Abstract
Interleukin-1β (IL-1β) is considered an important mediator in the pathogenesis of septic shock or bacterial meningitis. Its activity is specifically modulated by IL-1 receptor antagonist (IL-1Ra) and IL-1 soluble receptor type II (IL-1sRII). We now describe the time-course of IL-1β and these modulating agents in 59 patients with acute meningococcal infections, the prototype human disease of acute endotoxin exposure. Plasma IL-1β was increased only in severe shock and normalized within 12 to 24 hours, indicating that patients were admitted in an early stage of cytokine activation. Increased IL-1β values in cerebrospinal fluid (CSF ) were confined to patients with meningitis. Plasma IL-1Ra was elevated in both shock and nonshock patients, extremely high values being measured in severe shock. High concentrations of IL-1Ra in CSF were found in meningitis. Plasma IL-1Ra peaked shortly after IL-1β and decreased steeply in 1 to 2 days, followed by sustained moderately elevated levels in shock patients. Interestingly, IL-1sRII showed a completely different pattern. At admission, both nonshock and shock patients manifested a similar moderate increase of plasma IL-1sRII. However, during recovery plasma IL-1sRII further increased reaching maximal concentrations 3 to 5 days after admission, 1 to 2 days after normalization of IL-1Ra. In shock patients this increase was more prominent than in nonshock patients. It is hypothesized that this increase in plasma IL-1sRII can be explained by a synergistic effect of dexamethasone and endotoxin. A second interesting observation was that, unlike the pattern in plasma, IL-1sRII levels in CSF paralleled those of IL-1β and IL-1Ra. This suggests different modulation of IL-1β activity in the subarachnoid space and the plasma compartment. We conclude that: (1) During the early stage of meningococcal infections IL-1Ra modulates IL-1 activity, whereas during recovery IL-1sRII may be more important. (2) Modulation in CSF and in the plasma compartment are differentially regulated.
ACUTE MENINGOCOCCAL infections are the prototype disease of overwhelming endotoxin exposure in humans and are characterized by a generalized activation of the cytokine network.1 High plasma concentrations of endotoxin and proinflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) correlate well with the severity of shock, whereas in meningitis elevated concentrations are found in cerebrospinal fluid (CSF ).1-8 Proinflammatory cytokines are modulated by an early activation of antiinflammatory cytokines, including IL-1 receptor antagonist (IL-1Ra), IL-6, and IL-10.9-12 Further modulation takes place at cytokine receptor level by regulation of receptor density on target cells and the presence of soluble receptors.9,12-14 As such, we previously reported that in severe shock, TNF soluble receptors are increased and IL-6 soluble receptors (IL-6sR) are decreased, resulting in less activity for both ligand cytokines.9,12 14
The regulation of IL-1 activity is presumably more complex. The proinflammatory effects of IL-1 are mediated by IL-1α that remains mainly cell-associated and by IL-1β that is mostly released. These effects are restrained by IL-1Ra, a peptide that binds to IL-1 receptors (IL-1R) but lacks agonist activity.15 IL-1α is undetectable in plasma of patients with meningococcal infections, whereas plasma IL-1β is increased only in patients with severe shock.1,4,12 Plasma IL-1Ra is elevated in all patients with meningococcal infections but extremely high values are found during severe shock.9,12 IL-1 activity is also modulated by the density of cell-bound IL-1R and the presence of IL-1sR, generated by cleavage of the extracellular domain of IL-1R. Two types of IL-1R exist: IL-1R type I (IL-1RI), predominately expressed on the surface of T cells and fibroblasts with IL-1α, IL-1β, and IL-1Ra as ligands, and IL-1R type II (IL-1RII) primarily expressed on the surface of B cells and monocytes with IL-1β and, to a lesser extent, IL-1Ra as ligands.16,17 Transmission of IL-1 signals occurs via IL-1RI whereas IL-1RII functions merely as “decoy” receptor.18-20 Cell-bound IL-1RI and IL-1sRI bind IL-1α, IL-1β, and IL-1Ra with approximately similar kinetic constants. However, IL-1sRII preferentially binds IL-1β but has lost its affinity to IL-1Ra a 2,000-fold.21-24 Thus, the presence of IL-1 soluble receptors modulates IL-1 activity.23-25 Reportedly, plasma IL-1sRII is increased during sepsis.26 To date, no information is available on the relative contribution of IL-1Ra and IL-1sRII to the modulation of IL-1 activity during severe human infections.
The present study describes the pattern of these compounds in plasma and CSF of patients with acute life-threatening meningococcal infections at admission and the time course in plasma during the first week of recovery.
MATERIALS AND METHODS
From November 1990 to March 1996, 65 patients with bacteriologically proven acute meningococcal meningitis or shock were referred to the intensive care unit (ICU) of the University Hospital Nijmegen, The Netherlands, within 12 hours after their first hospital admission. From 54 of these patients, one or more serum or plasma samples were obtained at admission and at later time points. Five patients admitted to the ICU of the Eemland Hospital Amersfoort, The Netherlands, were sampled in a similar fashion.
Demographic and Prognostically Relevant Clinical and Laboratory Parameters
| . | Group A . | Group B . | Group C . |
|---|---|---|---|
| . | Meningitis Without Shock . | Meningitis With Shock . | Shock Without Meningitis . |
| No. female/male | 14/12 | 7/6 | 6/14 |
| Age, yr | 14 (1-64) | 5 (0-21) | 3‡ (0-30) |
| Duration of disease before admission, h | 24.0 (12.5-84) | 14.0ρ (3.0-30) | 12.01-155 (2.8-16.5) |
| Leukocytes in CSF, × 106/L | 12,300 (>100-70,000) | 4341-155 (101-19,100) | 15∥¶ (1-98) |
| Peripheral leukocytes*, × 109/L | 19.5 (10.5-29.9) | 11.4ρ (2.4-26.8) | 3.81-155 (1.4-13.6) |
| Platelets*, × 109/L | 158 (51-388) | 70‡ (39-325) | 471-155 (16-171) |
| Fibrinogen*, mg/L | 5,200 (3,500-12,000) | 2,4001-155 (230-4,115) | 1,1831-155 (90-3,732) |
| Lactate†, μmol/L | 2,123 (409-4,070) | 3,412‡ (1,806-7,013) | 5,1081-155 (1,529-14,087) |
| No. of fatalities | 2 (8%) | 1 (8%) | 6 (30%) |
| . | Group A . | Group B . | Group C . |
|---|---|---|---|
| . | Meningitis Without Shock . | Meningitis With Shock . | Shock Without Meningitis . |
| No. female/male | 14/12 | 7/6 | 6/14 |
| Age, yr | 14 (1-64) | 5 (0-21) | 3‡ (0-30) |
| Duration of disease before admission, h | 24.0 (12.5-84) | 14.0ρ (3.0-30) | 12.01-155 (2.8-16.5) |
| Leukocytes in CSF, × 106/L | 12,300 (>100-70,000) | 4341-155 (101-19,100) | 15∥¶ (1-98) |
| Peripheral leukocytes*, × 109/L | 19.5 (10.5-29.9) | 11.4ρ (2.4-26.8) | 3.81-155 (1.4-13.6) |
| Platelets*, × 109/L | 158 (51-388) | 70‡ (39-325) | 471-155 (16-171) |
| Fibrinogen*, mg/L | 5,200 (3,500-12,000) | 2,4001-155 (230-4,115) | 1,1831-155 (90-3,732) |
| Lactate†, μmol/L | 2,123 (409-4,070) | 3,412‡ (1,806-7,013) | 5,1081-155 (1,529-14,087) |
| No. of fatalities | 2 (8%) | 1 (8%) | 6 (30%) |
Medians are shown, range between brackets.
Abbreviation: CSF, cerebrospinal fluid.
Nadir* or maximum measured within the first 12 hours after admission.
Maximum† measured within the first 12 hours after admission.
Compared to group A:
P < .05.
ρ P < .01.
P < .001.
Compared to group B:
P < .001.
Admission Values of IL-1β, IL-1Ra, and IL-1sRII in Plasma and CSF
| . | Group A . | Group B . | Group C . |
|---|---|---|---|
| . | Meningitis Without Shock . | Meningitis With Shock . | Shock Without Meningitis . |
| IL-1β | |||
| Plasma concentration, pg/mL | 55 (<40-135) | 70 (<40-250) | 140† (<40-2,500) |
| CSF concentration, pg/mL | 1,750 (<400-2,850) | 490 (65-3,150) | 67.5‡ (<40-1,550) |
| IL-1Ra | |||
| Plasma concentration, pg/mL | 6,750 (2,000-30,000) | 10,600 (5,100-390,000) | 145,000‡ρ (6,480-805,000) |
| CSF concentration, pg/mL | 26,500 (920-140,000) | 5,500 (<600-140,000) | 1,800* (<600-6,800) |
| Soluble IL-1RII | |||
| Plasma concentration, ng/mL | 23.9 (17.9-57.9) | 29.5 (18-57.1) | 22.7 (15.8-51.2) |
| CSF concentration, ng/mL | 36.3 (7.0-160.4) | 9.1 (<2.5-58.0) | 3.1‡ (<2.5-3.8) |
| . | Group A . | Group B . | Group C . |
|---|---|---|---|
| . | Meningitis Without Shock . | Meningitis With Shock . | Shock Without Meningitis . |
| IL-1β | |||
| Plasma concentration, pg/mL | 55 (<40-135) | 70 (<40-250) | 140† (<40-2,500) |
| CSF concentration, pg/mL | 1,750 (<400-2,850) | 490 (65-3,150) | 67.5‡ (<40-1,550) |
| IL-1Ra | |||
| Plasma concentration, pg/mL | 6,750 (2,000-30,000) | 10,600 (5,100-390,000) | 145,000‡ρ (6,480-805,000) |
| CSF concentration, pg/mL | 26,500 (920-140,000) | 5,500 (<600-140,000) | 1,800* (<600-6,800) |
| Soluble IL-1RII | |||
| Plasma concentration, ng/mL | 23.9 (17.9-57.9) | 29.5 (18-57.1) | 22.7 (15.8-51.2) |
| CSF concentration, ng/mL | 36.3 (7.0-160.4) | 9.1 (<2.5-58.0) | 3.1‡ (<2.5-3.8) |
Median values are shown, range between brackets.
Compared to group A:
P < .05.
P < .01.
P < .001.
Compared to group B:
ρ P < .05.
Blood sampling in these 59 patients started median 2.6 hours (range, 0.0 to 14.2) after first hospital admission. The cytokine plasma concentrations at admission of 36 of these patients were reported before.12 From 45 patients, more than one sample was drawn at different time points. In 39 of them, daily samples from admission to death or recovery up to 7 days were drawn. A CSF sample obtained directly after admission was available from 25 patients.
Patients were prospectively classified in 3 groups, suffering from meningitis (group A, n = 26), meningitis and shock (group B, n = 13), or shock without meningitis (group C, n = 20). According to Halstensen et al,27 and as reported by us previously, outcome differs clearly among these groups, with mortality rates of 1.3%, 7.4%, and 28.6% in groups A through C, respectively. Meningitis was defined as the presence of nuchal rigidity or a CSF leukocyte count > 100 × 106/L. Shock was defined as the presence of systolic hypotension occurring within the first 12 hours after admission that was refractory to a fluid bolus and required inotropics or vasopressors. In infants younger than 4 years the systolic blood pressure had to be < 75 mm Hg, in children between 4 and 14 years < 85 mm Hg, and in patients older than 14 years < 100 mm Hg.12,27-29 Table 1 shows the demographic and prognostically relevant characteristics, such as gender, age, duration of disease before admission, leukocyte count in CSF, the nadir within the first 12 hours of peripheral leukocytes, platelets, and fibrinogen and the maximal serum lactate concentration.
All patients were treated with appropriate antibiotics and 57 received also steroids in a dosage equivalent to 0.6 mg/kg/d dexamethasone during 3 days. Of the 33 shock patients, 23 were treated with plasma exchange or whole blood exchange (PEBE) during the first 24 or 48 hours, as described before.30
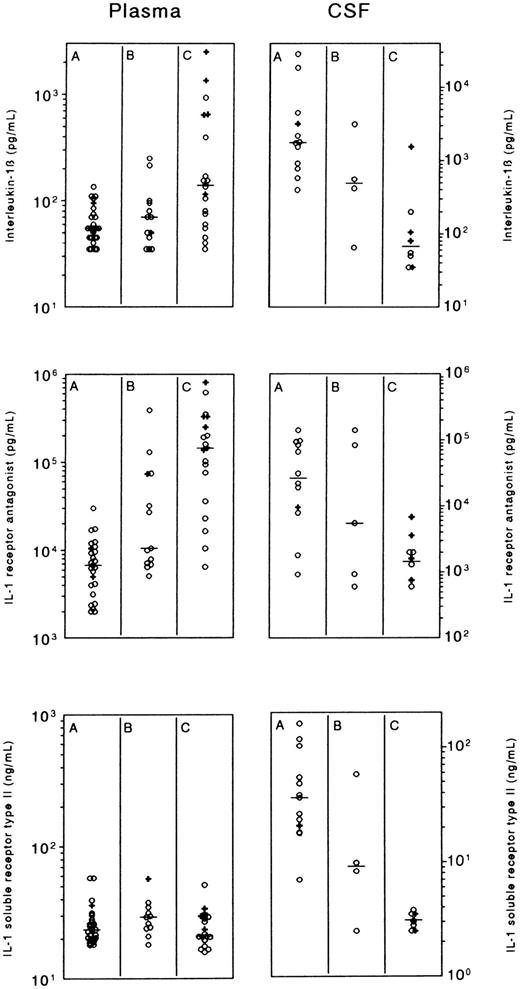
Plasma and CSF concentrations of interleukin-1β (IL-1β), IL-1 receptor antagonist (IL-1Ra), and IL-1 soluble receptor type II (IL-1sRII), in patients with meningococcal meningitis without shock (group A), meningitis with shock (group B), or shock without meningitis (group C). The left panel shows the maximal plasma concentration at the day of admission of IL-1β and IL-1Ra, and the first measured value of IL-1sRII. The right panel shows the CSF concentrations. Horizontal lines indicate medians; crosses refer to patients who died.
Plasma and CSF concentrations of interleukin-1β (IL-1β), IL-1 receptor antagonist (IL-1Ra), and IL-1 soluble receptor type II (IL-1sRII), in patients with meningococcal meningitis without shock (group A), meningitis with shock (group B), or shock without meningitis (group C). The left panel shows the maximal plasma concentration at the day of admission of IL-1β and IL-1Ra, and the first measured value of IL-1sRII. The right panel shows the CSF concentrations. Horizontal lines indicate medians; crosses refer to patients who died.
In total, 9 patients died: 2 patients of group A died because of cerebral herniation 2.5 hours after primary admission, 6 patients of group C died because of intractable shock after median 16.3 hours (range, 2.5 to 28), and 1 patient of group B died after 100 hours when treatment was stopped because of renal failure, severe metabolic acidosis, and mutilating limb gangrene. Among the 50 survivors, 2 patients of group A suffered from sensorineural deafness and 7 patients of groups B and C had serious ischemic sequaelae necessitating extensive plastic surgery.
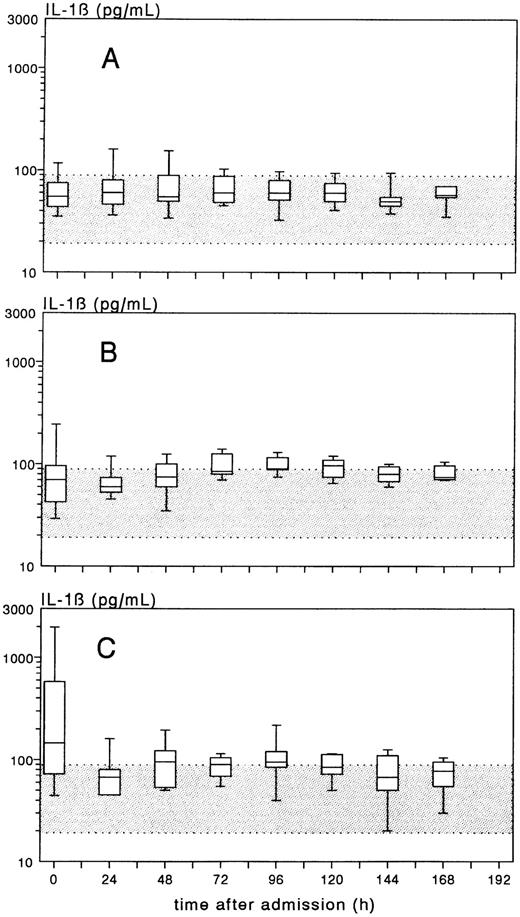
Time-course of the plasma concentration of IL-1β, expressed as box-plots, in patients with meningococcal meningitis without shock (group A), meningitis plus shock (group B), or shock without meningitis (group C). The gray band indicates the normal range of IL-1β (mean ± 2 × SD).
Time-course of the plasma concentration of IL-1β, expressed as box-plots, in patients with meningococcal meningitis without shock (group A), meningitis plus shock (group B), or shock without meningitis (group C). The gray band indicates the normal range of IL-1β (mean ± 2 × SD).
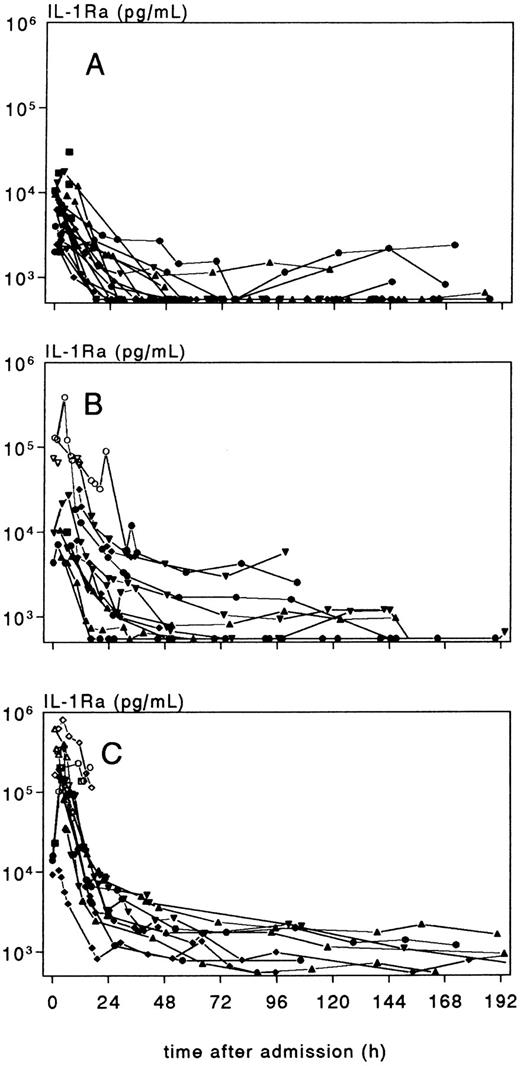
Individual time-course of plasma IL-1Ra concentration, in patients with meningococcal meningitis without shock (group A), meningitis plus shock (group B), and shock without meningitis (group C). Values < 25,000 pg/mL, indicated with an open marker, are estimates based on diluted samples.
Individual time-course of plasma IL-1Ra concentration, in patients with meningococcal meningitis without shock (group A), meningitis plus shock (group B), and shock without meningitis (group C). Values < 25,000 pg/mL, indicated with an open marker, are estimates based on diluted samples.
IL-1β and IL-1Ra were measured by radioimmunoassays (RIA) as described previously.31 IL-1β and IL-1Ra showed no cross-reaction. However, IL-1sRII at higher concentrations (> 100 ng/mL) interfered with the IL-1β assay, by capturing labeled IL-1β. As a consequence, IL-1β concentrations were slightly overestimated when IL-1sRII concentrations were above 100 ng/mL. For IL-1β, 100-μL sample was used, resulting in a lower detection limit of 40 pg/mL and an upper limit of 2,500 pg/mL. High (CSF ) samples were properly diluted. The mean IL-1β plasma concentration ± SD in 143 healthy controls was 55 ± 18 pg/mL. The normal IL-1β concentration in CSF (n = 8) was 76 ± 10 pg/mL. The IL-1Ra assay lacked parallelity,12 therefore a fixed amount of 10 μL was used for all assays. Concentrations exceeding the upper detection limit of 25,000 pg/mL are estimates based on measurements in further diluted samples. Normal plasma and CSF concentrations fell below the detection limit of the assay (< 600 pg/mL). IL-1sRII was measured by a specific RIA similar to that for IL-1β and IL-1Ra. For the assay, 25-μL sample was used, giving a detection range of 2.5 to 320 ng/mL. With this assay, values in serum appeared to be reproducibly higher than plasma values, thus IL-1sRII data obtained in serum were corrected according to the following formula: plasma value = 0.775 × serum value + 3.17 (formula calculated from 29 paired serum and plasma samples [r = .995]). Normal values of IL-1sRII were for plasma (n = 36) 16.5 ± 2.8 ng/mL and for CSF (n = 9) < 2.5 ng/mL.
Statistical comparison between groups was performed with analysis of variance and Dunn's test or two-sided Fisher's exact test.
RESULTS
The plasma and CSF admission values, ie, the maximum value of IL-1β and IL-1Ra during the day of admission (vide infra), and the first value of IL-1sRII, are shown in Fig 1 and Table 2.
Increased plasma IL-1β concentrations, defined as > 91 pg/mL (mean plus 2 × SD of healthy controls) were found in 17 out of the 33 (51%) group B and C patients, all with very profound shock, among them 6 of the 7 fatalities. In 8 of the 10 shock patients (80%) with increased IL-1β values and more than one sample within the first 6 hours, IL-1β decreased rapidly, whereas in 2 (20%) a short-lasting increase occurred. After 12 to 24 hours, plasma IL-1β levels reached a stable level in all patients. PEBE did not affect the course of IL-1β. During recovery, the median IL-1β plasma concentrations in groups B and C increased somewhat, with a maximum of 97.5 pg/mL (median) on day 5 in group B and 95.0 pg/mL on day 4 in group C (Fig 2). IL-1β in CSF of groups A, B, and C showed a reciprocal pattern, with the highest values in meningitis patients of group A, and the lowest values in patients with shock without meningitis of group C.
Plasma IL-1Ra concentrations were increased at admission in all patients but an impressive elevation was observed in severe shock. During recovery, plasma IL-1Ra rapidly declined within 1 to 2 days (Fig 3), followed by a more sustained and moderate elevation in shock patients. In 5 (36%) of the 14 shock patients who had more than one sample within the first 6 hours, IL-1Ra decreased directly after admission, whereas in the remaining 64%, the decrease was preceded by an initial increase. There was no effect of PEBE on the IL-1Ra pattern. After 4 to 5 days, those patients who experienced a second febrile episode coinciding with a rash and/or sterile reactive arthritis, had again an increase of IL-1Ra. The pattern of IL-1Ra in CSF was similar to that of IL-1β, with the highest values in meningitis (group A) and the lowest values in shock patients without meningitis (group C).
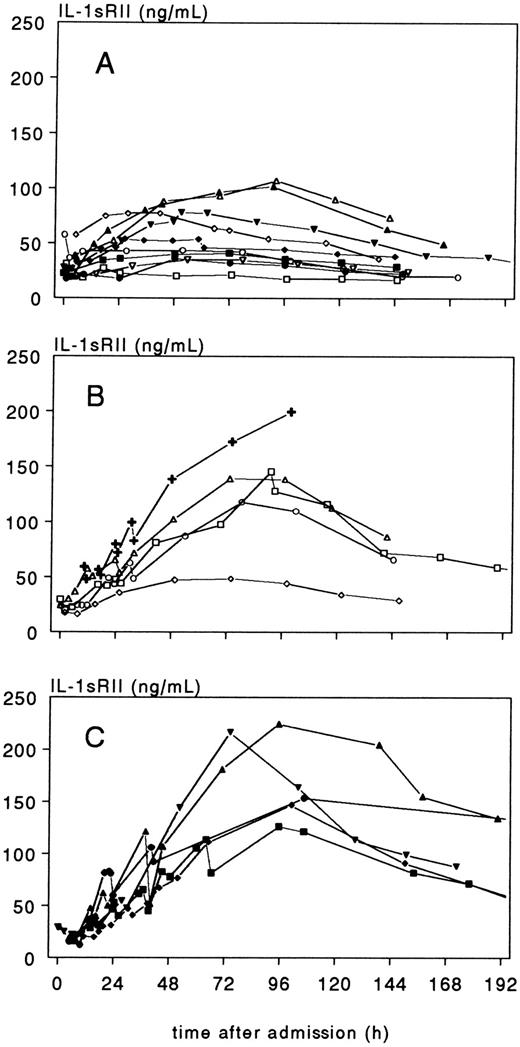
In contrast to IL-1β and IL-1Ra, plasma IL-1sRII concentrations at admission did not differ significantly between groups A, B and C, and were approximately 25 ng/mL in all groups. IL-1sRII transiently decreased during PEBE sessions, especially at day 2 when IL-1sRII concentrations were high (> 100 ng/mL). Most remarkably, IL-1sRII behaved completely different from IL-1β and IL-1Ra during recovery (Fig 4). In 18 of the 20 patients with sequential measurements, IL-1sRII increased during recovery reaching maximal concentrations in group A of median 65.6 ng/mL (range, 35.1 to 107.3) at median 53 hours (range, 26.5 to 92) after admission, and in groups B and C of median 145.4 ng/mL (range, 48.6 to 224.8; P = .0009 v group A) at 93.3 hours (range, 73 to 107) after admission. This peak coincided with the moderate increase in IL-1β as shown in Fig 2. In contrast to plasma concentrations, CSF concentrations of IL-1sRII discriminated well between groups A, B, and C, with the highest values in meningitis patients of group A and the lowest values in shock patients of group C.
Individual time-course of plasma IL-1sRII concentration, in 10 patients with meningococcal meningitis without shock (group A), 5 patients with meningitis and shock (group B), and 5 patients with shock without meningitis (group C). The crosses in (B) refer to a patient who died 102 hours after admission.
Individual time-course of plasma IL-1sRII concentration, in 10 patients with meningococcal meningitis without shock (group A), 5 patients with meningitis and shock (group B), and 5 patients with shock without meningitis (group C). The crosses in (B) refer to a patient who died 102 hours after admission.
DISCUSSION
In this study, IL-1β and its natural antagonists IL-1Ra and IL-1sRII were measured in plasma and CSF from patients with the prototype disease of acute endotoxin exposure, ie, acute meningococcal infections.
Elevated plasma IL-1β concentrations were found only in patients with severe shock at a very early stage. Within 12 to 24 hours after admission, plasma IL-1β levels normalized. In the majority of these patients (80%), IL-1β decreased rapidly from admission onwards, indicating that IL-1β activation occurred in a very early preclinical stage of the disease. In patients with meningitis without shock, IL-1β was increased in CSF reflecting compartmentalization of IL-1β synthesis, a distinctive characteristic of meningococcal infections.6-8 32
In one of our earlier studies, we reported an increase in plasma IL-1β concentrations during recovery of meningococcal infections, between 45 and 118 hours after admission.33 In that study IL-1β was measured in chloroform-extracted samples using a slightly different RIA.34 In subsequent measurements, using the D10-cell bioassay and other immunochemical assays, we were unable to substantiate this observation. In the present study, an improved and adapted RIA was used.31 It now appears that, during recovery IL-1β increased only slightly (Fig 2), and that this increase could be explained by interference of high IL-1sRII concentrations. This observation underscores once again the impact of soluble cytokine receptors on the measurement of the ligand cytokine.22,35 36
Elevated plasma IL-1Ra concentrations are found after various physical and infectious stimuli.31,37-39 Like IL-6, IL-1Ra can be considered an easy and reliable indicator of activation of the cytokine network.12 In the present study, IL-1Ra was increased at admission in all shock and nonshock patients. However, in patients with severe shock, very high plasma concentrations were present (median value for fatalities 250,000 pg/mL, range 73,500 to 805,000). Of the shock patients, 64% had a short increase of IL-1Ra after admission in contrast to 20% for IL-1β (P = .04). This supports the experimental observation that, after endotoxin infusion, plasma IL-1Ra increases shortly following IL-1β synthesis.40 It also underlines the fact that patients with meningococcal sepsis enter the hospital in a very early stage of cytokine activation and that apparently the devastating pathophysiological derangement occurs within a few hours after the first clinical signs and symptoms. As expected, IL-1Ra accumulation in patients with meningitis without shock occurred merely in the subarachnoid space.
Remarkably, the pattern of plasma IL-1sRII was entirely different from that of IL-1β and IL-1Ra. Both upregulation of the “decoy” receptor IL-1RII and shedding of this receptor are antiinflammatory processes. Shed IL-1RII has even stronger antiinflammatory potencies than cell-bound IL-1RII, because as compared to IL-1Ra, shed receptors bind IL-1β with higher affinity.23 Short-lasting studies in humans after endotoxin infusion failed to show increased plasma IL-1sRII levels.41,42 However, during sepsis and after steroid therapy, IL-1RII mRNA is found to be upregulated and increased densities of cell-bound IL-1RII on neutrophils are measured.20,43-46 Reportedly, during sepsis plasma levels of IL-1sRII are only moderately elevated.26 42 Our findings are in agreement with these observations although we measured equally increased IL-1sRII plasma concentrations at admission in patients without or with shock. However, in contrast to previous studies in septic patients, we observed a gradual increase of IL-1sRII during recovery, reaching maximum levels 3 to 4 days after IL-1β activation and 1 to 2 days after subsidence of plasma IL-1Ra.
Currently, the mechanism of this intriguing pattern is unclear. Several factors may have influenced this pattern. Meningococcal sepsis is characterized by massive complement activation,47,48 and reportedly, C5a is a potent inducer of IL-1RII release from neutrophils.49 However, the major difference between the present study and previous studies in human sepsis26,42 is that all patients in the present study received dexamethasone. Dexamethasone in vitro stimulates IL-1sRII mRNA expression and increases the density of the cell-bound receptor.43-46 Lipopolysaccharide (LPS) alone is unable to affect IL-1RII synthesis.50 However, as was recently shown,50 the combination of LPS with dexamethasone induces substantial shedding of IL-1sRII in in vitro experiments. Our clinical data support such a synergistic effect of dexamethasone, and suggest that the magnitude of the antiinflammatory action of dexamethasone depends on the severity of the initial inflammatory signal. In the present study, nonshock and shock patients received equal doses of dexamethasone; however, the highest IL-1sRII concentrations were found in shock patients of groups B and C, who reportedly display the highest levels of endotoxemia and complement-activation.2,12,47 48
A second remarkable observation in the present study is that, unlike the concentration of IL-1sRII in plasma, the concentration in CSF parallels that of IL-1β and IL-1Ra, with the highest concentration in meningitis patients. Thus, cytokine activation and regulation in the CSF-compartment in meningococcal meningitis is not a mere copy of the generalized cytokine activation in meningococcal sepsis.5-8 It is tempting to speculate that IL-1sRII in the CSF-compartment originates from the activated neutrophils that have invaded this extravascular space.
In previous studies, we have shown that TNF soluble receptors are increased and IL-6sR is decreased early in meningococcal infections.9,12 14 We now show that IL-1β activity is modulated in a more complex fashion. Systemic IL-1 activity is mitigated by IL-1Ra at an early stage of the infection and by IL-1sRII at a later stage, whereas in the subarachnoid space, both antagonists are increased simultaneously early during the infection.
ACKNOWLEDGMENT
We thank M.B. Widmer (Immunex, Seattle) for the kindful supply of IL-1sRII.
Address reprint requests to Marcel van Deuren, MD, Department of Internal Medicine, University Hospital Nijmegen, PO Box 9101, 6500 HB Nijmegen, The Netherlands.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal