330 mOsm)27 for measurement of internal Na and K content by atomic absorption spectrometry.
Erythrocyte adenosine triphosphate (ATP) and 2,3-diphosphoglyceric acid (2,3-DPG) content was measured after perchloroacetic acid extraction using standard biochemical techniques.
Measurements of erythrocyte K-Cl cotransport.K-Cl cotransport was measured in fresh mouse erythrocytes as the chloride-dependent K efflux and volume-dependent K efflux.28 Net K efflux from fresh cells was measured in hypotonic (260 mOsm) Na media (340 mOsm is required to obtain isotonicity for mouse erythrocytes). Chloride-dependent and volume-dependent K efflux was calculated as the difference between K efflux in chloride and sulfamate hypotonic media. All media contained (in mmol/L) 1 MgCl2 , 10 glucose, 1 ouabain, 0.01 bumetanide, and 10 Tris-MOPS (pH 7.40 at 37°C). Efflux was calculated from K concentration in the supernatant at 5 and 25 minutes.20,27 28
Statistical analysis.All values are the mean ± SD. For each group of mice, comparisons of separate variables between the baseline state and after 14 and 28 days of treatment were performed using two-tailed Student's t-test. Comparison of more than two groups was performed by one-way ANOVA with Tukey's test for post hoc comparison of the means.29 Correlations were assessed by calculation of Pearson's correlation coefficient.
RESULTS
Effects of different Mg dietary intakes on serum and erythrocyte Mg levels in C57BL/6 control and β thal mice.Table 1 presents baseline hematologic data, erythrocyte cation content, and phthalate density profiles for β thal and C57BL/6 control mice. As shown in previous publications, β thal mouse erythrocytes are dehydrated compared with C57BL/6 control erythrocytes, due to a large reduction in cell K content. Hematologic parameters in β thal mice are essentially stable over a 3-month period (data not shown). β thal mice have significantly lower serum Mg when compared with C57BL/6 controls, whereas erythrocyte Mg content is not significantly different (Table 1).
The effects of different Mg dietary intakes on serum and erythrocyte Mg levels in β thal and C57BL/6 control mice are shown in Tables 2-5. After 14 and 28 days of treatment with a low-Mg diet, there was a significant decrease in serum and erythrocyte Mg content when compared with the standard diet in both β thal and C57BL/6 control mice (P < .05; Tables 2 and 3). At 28 days, a low-Mg diet resulted in serum and erythrocyte Mg levels that were significantly lower in the β thal mouse group than in C57BL/6 controls (P < .005; Tables 2 and 3).
Effect of Low-Mg Diet in β Thal Mice
| Parameter . | Baseline (n = 6) . | Day 14 (n = 6) . | Day 28 (n = 6) . |
|---|---|---|---|
| Hct (%) | 32.8 ± 1.5 | 29.9 ± 1.73-150 | 21.4 ± 0.83-150 |
| Hb (g/dL) | 10.2 ± 0.4 | 8.4 ± 0.43-150 | 6.7 ± 0.13-150 |
| MCHC (g/dL) | 32.1 ± 1.0 | 33.2 ± 0.6† | 33.3 ± 0.7† |
| D50 | 1.102 ± 0.001 | 1.104 ± 0.0013-150 | 1.107 ± 0.0023-150 |
| Reticulocytes (%) | 28.3 ± 3.2 | 39.5 ± 4.83-150 | 42.8 ± 7.23-150 |
| Corrected reticulocyte count (%) | 20.5 ± 2.3 | 26.1 ± 3.23-150 | 20.3 ± 3.4 |
| Serum Mg (mmol/L) | 1.9 ± 0.3 | 0.6 ± 0.13-150 | 0.3 ± 0.13-150 |
| Erythrocyte Mg (mmol/kg Hb) | 9.1 ± 0.3 | 8.7 ± 0.2† | 6.4 ± 0.13-150 |
| Cell Na (mmol/kg Hb) | 51.1 ± 1.7 | 54.2 ± 1.7† | 55.0 ± 1.53-150 |
| Cell K (mmol/kg Hb) | 291 ± 10 | 242 ± 8.23-150 | 251.1 ± 213-150 |
| Cell Na + K (mmol/kg Hb) | 342.3 ± 13.7 | 302 ± 7.93-150 | 306 ± 11.23-150 |
| K-Cl cotransport (mmol/L cells⋅h) | 11.9 ± 0.9 | 29.7 ± 7.63-150 | 24.9 ± 6.43-150 |
| Parameter . | Baseline (n = 6) . | Day 14 (n = 6) . | Day 28 (n = 6) . |
|---|---|---|---|
| Hct (%) | 32.8 ± 1.5 | 29.9 ± 1.73-150 | 21.4 ± 0.83-150 |
| Hb (g/dL) | 10.2 ± 0.4 | 8.4 ± 0.43-150 | 6.7 ± 0.13-150 |
| MCHC (g/dL) | 32.1 ± 1.0 | 33.2 ± 0.6† | 33.3 ± 0.7† |
| D50 | 1.102 ± 0.001 | 1.104 ± 0.0013-150 | 1.107 ± 0.0023-150 |
| Reticulocytes (%) | 28.3 ± 3.2 | 39.5 ± 4.83-150 | 42.8 ± 7.23-150 |
| Corrected reticulocyte count (%) | 20.5 ± 2.3 | 26.1 ± 3.23-150 | 20.3 ± 3.4 |
| Serum Mg (mmol/L) | 1.9 ± 0.3 | 0.6 ± 0.13-150 | 0.3 ± 0.13-150 |
| Erythrocyte Mg (mmol/kg Hb) | 9.1 ± 0.3 | 8.7 ± 0.2† | 6.4 ± 0.13-150 |
| Cell Na (mmol/kg Hb) | 51.1 ± 1.7 | 54.2 ± 1.7† | 55.0 ± 1.53-150 |
| Cell K (mmol/kg Hb) | 291 ± 10 | 242 ± 8.23-150 | 251.1 ± 213-150 |
| Cell Na + K (mmol/kg Hb) | 342.3 ± 13.7 | 302 ± 7.93-150 | 306 ± 11.23-150 |
| K-Cl cotransport (mmol/L cells⋅h) | 11.9 ± 0.9 | 29.7 ± 7.63-150 | 24.9 ± 6.43-150 |
Data are presented as the mean ± SD. Corrected reticulocyte count based on baseline 45.2% Hct of C57BL/6 controls (see Table 1).
P < .005, † P < .05: v baseline.
Effect of High-Mg Diet in C57BL6 Control Mice
| Parameter . | Baseline (n = 6) . | Day 14 (n = 6) . | Day 28 (n = 6) . |
|---|---|---|---|
| Hct (%) | 45.4 ± 1.6 | 46.8 ± 0.1 | 45.2 ± 0.5 |
| Hb (g/dL) | 14.2 ± 0.5 | 15.1 ± 0.2 | 14.4 ± 0.5 |
| MCHC (g/dL) | 29.8 ± 1.6 | 31.2 ± 0.5 | 30.8 ± 0.2 |
| D50 | 1.090 ± 0.001 | 1.088 ± 0.002 | 1.088 ± 0.002 |
| Reticulocytes (%) | 3.6 ± 0.7 | 3.2 ± 1.3 | 3.4 ± 0.8 |
| Corrected reticulocyte count (%) | 3.6 ± 0.7 | 3.3 ± 1.3 | 3.4 ± 0.8 |
| Serum Mg (mmol/L) | 2.8 ± 0.1 | 4.7 ± 0.24-150 | 5.1 ± 0.34-150 |
| Erythrocyte Mg (mmol/kg Hb) | 8.9 ± 0.3 | 9.6 ± 0.2† | 9.7 ± 0.1† |
| Cell Na (mmol/kg Hb) | 53.1 ± 3.6 | 51.6 ± 2.7 | 50.3 ± 2.6 |
| Cell K (mmol/kg Hb) | 421.2 ± 21 | 492.2 ± 25.34-150 | 476.5 ± 244-150 |
| Cell Na + K (mmol/kg Hb) | 474.1 ± 12.3 | 543 ± 28.14-150 | 526.8 ± 24.74-150 |
| K-Cl cotransport (mmol/L cells⋅h) | 4.5 ± 0.8 | 2.7 ± 0.54-150 | 2.8 ± 0.64-150 |
| Parameter . | Baseline (n = 6) . | Day 14 (n = 6) . | Day 28 (n = 6) . |
|---|---|---|---|
| Hct (%) | 45.4 ± 1.6 | 46.8 ± 0.1 | 45.2 ± 0.5 |
| Hb (g/dL) | 14.2 ± 0.5 | 15.1 ± 0.2 | 14.4 ± 0.5 |
| MCHC (g/dL) | 29.8 ± 1.6 | 31.2 ± 0.5 | 30.8 ± 0.2 |
| D50 | 1.090 ± 0.001 | 1.088 ± 0.002 | 1.088 ± 0.002 |
| Reticulocytes (%) | 3.6 ± 0.7 | 3.2 ± 1.3 | 3.4 ± 0.8 |
| Corrected reticulocyte count (%) | 3.6 ± 0.7 | 3.3 ± 1.3 | 3.4 ± 0.8 |
| Serum Mg (mmol/L) | 2.8 ± 0.1 | 4.7 ± 0.24-150 | 5.1 ± 0.34-150 |
| Erythrocyte Mg (mmol/kg Hb) | 8.9 ± 0.3 | 9.6 ± 0.2† | 9.7 ± 0.1† |
| Cell Na (mmol/kg Hb) | 53.1 ± 3.6 | 51.6 ± 2.7 | 50.3 ± 2.6 |
| Cell K (mmol/kg Hb) | 421.2 ± 21 | 492.2 ± 25.34-150 | 476.5 ± 244-150 |
| Cell Na + K (mmol/kg Hb) | 474.1 ± 12.3 | 543 ± 28.14-150 | 526.8 ± 24.74-150 |
| K-Cl cotransport (mmol/L cells⋅h) | 4.5 ± 0.8 | 2.7 ± 0.54-150 | 2.8 ± 0.64-150 |
Data are presented as the mean ± SD.
P < .005, † P < .05: v baseline.
Effect of High-Mg Diet in β Thal Mice
| Parameter . | Baseline (n = 6) . | Day 14 (n = 6) . | Day 28 (n = 6) . |
|---|---|---|---|
| Hct (%) | 30.9 ± 1.5 | 34.7 ± 1.05-150 | 35.6 ± 0.65-150 |
| Hb (g/dL) | 9.5 ± 0.6 | 10.7 ± 0.55-150 | 11.0 ± 0.45-150 |
| MCHC (g/dL) | 32.5 ± 1.4 | 31.2 ± 1.6 | 30.2 ± 0.4† |
| D50 | 1.103 ± 0.002 | 1.097 ± 0.0015-150 | 1.096 ± 0.0015-150 |
| Reticulocytes (%) | 29.2 ± 1.8 | 25.2 ± 3.75-150 | 22.0 ± 3.75-150 |
| Corrected reticulocyte count (%) | 20.0 ± 1.2 | 19.3 ± 2.8 | 17.3 ± 2.9 |
| Serum Mg (mmol/L) | 1.8 ± 0.2 | 3.8 ± 0.45-150 | 4.2 ± 0.65-150 |
| Erythrocyte Mg (mmol/kg Hb) | 8.9 ± 0.2 | 13.8 ± 0.25-150 | 15.0 ± 0.15-150 |
| Cell Na (mmol/kg Hb) | 50.3 ± 4.8 | 49.5 ± 3.9 | 50.2 ± 5.1 |
| Cell K (mmol/kg Hb) | 279 ± 11.5 | 401 ± 485-150 | 382 ± 215-150 |
| Cell Na + K (mmol/kg Hb) | 329.3 ± 18 | 450.8 ± 335-150 | 432.2 ± 12.15-150 |
| K-Cl cotransport (mmol/L cells⋅h) | 11.3 ± 1.5 | 6.8 ± 0.85-150 | 5.0 ± 2.35-150 |
| Parameter . | Baseline (n = 6) . | Day 14 (n = 6) . | Day 28 (n = 6) . |
|---|---|---|---|
| Hct (%) | 30.9 ± 1.5 | 34.7 ± 1.05-150 | 35.6 ± 0.65-150 |
| Hb (g/dL) | 9.5 ± 0.6 | 10.7 ± 0.55-150 | 11.0 ± 0.45-150 |
| MCHC (g/dL) | 32.5 ± 1.4 | 31.2 ± 1.6 | 30.2 ± 0.4† |
| D50 | 1.103 ± 0.002 | 1.097 ± 0.0015-150 | 1.096 ± 0.0015-150 |
| Reticulocytes (%) | 29.2 ± 1.8 | 25.2 ± 3.75-150 | 22.0 ± 3.75-150 |
| Corrected reticulocyte count (%) | 20.0 ± 1.2 | 19.3 ± 2.8 | 17.3 ± 2.9 |
| Serum Mg (mmol/L) | 1.8 ± 0.2 | 3.8 ± 0.45-150 | 4.2 ± 0.65-150 |
| Erythrocyte Mg (mmol/kg Hb) | 8.9 ± 0.2 | 13.8 ± 0.25-150 | 15.0 ± 0.15-150 |
| Cell Na (mmol/kg Hb) | 50.3 ± 4.8 | 49.5 ± 3.9 | 50.2 ± 5.1 |
| Cell K (mmol/kg Hb) | 279 ± 11.5 | 401 ± 485-150 | 382 ± 215-150 |
| Cell Na + K (mmol/kg Hb) | 329.3 ± 18 | 450.8 ± 335-150 | 432.2 ± 12.15-150 |
| K-Cl cotransport (mmol/L cells⋅h) | 11.3 ± 1.5 | 6.8 ± 0.85-150 | 5.0 ± 2.35-150 |
Data are presented as the mean ± SD. Corrected reticulocyte count based on baseline 45.2% Hct of C57BL/6 controls (see Table 1).
P < .005, † P < .05: v baseline.
In β thal mice and C57BL/6 controls, a high-Mg diet resulted in a significant increase in both serum and erythrocyte Mg compared with the standard diet (P < .05; Tables 4 and 5). With 14 days of a high-Mg diet, serum Mg in β thal mice achieved values similar to those observed in C57BL/6 controls, while the Mg content of β thal mouse erythrocytes became significantly higher than that of C57BL/6 controls exposed to either the standard diet or high-Mg diet (P < .005; Tables 4 and 5).
Effects of different Mg dietary intakes on anemia, MCHC, and erythrocyte density of β thal and C57BL/6 mice.In β thal mice, a Mg-restricted diet induced a marked reduction in Hct and Hb and a significant increase in MCHC and D50 (Table 3). The percentage of reticulocytes was significantly increased at days 14 and 28, but the corrected reticulocyte count increased only at day 14 and was similar to baseline levels at day 28 (Table 3). C57BL/6 controls showed a transient increase in reticulocyte count at 14 days only, and not until day 24 did a decrease in Hct and Hb with increased MCHC and D50 become evident (Table 2).
When β thal mice were treated with a high-Mg diet, a marked increase in Hct and Hb and a significant reduction in D50 and MCHC were noted at day 28 (Table 5). We observed no significant changes in corrected reticulocyte counts (Table 5). Values for MCHC and D50 at day 28 were similar to those observed in C57BL/6 control mice exposed to either normal or high-Mg diets. In C57BL/6 controls, the high-Mg diet group showed a transient increase in Hb at day 14 with no significant changes in Hct, MCHC, or D50 at either the 14-day or 28-day time points (Table 4).
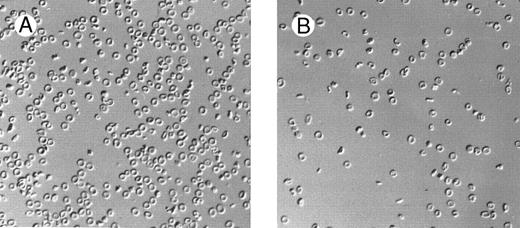
Marked changes in erythrocyte morphology were observed in β thal mice concomitant with the changes in dietary Mg intake (Fig 1). The high-Mg diet group showed a decreased proportion of abnormal cells (target cells, dehydrated cells, and cell fragmentation). In contrast, the low-Mg diet group showed an increase of all morphologic abnormalities. In C57BL/6 control mice, the low-Mg diet group showed changes in erythrocyte morphology, ie, poikilocytosis, with some cells being elongated or spherocytic, with increased Hb concentration. The high-Mg diet had little or no effect on the morphology of C57BL/6 control mouse erythrocytes.
Effect of dietary Mg on erythrocyte morphology. Red blood cell morphology in β thal mice following 28 days of high-Mg diet (A) and low-Mg diet (B).
Effect of dietary Mg on erythrocyte morphology. Red blood cell morphology in β thal mice following 28 days of high-Mg diet (A) and low-Mg diet (B).
Effects of different Mg dietary intakes on erythrocyte cation content and K-Cl cotransport activity in C57BL/6 and β thal mice.At baseline, β thal erythrocytes showed a lower K content (Table 1) and increased K-Cl cotransport activity compared with C57BL/6 controls (Tables 2 and 3). Relative cell dehydration and increased K-Cl cotransport have been observed in human β-thalassemia.30 31
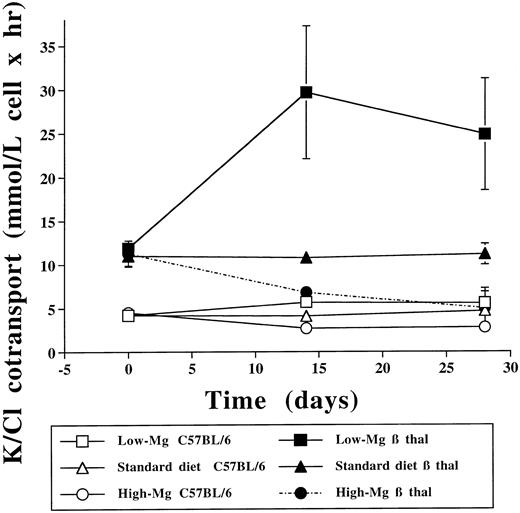
The low-Mg diet group showed a significant increase of K-Cl cotransport in both β thal and C57BL/6 control erythrocytes compared with the standard diet group (P < .05) and the high-Mg diet group (P < .005; Tables 2 and 3 and Fig 2). Conversely, 14 days of a high-Mg diet was followed by a significant reduction in the activity of K-Cl cotransport in both β thal and C57BL/6 control erythrocytes (Tables 4 and 5 and Fig 2). At 28 days, K-Cl cotransport activity in C57BL/6 control mice exposed to a high-Mg diet returned to values that were indistinguishable from baseline but still higher than in C57BL/6 control mice treated with the low-Mg diet. The marked decrease in K-Cl cotransport observed in β thal mice treated with the high-Mg diet persisted at 28 days, reaching values similar to those observed in C57BL/6 controls exposed to a standard diet. Cell age is an important determinant of K-Cl cotransport in human erythrocytes.32 Since there were no substantial changes in the corrected reticulocyte counts at day 28 (Tables 2-5), the changes in K-Cl cotransport activity are most likely due to the variations in erythrocyte Mg rather than to changes in cell age.
Effect of dietary Mg on erythrocyte K-Cl cotransport. Erythrocyte K-Cl cotransport activity in C57BL/6 control and β thal mice at baseline and after 14 and 28 days at 3 different dietary Mg intakes.
Effect of dietary Mg on erythrocyte K-Cl cotransport. Erythrocyte K-Cl cotransport activity in C57BL/6 control and β thal mice at baseline and after 14 and 28 days at 3 different dietary Mg intakes.
Changes in K-Cl cotransport activity were associated with changes in erythrocyte K content. The low-Mg diet produced a significant reduction in cell K content in both β thal and C57BL/6 control mice compared with either the standard-Mg diet (P < .02) or high-Mg diet (P < .005; Tables 2 and 3). Erythrocyte K content increased significantly when β thal and C57BL/6 control mice were treated with the high-Mg diet (P < .05; Tables 4 and 5).
No significant changes in the erythrocyte Na content of C57BL/6 control mice were observed in this study (Tables 2 and 4). β thal mice that received a low-Mg diet showed an increased erythrocyte Na content and those receiving a high-Mg diet showed a decreased Na content compared with the standard diet group (P < .05; Tables 3 and 5).
No significant differences in erythrocyte 2,3-DPG were observed between low- and high-Mg intake regimens (Table 6) in both β thal and C57BL/6 control mice. In β thal mice only, a low-Mg diet induced a significant increase in erythrocyte ATP content compared with a high-Mg diet.
Erythrocyte 2,3-DPG and ATP Content in β Thal and C57BL/6 Control Mice After 28 Days of High-Mg and Low-Mg Diets
| . | High-Mg Diet . | Low-Mg Diet . |
|---|---|---|
| Erythrocyte 2,3-DPG (μmol/g Hb) | ||
| β thal | 32.8 ± 8.6 | 37.3 ± 3.7 |
| C57BL/6 | 37.0 ± 7.4 | 42.5 ± 3.0 |
| Erythrocyte ATP (μmol/g Hb) | ||
| β thal | 4.07 ± 1.08 | 7.1 ± 0.96-150 |
| C57BL/6 | 4.06 ± 2.3 | 4.1 ± 1.2 |
| . | High-Mg Diet . | Low-Mg Diet . |
|---|---|---|
| Erythrocyte 2,3-DPG (μmol/g Hb) | ||
| β thal | 32.8 ± 8.6 | 37.3 ± 3.7 |
| C57BL/6 | 37.0 ± 7.4 | 42.5 ± 3.0 |
| Erythrocyte ATP (μmol/g Hb) | ||
| β thal | 4.07 ± 1.08 | 7.1 ± 0.96-150 |
| C57BL/6 | 4.06 ± 2.3 | 4.1 ± 1.2 |
Determinations were made after 28 days of high-Mg and low-Mg diets. Data are presented as the mean ± SD (n = 6 for each experimental condition).
P < .005 v high-Mg diet.
DISCUSSION
Membrane damage due to an excess of α chains is an important determinant in the anemia of β-thalassemia and may lead to the activation of K-Cl cotransport and relative erythrocyte dehydration. Since increasing erythrocyte Mg inhibits K-Cl cotransport and cell dehydration, and since reduced serum Mg has been reported in patients with β-thalassemia major, we studied the effect of varying dietary Mg intake in a murine model of β-thalassemia (Hbbth1), which also shows reduced serum Mg.
We have shown here that Mg intake modulates the severity of anemia in β thal mice and affects ion transport and erythrocyte hydration status. The anemia of β thal mice is worsened by Mg deficiency and is improved by Mg supplementation. These two phenomena will be discussed later, as will their implication for human β-thalassemia.
Dietary Mg deficiency worsens anemia in β thal mice.Both β thal and C57BL/6 control mice demonstrated decreased Hb and Hct (Tables 2 and 3) when exposed to a low-Mg diet. The corrected reticulocyte counts increased at day 14 and returned to baseline at day 28 in both strains (Tables 2 and 3). Thus, the pathogenesis of the observed anemia is not exclusively due to hemolysis, and a production deficit or an increased ineffective erythropoiesis may also play a role.
These results are not unexpected, since it had been shown in rats that severe dietary Mg restriction induces anemia with alterations in erythrocyte metabolism that include decreased glucose utilization, lactate production, and erythrocyte ATP and 2,3-DPG.33 Our data indicate that in C57BL/6 mice, erythrocyte ATP and 2,3-DPG contents of low- and high-Mg diets are similar (Table 6). In β thal mice, a low-Mg diet induced a significant increase in ATP compared with a high-Mg diet (Table 6). Thus, ATP or 2,3-DPG deficiency is not involved in the pathogenesis of anemia induced by Mg deficiency in mice. Mg deficiency in hamsters increases the susceptibility to free radical injury,34 and in rats it leads to a significant reduction in erythrocyte glutathione, which is reversed by administration of vitamin E or D-propranolol.35 The structure of the rat cell membrane is also altered by Mg deprivation, with formation of characteristic round or oval plaques.36 Intravascular hemolysis may also play a significant role in Mg-depleted rats.33 37
In human erythrocytes, in vitro studies have shown that Mg depletion affects the stability of the erythrocyte membrane with no changes in elasticity, measured as shear elastic modulus, and leads to echinocytosis and increased thermal sensitivity, possibly indicating altered cytoskeletal interactions.38 These parameters could not be tested in the present study, and may play a role in the anemia we observed with the low-Mg diet.
In erythrocytes of Mg-deficient mice, we observed no changes in cell Na and a decrease in cell K (Tables 2 and 3). These results can be explained on the basis of the concomitant increase in K-Cl cotransport activity. Mg deficiency in rats leads to increased cell Na and unchanged cell K, mediated by a reduction in the activity of the ouabain-sensitive Na-K pump and increased passive permeability to Na.39 Mouse erythrocytes have transport characteristics similar to human erythrocytes,27 whereas rat erythrocytes have increased rates of transport via the Na-K pump, Na-K-Cl cotransport, and markedly increased (fivefold to sixfold) passive Na permeability.40
Relative cell dehydration is associated with the anemia of thalassemia. Using the same mouse model, we have previously demonstrated that although dehydration can be prevented by oral administration of clotrimazole, this change leads to no significant improvement of the anemia.20 The improvement of anemia induced by hydroxyurea or rHuEPO was associated with an increased erythrocyte hydration and K content in β thal mice.
Dietary Mg supplementation improves the anemia of β thal mice.Dietary Mg supplementation improves the anemia (increased Hb, Table 5) and the erythrocyte phenotype (Fig 1) of β thal mice. There have been no previous reports on the beneficial effects of Mg supplementation in human or mouse β-thalassemia. Although the diet in our control groups with standard diet matches the established daily requirements for Mg and serum and erythrocyte Mg were stable in animals treated for 28 days with the standard diet (data not shown), we cannot rule out that β thal mice might have increased Mg turnover and/or Mg requirements versus normal control mice. There is some indirect evidence that changes in erythrocyte Mg may be helpful in detecting the response to Mg supplementation in normal human subjects with marginal Mg deficiency.41 It is interesting that the high-Mg diet induced a similar change in serum Mg in β thal and C57BL/6 control mice but there was a much greater increase in erythrocyte Mg in β thal mice (Tables 4 and 5). The large increase in erythrocyte Mg observed in β thal mice after Mg supplementation (Table 5) could be interpreted as evidence of Mg deficiency41 or indicate a different handling of Mg by the thalassemic red blood cell membrane.
The dramatic effects of Mg deprivation on erythrocyte membrane structure suggest that Mg supplements may improve “membrane stability” in thalassemic mouse erythrocytes. If the reduced serum Mg (Table 1) plays a direct role in the hemolysis of thalassemic mouse erythrocytes (as it seems to in rat erythrocytes37 ), restoring normal or above-normal serum Mg levels may reduce hemolysis independently of the changes in erythrocyte Mg. Whereas β thal erythrocytes maintain normal erythrocyte Mg despite the reduced serum Mg, two mouse strains have been described with increased (MGH) or decreased (MGL) serum and erythrocyte Mg.42 These two strains have significant differences in cell Hb concentration, K content, and K-Cl cotransport (De Franceschi and Brugnara, unpublished results, February 1997).
Based on our previous results with oral administration of clotrimazole in this mouse model, cell dehydration per se does not play a role in the hemolysis of thalassemic mouse erythrocytes.20 An increased erythrocyte volume and K content and a reduced density may reflect improved erythrocyte survival related to increased plasma or erythrocyte Mg concentration, as observed in this study.
In a transgenic model for sickle cell anemia, Mg deficiency induces anemia with cell dehydration and increased morphologic abnormalities of erythrocytes, whereas Mg supplementation leads to improvement of the anemia and reduced dehydration.2 The positive effects of dietary Mg supplementation in sickle cell mouse models can be explained by the benefits of a reduction in cellular Hb S concentration, with consequent reduced Hb S polymerization and sickling. It is also a possibility that certain hemolytic disorders of mouse and human lead to increased requirements for Mg or that Mg supplements “stabilize” damaged red blood cell membranes. In this case, the beneficial effect of Mg may be due to both specific interactions with K-Cl cotransport and other effects on the red blood cell membrane.
Mg and human β-thalassemia.The results obtained in this mouse model of β-thalassemia are relevant to the pathophysiology and possible therapy for the human disease. Although the daily requirement for Mg in rodents (400 mg Mg/kg body weight) is much higher (approximately 70-fold) than for humans (400 mg/d),23 the extent of Mg supplementation we used in this mouse model (2.5-fold the daily requirement) is not much greater than that used in previous studies in normal human subjects.43 44 The human studies added to the regular diet the equivalent of the daily recommended amount of Mg, with no reported side effects.
In heterozygous β-thalassemia and untransfused β-thalassemia intermedia, a reduced erythrocyte Mg content has been observed with normal serum Mg.11,12 Patients with homozygous β-thalassemia have been shown to be in a state of Mg deficit, with markedly reduced serum Mg levels and normal or above-normal erythrocyte Mg content.10,45 There is no clear explanation for the hypomagnesemia of homozygous β-thalassemia, which may be severe enough to induce clinically relevant neuromuscular symptoms.10 Mg absorption is normal in thalassemia,46 but urinary Mg excretion is reduced.45 It has been speculated that hypomagnesemia could be due to chelation by citrate in chronically transfused patients, or could just be a consequence of the cellular iron overload.10
In conclusion, the present study shows that Mg supplementation improves the anemia of β thal mice. It also compensates for the abnormal erythrocyte K loss by decreasing the activity of the K-Cl cotransport system. These results indicate that Mg supplementation should be tried as a possible new therapeutic strategy to improve anemia in human β-thalassemia intermedia.
Supported by grants from the National Heart, Lung, and Blood Institute (P60-HL15157) and the National Institute of Diabetes and Digestive and Kidney Diseases (R01-DK50422), National Institutes of Health.
Address reprint requests to Carlo Brugnara, MD, Department of Laboratory Medicine, The Children's Hospital, 300 Longwood Ave, Bader 760, Boston, MA 02115.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal