Abstract
Prostacyclin (prostaglandin I2, PGI2) is a potent vasodilator and inhibitor of platelet aggregation. Although it is well known that the specific receptor for prostacyclin (PGI2-R) is abundantly expressed on platelets, PGI2-R expression in megakaryocytes is poorly understood. In this study, we examined its expression in leukemic or normal megakaryocytes. PGI2-R mRNA was expressed in human leukemic cell lines of megakaryocytic nature as evaluated by Northern blot analysis. Phorbol 12-myristate 13-acetate (PMA), interleukin-1 (IL-1), IL-3, IL-6, granulocyte-macrophage colony-stimulating factor (GM-CSF ), thrombopoietin (TPO), and tumor necrosis factor-α (TNF-α) enhanced PGI2-R mRNA expression. The enhancement of PGI2-R expression by PMA and TPO was associated with the upregulation of platelet factor 4 or glycoprotein IIb mRNA expression. Iloprost, an agonist of prostacyclin, induced significant cyclic (c)AMP synthesis in these leukemic cells indicating that interaction of PGI2-R and its ligand can induce postreceptor signal transduction. Furthermore, iloprost-induced cAMP synthesis was enhanced by the pretreatment with PMA or the cytokines that promoted PGI2-R expression. PMA and TPO also increased the specific binding of [3H]iloprost to these cells. Pooled normal megakaryocytic colonies from TPO-containing semisolid culture of purified human CD34+ cells expressed PGI2-R, which were increased as the megakaryocytes matured with the peak expression before proplatelet formation, as evaluated by semiquantitative reverse transcription-polymerase chain reaction (RT-PCR). These results indicate that PGI2-R is expressed in human megakaryocytes and is upregulated by cytokines involved in thrombopoiesis or inflammation. Also, it was indicated that megakaryocytic maturation accompanies enhancement of PGI2-R expression.
PROSTANOIDS ARE cyclooxygenase metabolites of arachidonic acid and are one of the regulatory factors maintaining homeostasis in the body such as vitamins and hormones. Prostanoids, synthesized in various organs, act locally and are immediately degenerated in situ or inactivated during one passage through the lung. Prostacyclin (prostaglandin I2, PGI2) and thromboxane A2 (TxA2) are the major prostanoids regulating the homeostasis of the blood-vessel system. They mostly counteract each other and are also opposite each other in their blood concentrations in ischemic heart disease; while the blood level of prostacyclin decreases, that of TxA2 increases.1-3 Prostacyclin is a potent vasodilator and inhibitor of platelet aggregation. The major source of which is arterial endothelial cells.4 With these biological activities, prostacyclin and its analogues have been clinically used to improve pulmonary hypertension5 or vaso-occlusive disorders such as collagen disease-associated Raynaud's syndrome.6 Prostacyclin exhibits its activities via binding to its specific membrane receptor, PGI2-R which exists widely in the heart, aorta, kidney, and platelets.7-10 It is well known that PGI2-R is abundantly expressed on platelets. The number of PGI2-R on platelets decreases in the active phase of spontaneous angina pectoris11 and in acute myocardial infarction,12 while that of TxA2 receptor increases in patients with acute myocardial infarction.13 In contrast to well documented PGI2-R expression on platelets, expression on megakaryocytes, the precursor of platelets, is poorly understood. To our knowledge, studies concerning PGI2-R expression on megakaryocytes have been performed only by two groups; they showed binding of prostanoids to human leukemic cell lines with megakaryocytic features (HEL or MEG-01).14 15 However, there has been no study examining the PGI2-R expression at the transcriptional level.
MATERIALS AND METHODS
Cell Lines and Culture
Human hematopoietic cell lines, HEL (megakaryocytic/erythroid),17 CMK (megakaryocytic),18 CMK11-5 (megakaryocytic),19 NS-Meg (megakaryocytic/erythroid),20 MEG-01 (megakaryocytic),21 KU812 (basophilic),22 JK-1 (erythroid),23 K562 (megakaryocytic/erythroid),24 HL-60 (granuloid),25 THP-1 (monocytoid),26 MOLT4 (T-lymphoid),27 MM-S1 (myeloma),28 and U266 (myeloma)29 were examined. As megakaryocytic cell lines, HEL, CMK and its subline CMK11-5, and NS-Meg were used. HEL, KU812, K562, HL-60, THP-1, and MOLT4 were provided by Japanese Cancer Research Resources Bank (Tokyo). CMK and CMK11-5 were kindly provided by Dr Takeyuki Sato of Chiba University, Chiba, Japan. CMK11-5 was derived from CMK as a subline exhibiting greater megakaryocytic maturation. Meg-01 was provided by Dr R. M. Nüsig of the University of Konstanz, Konstanz, Germany. NS-Meg was established in our laboratory from a patient with chronic myeloid leukemia in megakaryocytic crisis and possesses both erythroid and megakaryocytic features. JK-1 and MM-S1 were also established in our laboratory. Cells were cultured in RPMI 1640 medium (Sigma Chemical, St Louis, MO) with 10% fetal calf serum (FCS) (Life Technologies, Grand Island, NY) at an initial cell density of 1 to 5 × 105/mL.
Recombinant Human Cytokines and Chemical Reagents
Recombinant human cytokines and their respective concentrations employed in culture are as follows: interleukin-1α (IL-1α) (100 U/mL), IL-1β (10 ng/mL), IL-3 (10 ng/mL), IL-6 (10 ng/mL), granulocyte colony-stimulating factor (G-CSF ) (10 ng/mL), granulocyte-macrophage (GM)-CSF (10 ng/mL), tumor necrosis factor-α (TNF-α) (10 ng/mL), erythropoietin (EPO) (5 U/mL), and thrombopoietin (TPO) (10 ng/mL). IL-1α and TNFα was kindly provided by Dainippon Pharmaceutical Co (Osaka, Japan), IL-1β by Otsuka Pharmaceutical Co (Tokushima, Japan), IL-6, G-CSF, and EPO by Chugai Pharmaceutical Co (Tokyo, Japan), and IL-3, GM-CSF, and TPO by Kirin Brewery Co (Maebashi, Japan). Phorbol 12-myristate 13-acetate (PMA) (Sigma) was added to the culture at a concentration of 10 nmol/L.
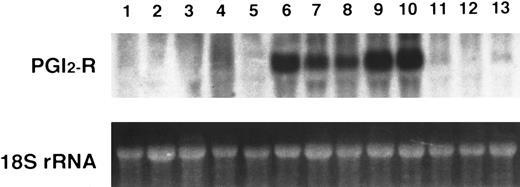
Northern blot analysis of PGI2-R mRNA in human hematopoietic cell lines. A total of 20 μg of total RNA from respective cell lines was electrophoresed in 1.5 % agarose-formaldehyde gel and hybridized with 1.9 kb EcoRI fragment of phIPR1 for PGI2-R cDNA. The 18S ribosomal RNA was used as the internal control. Lane 1, MOLT4; lane 2, MM-S1; lane 3, U266; lane 4, THP-1; lane 5, HL60; lane 6, KU812; lane 7, HEL; lane 8, NS-Meg; lane 9, CMK11-5; lane 10, CMK; lane 11, Meg-01; lane 12, K562; lane 13, JK-1. The experiment is representative of three performed.
Northern blot analysis of PGI2-R mRNA in human hematopoietic cell lines. A total of 20 μg of total RNA from respective cell lines was electrophoresed in 1.5 % agarose-formaldehyde gel and hybridized with 1.9 kb EcoRI fragment of phIPR1 for PGI2-R cDNA. The 18S ribosomal RNA was used as the internal control. Lane 1, MOLT4; lane 2, MM-S1; lane 3, U266; lane 4, THP-1; lane 5, HL60; lane 6, KU812; lane 7, HEL; lane 8, NS-Meg; lane 9, CMK11-5; lane 10, CMK; lane 11, Meg-01; lane 12, K562; lane 13, JK-1. The experiment is representative of three performed.
Northern Blot Analysis
Cells were harvested and total RNA was extracted by the acid phenol method using TRIzol (Life Technologies). A total of 20 μg of the RNA was electrophoresed on 1.5% agarose-formaldehyde gel and transferred onto GeneScreen Plus (Du Pont-New England Nuclear, Boston, MA). Probes for hybridization were the 1.9 kb EcoRI fragment of phIPR18: prostacyclin receptor cDNA, 365 bp reverse transcription-polymerase chain reaction (RT-PCR) product of platelet factor 4 (PF4),30 which is mentioned later, and 861 bp RT-PCR product of glycoprotein IIb (GPIIb).31 Hybridization was performed at 42°C for 20 hours in 5× SSPE, 50% formamide, 5× Denhardt's solution, 1% sodium dodecyl sulfate (SDS), and 100 μg/mL heat-denatured salmon sperm DNA. The membrane was washed in 2× SSC for 15 minutes at room temperature, in 2× SSC, 1% SDS for 45 minutes at 60°C, in 0.1× SSC for 30 minutes at room temperature, twice respectively. All experiments, including cell culture, RNA extraction, and Northern blot hybridization were performed in triplicate, except the experiment shown in Fig 1. Signals on the autoradiograms were quantified by densitometry.
Cyclic (c)AMP Assay
Cells were first preincubated with PMA or cytokines (IL-3, IL-6, GM-CSF, TPO, IL-1β, TNFα) for 24 hours, then incubated in a 24-well microplate (Corning, NY) (1 × 105 cells/well) in 500 μL of RPMI1640 containing 0.5 mmol/L 3-isobutyl-1-methylxanthine (Sigma) and 0.1 % bovine serum albumin (BSA) (Sigma) at 37°C for 10 minutes then iloprost (Amersham, Buckinghamshire, UK), an agonist of prostacyclin (final concentration: 100 nmol/L), or forskolin (Sigma; 1 μmol/L) was added to each well. Incubation was continued for another 30 minutes. Because of the high instability of prostacyclin, we used the agonist. The reaction was terminated by the addition of 500 μL of 12% trichloroacetic acid. The cAMP level was measured in triplicate using an 125I cAMP assay kit (Yamasa Shoyu, Chiba, Japan).
Binding Assay
HEL and NS-Meg cells were precultured for 48 hours with or without (control) either PMA or TPO, then harvested and washed twice with RPMI 1640 medium, then suspended (1 × 106 cells/tube) in 20 mmol/L Tris-HCl pH 7.4. Binding assay was performed with 50 nmol/L of [3H]iloprost (Amersham). After incubation at 30°C for 1 hour, the reaction was terminated by an addition of 5 mL of ice-cold 10 mmol/L Tris-HCl pH 7.4 buffer. This mixture was rapidly filtered through a Whatman GF/C filter (Whatman International, Maidstone, UK). The filter was washed four times with 5 mL of the same buffer and the radioactivity was measured in 5 mL of Cleasol scintillation cocktail (Nacalai Tesque, Kyoto, Japan). Nonspecific binding was determined by the addition of 500-fold excess of unlabeled iloprost to the incubation mixture. The specific binding was calculated by subtraction of the nonspecific binding from the total binding.
Separation of Human CD34-Positive Cells and Megakaryocytic Colony Formation
Cord blood was obtained with informed consent from patients who underwent Caesarian section. Mononuclear cells were separated by centrifugation (400g, 30 minutes) over 60% Percoll (Pharmacia Fine Chemicals, Uppsala, Sweden), then suspended in RPMI 1640 with 10% FCS and incubated for 2 hours in 60 × 15 mm plastic culture dishes (Becton Dickinson, Lincoln Park, NJ) at 37°C in humidified 5% CO2 in air. The plastic nonadherent cells were adjusted to 2 × 108 cells/mL in phosphate-buffered saline without Ca2+ and Mg2+ (PBS[−]) with 2% BSA and mixed with mouse antihuman CD34 antibody-coated magnetic beads (M-450; Dynal, Oslo, Norway) at a ratio of 1 bead per cell. The mixture was then incubated under gentle rotation for 30 minutes at 4°C. Thereafter, cells that attached to the beads were separated from nonattached cells with a magnetic particle concentrator (MPC-6; Dynal) for 2 minutes and this procedure was repeated four to five times. Positively selected cells were then resuspended in 100 μL of PBS(−) with 2% BSA plus 100 μL of DETACHaBEADS (Dynal). After a 45-minute incubation under gentle rotation at room temperature, beads were released from cells by consecutive separation with a magnetic particle concentrator. Isolated cells were counted with a hemocytometer and cell viability was determined by trypan blue dye exclusion. The proportions of CD34+ cells in the isolated fraction were 78% to 85% as determined by the fluorescent microscopy (Olympus, Tokyo, Japan). Megakaryocytic colony formation was performed according to the method described previously32 with some modifications. Briefly, CD34+ cells (5 × 104/mL) were mixed with Iscove's modified Dulbecco's MEM (IMDM) (Life Technologies), 30% human plasma, 50 ng/mL TPO, 50 mmol/L 2-mercaptoethanol (Sigma), and 0.9% methylcellulose (Dow Chemicals, Quebec, Canada). A total of 1-mL aliquots of this mixture was cultured in 35 × 10 mm plastic dishes (Nunc, Naperville, IL) at 37°C in humidified 5% CO2 in air.
RT-PCR of Megakaryocytic Colonies
Megakaryocytic colonies consisting of more than 50 cells were plucked at days 6, 9, and 14 of culture under an inverted microscope (Olympus). Two hundred colonies were collected on each day and total RNA was extracted using TRIzol. RT-PCR high kit (Toyobo, Osaka, Japan) was used for RT-PCR. Briefly, 1 μg of RNA was reverse transcribed in 20 μL of 1× RTase Buffer, 1 mmol/L dNTPs, 1.25 mmol/L oligo(dT), 10 U RNase Inhibitor, 20 U M-MLV Reverse Transcriptase (RNaseH−). RT was performed at 30°C for 10 minutes, 42°C for 60 minutes, and 99°C for 5 minutes. The reverse transcribed cDNA pool was amplified by PCR with specific primers for PGI2-R, PF4, and a housekeeping gene, glyceraldehyde 3-phosphate dehydrogenase (G3PDH) gene as an internal control. The PGI2-R primers were TGCTCCCTGCCTCTCACGAT, sense primer and TGGCTTCTGCTTTGGACGAC, antisense primer. The PF4 primers were TTCCCATCGCACTGAGCACTG, sense primer and GCAGCTAGTAGCTAACTCTCCAAAAG, antisense primer. The G3PDH primers were ACCACAGTCCATGCCATCAC, sense primer and TCCACCACCCTGTTGCTGTA, antisense primer. PCR reaction mixture contained 5 μL of cDNA pool, 2.5 μL of Plus Buffer from the RT-PCR high kit, 0.4 μmol/L each specific primers, 0.5 U of rTaq DNA polymerase in a total volume of 25 μL. PCR for PGI2-R was performed on the GeneAmp PCR System 2400 (Perkin Elmer, Norwalk, CT) for 35 cycles (a cycle of 94°C for 30 seconds, 58°C for 30 seconds, 72°C for 1 minute) and PCR for PF4 and G3PDH, for 30 cycles (a cycle of 94°C for 30 seconds, 60°C for 30 seconds, 72°C for 1 minute).
Effect of PMA on the expression of PGI2-R, PF4, and GPIIb mRNA in HEL, NS-Meg, and CMK11-5 cells. PMA was added at the initiation of culture and cells harvested at the indicated times were examined by Northern analysis. The figure shows the representative results from triplicate experiments repeated twice. PMA enhanced PGI2-R expression in all of these cell lines. The time and intensity of maximum PGI2-R expression was as follows: HEL, 48 hours (threefold), NS-Meg, 48 hours (fivefold), and CMK11-5, 72 hours (eightfold). PMA enhanced the expression of GPIIb mRNA in these cell lines and that of PF4 mRNA in HEL cells. The controls were the cells cultured without PMA. They yielded no enhancement of PGI2-R for the entire culture periods except a little enhancement in HEL at 48 hours (48-hour controls are shown). All of the PMA-stimulated expressions were significantly different from respective control expressions in all of these cell lines (P < .01).
Effect of PMA on the expression of PGI2-R, PF4, and GPIIb mRNA in HEL, NS-Meg, and CMK11-5 cells. PMA was added at the initiation of culture and cells harvested at the indicated times were examined by Northern analysis. The figure shows the representative results from triplicate experiments repeated twice. PMA enhanced PGI2-R expression in all of these cell lines. The time and intensity of maximum PGI2-R expression was as follows: HEL, 48 hours (threefold), NS-Meg, 48 hours (fivefold), and CMK11-5, 72 hours (eightfold). PMA enhanced the expression of GPIIb mRNA in these cell lines and that of PF4 mRNA in HEL cells. The controls were the cells cultured without PMA. They yielded no enhancement of PGI2-R for the entire culture periods except a little enhancement in HEL at 48 hours (48-hour controls are shown). All of the PMA-stimulated expressions were significantly different from respective control expressions in all of these cell lines (P < .01).
Statistical Analysis
The differences in the values obtained were analyzed by the analysis of variance (ANOVA).
RESULTS
Expression of PGI2-R mRNA in Hematopoietic Cell Lines
To determine whether PGI2-R expression is cell lineage-specific, we examined 13 hematopoietic cell lines by Northern blot analysis. As shown in Fig 1, abundant expression was noted in KU812, HEL, NS-Meg, CMK11-5, and CMK cells, and to a lesser amount in MEG-01 and JK-1 cells, while we found no detectable band in MOLT4, MM-S1, U266, THP-1, HL60, and K562 cells. PGI2-R mRNA, therefore, was expressed in all megakaryocytic (CMK11-5, CMK, MEG-01) and megakaryocytic/erythroid (HEL, NS-Meg) cells examined, except for K562 cells.
Effect of PMA on the Expression of PGI2-R, PF4, and GPIIb mRNA in Megakaryocytic Cells
HEL, NS-Meg, CMK11-5, and CMK cells were challenged with 10 nmol/L PMA and the expression of PGI2-R was determined by Northern blot analysis. We noted time-dependent increases of PGI2-R expression by PMA treatment in all of these cell lines (HEL, NS-Meg, CMK11-5, Fig 2) (CMK, data not shown). Maximum expression was observed at 48 hours in HEL cells (threefold, compared with control culture without any stimulus), 48 hours in NS-Meg cells (fivefold), 72 hours in CMK11-5 cells (eightfold), and 24 hours in CMK cells (threefold) from the initiation of culture. Expression of mRNA for GPIIb was also increased time-dependently by PMA in all of these cell lines, while PF4 was enhanced only in HEL cells.
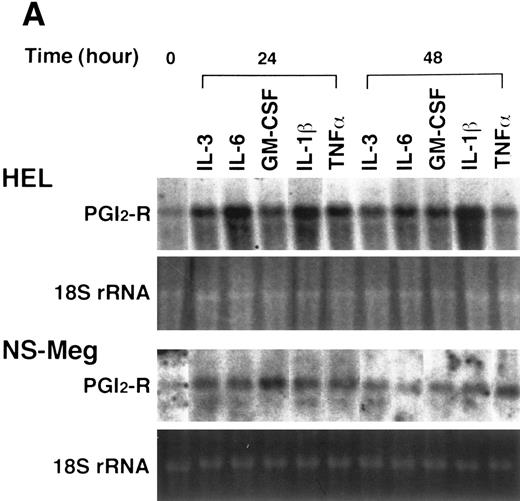
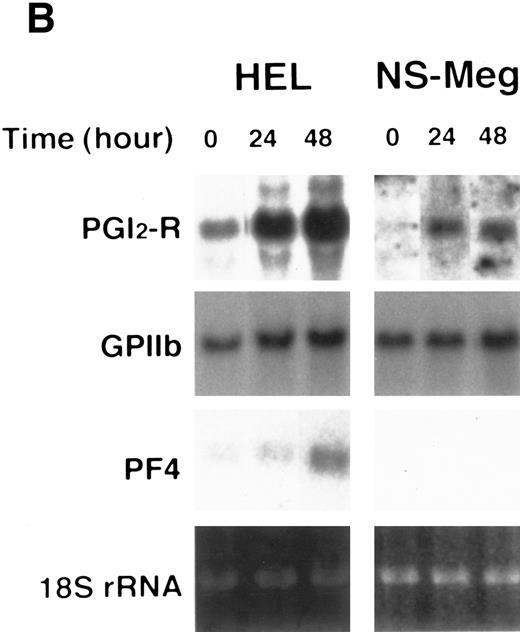
Effect of cytokines on the expression of PGI2-R in HEL and NS-Meg cells. At the initiation of culture, either IL-3, IL-6, GM-CSF, TPO, IL-1β, or TNF-α was added to the culture and cells harvested at the indicated times were examined by Northern analysis. The controls were the cells cultured without cytokines for the respective culture periods (data not shown, similar to the controls for Fig 2). The figure shows representative results from triplicate experiments repeated twice. (A) All cytokines upregulated the expression of PGI2-R mRNA. (B) Effect of TPO on the expression of PGI2-R, PF4, GPIIb mRNA in HEL and NS-Meg cells. TPO increased the expression of PGI2-R, PF4, and GPIIb mRNA in HEL cells, and PGI2-R and GPIIb in NS-Meg cells.
Effect of cytokines on the expression of PGI2-R in HEL and NS-Meg cells. At the initiation of culture, either IL-3, IL-6, GM-CSF, TPO, IL-1β, or TNF-α was added to the culture and cells harvested at the indicated times were examined by Northern analysis. The controls were the cells cultured without cytokines for the respective culture periods (data not shown, similar to the controls for Fig 2). The figure shows representative results from triplicate experiments repeated twice. (A) All cytokines upregulated the expression of PGI2-R mRNA. (B) Effect of TPO on the expression of PGI2-R, PF4, GPIIb mRNA in HEL and NS-Meg cells. TPO increased the expression of PGI2-R, PF4, and GPIIb mRNA in HEL cells, and PGI2-R and GPIIb in NS-Meg cells.
Effect of Cytokines on the Expression of PGI2-R, PF4, and GPIIb
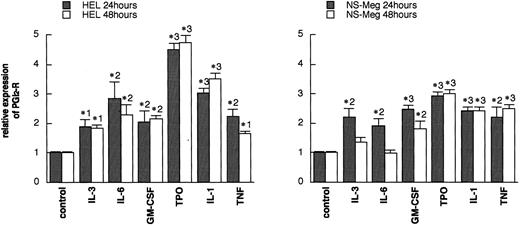
HEL and NS-Meg cells were challenged with various cytokines as indicated in Fig 3A and B. IL-3, IL-6, GM-CSF, TPO, IL-1β, and TNF-α upregulated the expression of PGI2-R, although intensity of the maximum expression induced and the time course by these stimuli differed depending on cytokines used and cell lines examined. The effect of IL-1α was similar to IL-1β. G-CSF and EPO did not induce significant increases in PGI2-R expression (data not shown). The results obtained by densitometry are summarized in Fig 4. In HEL cells, both PF4 and GPIIb expression were enhanced by TPO, while in NS-Meg cells, which lack PF4, TPO intensified only GPIIb expression (Fig 3B). All cytokines, except TPO, did not significantly increase the expression of PF4 or GPIIb mRNA in these cells (data not shown).
Analysis of the Northern blottings in Fig 3A and B by densitometry. Each value (mean ± SE, n = 3) shows the ratio of PGI2-R expression after 24 or 48 hours of culture versus the respective control expression. *1, P < .05; *2, P < .01; *3, P < .001 as compared with the respective controls.
Analysis of the Northern blottings in Fig 3A and B by densitometry. Each value (mean ± SE, n = 3) shows the ratio of PGI2-R expression after 24 or 48 hours of culture versus the respective control expression. *1, P < .05; *2, P < .01; *3, P < .001 as compared with the respective controls.
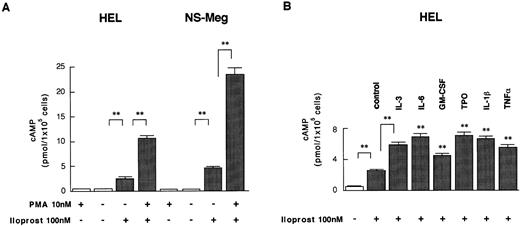
Induction of cAMP Synthesis by Iloprost and the Effect of PMA or Cytokines on cAMP Synthesis
We examined whether iloprost could induce postreceptor signal transduction through its specific receptor (ie, PGI2-R) coupled with Gs type guanosine triphosphate (GTP) binding protein.7 Addition of iloprost to the cultures of HEL and NS-Meg cells, which were shown to express PGI2-R, induced significant increases of cAMP (Fig 5A). Next we examined whether cAMP synthesis was increased following upregulation of PGI2-R. PMA pretreatment increased iloprost-induced cAMP synthesis 4.2-fold in HEL cells and 5.0-fold in NS-Meg cells when compared with nontreated cells. Pretreatment either with IL-3, IL-6, GM-CSF, TPO, IL-1β, or TNF-α also elevated iloprost-induced cAMP synthesis in HEL cells. All of the pretreatments (without iloprost) yielded no significant increase of cAMP in the cells from each cell line when compared with nontreated cells (Fig 5A and B). cAMP levels were not changed by the difference in incubation time with iloprost from 15 to 60 minutes, and forskolin increased cAMP synthesis without significant difference between PMA- or cytokine-pretreated cells and nontreated ones (Fig 5C).
Induction of cAMP synthesis by iloprost or forskolin and the effect of the pretreatments on the synthesis in HEL or NS-Meg cells. Each value (mean ± SE, n = 3) shows the amount of cAMP synthesized by 1 × 105 cells. Cells were first preincubated with PMA or respective cytokines for 24 hours, then incubated in a 24-well microplate (1 × 105 cells/well) in 500 μL of RPMI 1640 containing 0.5 mmol/L 3-isobutyl-1-methylxanthine and 0.1% BSA at 37°C for 10 minutes and then iloprost or forskolin was added to each well. Incubation was continued for another 30 minutes. (A) and (B) Addition of iloprost increased significantly (**, P < .001) the synthesis of cAMP in each cell line. Addition of iloprost to the cells that had been preincubated for 24 hours with PMA or cytokines further increased (**, P < .001) cAMP synthesis as compared with the untreated cells of each cell line. The pretreatments with the cytokines (without iloprost) yielded no significant increase of cAMP in the cells from each cell line compared with nontreated cells (data not shown). (C) Forskolin increased cAMP synthesis without significant difference between PMA- or cytokine-pretreated cells and nontreated ones (control).
Induction of cAMP synthesis by iloprost or forskolin and the effect of the pretreatments on the synthesis in HEL or NS-Meg cells. Each value (mean ± SE, n = 3) shows the amount of cAMP synthesized by 1 × 105 cells. Cells were first preincubated with PMA or respective cytokines for 24 hours, then incubated in a 24-well microplate (1 × 105 cells/well) in 500 μL of RPMI 1640 containing 0.5 mmol/L 3-isobutyl-1-methylxanthine and 0.1% BSA at 37°C for 10 minutes and then iloprost or forskolin was added to each well. Incubation was continued for another 30 minutes. (A) and (B) Addition of iloprost increased significantly (**, P < .001) the synthesis of cAMP in each cell line. Addition of iloprost to the cells that had been preincubated for 24 hours with PMA or cytokines further increased (**, P < .001) cAMP synthesis as compared with the untreated cells of each cell line. The pretreatments with the cytokines (without iloprost) yielded no significant increase of cAMP in the cells from each cell line compared with nontreated cells (data not shown). (C) Forskolin increased cAMP synthesis without significant difference between PMA- or cytokine-pretreated cells and nontreated ones (control).
Effect of PMA or TPO on Binding Capacity of Iloprost to HEL and NS-Meg Cells
Specific [3H]iloprost binding was observed in nontreated HEL (mean ± standard error [SE]: 1.3 × 105 ± 5.3 × 103 dpm/1 × 106 cells) and NS-Meg cells (4.2 × 104 ± 3.9 × 103). Preincubation with PMA or TPO for 48 hours increased the binding capacity of the ligand up to sixfold and threefold in HEL cells and fivefold and twofold in NS-Meg cells, respectively, when compared with control values (nontreated cells) (Fig 6).
Effect of PMA and TPO on PGI2-R expression. HEL () and NS-Meg (▪) cells were preincubated for 48 hours with or without (control) either PMA or TPO. PGI2-R expression was evaluated by specific [3H]iloprost binding to the cells. Values (mean ± SE, n = 4) are expressed as a ratio of scintillation count of the control cells (nontreated HEL, 1.3 × 105 ± 5.3 × 103 dpm/1 × 106 cells, nontreated NS-Meg, 4.2 × 104 ± 3.9 × 103). Preincubation with PMA or TPO increased the binding capacity of the ligand up to sixfold and threefold in HEL cells and fivefold and twofold in NS-Meg cells, respectively, when compared with control values. The experiment is representative of three performed.
Effect of PMA and TPO on PGI2-R expression. HEL () and NS-Meg (▪) cells were preincubated for 48 hours with or without (control) either PMA or TPO. PGI2-R expression was evaluated by specific [3H]iloprost binding to the cells. Values (mean ± SE, n = 4) are expressed as a ratio of scintillation count of the control cells (nontreated HEL, 1.3 × 105 ± 5.3 × 103 dpm/1 × 106 cells, nontreated NS-Meg, 4.2 × 104 ± 3.9 × 103). Preincubation with PMA or TPO increased the binding capacity of the ligand up to sixfold and threefold in HEL cells and fivefold and twofold in NS-Meg cells, respectively, when compared with control values. The experiment is representative of three performed.
Normal Human Megakaryocytic Colonies
In methylcellulose semisolid cultures of cord blood CD34+ cells stimulated by TPO, we noted a number of megakaryocytic colonies consisting of more than 50 megakaryocytes, as early as 6 days after culture. The megakaryocytic nature of the colonies was confirmed by immunocytochemistry with anti-CD41a antibodies, as previously reported.32 In accordance with the report by Nishihira et al,33 we detected no other types of hemopoietic colonies until after 15 days of culture. On days 6, 9, and 14 of culture, more than 200 megakaryocytic colonies were plucked and pooled, respectively. Cytospin preparations were made from a part of each cell suspension and the diameter of individual cells was determined with a micrometer. The size of cells increased as the culture advanced; day 6, 16.74 ± 4.33 μm (mean ± standard deviation [SD]) (n = 205), day 9, 19.43 ± 4.85 μm (n = 218), and day 14, 22.43 ± 4.62 μm (n = 242). The cytoplasm of cells plucked on days 9, 11, and 14 had fine azulophilic granules. In contrast, the cytoplasm of cells on day 6 was mostly basophilic without granules. Proplatelet formation32 was observed in situ after 11 days of culture.
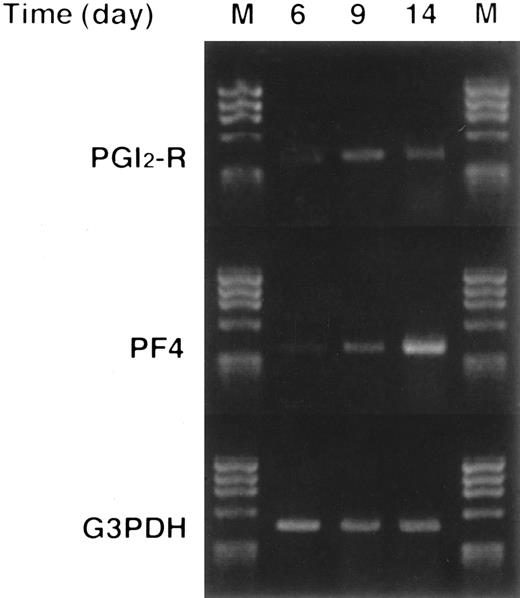
Semiquantitative RT-PCR for PGI2-R Expression in Megakaryocytic Colonies
We next extracted total RNA from each cell suspension. Evaluation of PGI2-R gene expression was performed by semiquantitative RT-PCR. We also assayed PF4 transcripts to compare with the kinetics of PGI2-R transcriptional levels. PGI2-R expression peaked on day 9, then declined toward day 14. In contrast, expression of PF4 increased towards day 14 (Fig 7).
Expression of PGI2-R and PF4 mRNA in normal human megakaryocytes as evaluated by semiquantitative RT-PCR. More than 200 megakaryocytic colonies were plucked and pooled on the indicated days of culture, total RNA was extracted, and RT-PCR was performed. The amount of transcripts for PGI2-R or PF4 was analyzed and compared with that of G3PDH. M, size marker (φX174/HaeIII digest). This figure shows the representative results from experiments repeated three times.
Expression of PGI2-R and PF4 mRNA in normal human megakaryocytes as evaluated by semiquantitative RT-PCR. More than 200 megakaryocytic colonies were plucked and pooled on the indicated days of culture, total RNA was extracted, and RT-PCR was performed. The amount of transcripts for PGI2-R or PF4 was analyzed and compared with that of G3PDH. M, size marker (φX174/HaeIII digest). This figure shows the representative results from experiments repeated three times.
DISCUSSION
Prostacyclin synthesized mainly by arterial endothelial cells is the most potent inhibitor of platelet aggregation. This action is mediated through the specific receptor for prostacyclin. Because platelets possess only a small amount of remnant RNA transcribed from the nucleus of the megakaryocytes, supply of new receptors on platelet membranes is trivial. Eventually, the expressed receptor on platelets must have originated from the megakaryocyte. In this study, we examined the expression of PGI2-R in megakaryocytes. As for leukemic cell lines, all megakaryocytic and megakaryocytic/erythroid cells expressed PGI2-R mRNA except K562 cells. Furthermore, normal megakaryocytic colonies were shown to express the receptor. Thus, the expression of PGI2-R was first demonstrated at the transcriptional level either in leukemic or normal megakaryocytes.
PMA, which is known to induce megakaryocytic maturation, enhanced PGI2-R expression in megakaryocytic or megakaryocytic/erythroid cell lines with the upregulation of PF4 and GPIIb, a platelet-specific protein in α-granules and an adhesion molecule, respectively. TPO, the potent promoter of megakaryocytopoiesis, exhibited similar effects. IL-3, IL-6, and GM-CSF, which are also known to induce megakaryocytic proliferation or maturation, enhanced PGI2-R expression without significant enhancement of PF4 or GPIIb expression. These results indicate that PGI2-R expression in megakaryocytes is upregulated by the cytokines involved in megakaryocytopoiesis. Furthermore, megakaryocytic maturation appears to be associated with the upregulation of PGI2-R expression, as indicated by PMA or TPO stimulation.
The upregulation of PGI2-R mRNA expression by PMA or cytokines was supported by iloprost-induced cAMP synthesis and [3H]iloprost binding experiments. Pretreatment of leukemic megakaryocytes with PMA, IL-3, IL-6, GM-CSF, TPO, IL-1β, or TNF-α enhanced cAMP synthesis. Because ligands of PGI2-R trigger postreceptor signal transduction and subsequently induce cAMP synthesis, PMA- or cytokine-enhanced cAMP synthesis may confirm that PMA and cytokines increased the number of PGI2-R on these cells. In fact, PMA and TPO increased the binding capacity of the ligand to PGI2-R.
It is of interest to examine the time course pattern of PGI2-R expression during megakaryocytopoiesis. Leukemic megakaryocytes used in this study were thought to be originated from a megakaryocytic progenitor (CFU-Meg) or an oligopotent progenitor for both megakaryocytic and erythroid lineages. Untreated leukemic cells are morphologically blastic and do not undergo terminal differentiation such as proplatelet formation even after PMA treatment. Nevertheless, untreated cells were shown to express PGI2-R; therefore, PGI2-R expression may be initiated in immature megakaryocytes nearly progenitors and upregulated along PMA or cytokine-induced megakaryocytic maturation. While in normal megakaryocytic colonies induced by TPO, PGI2-R mRNA expression peaked at 9 days, but not at 14 days of CFU-Meg culture. In our culture system, proplatelet formation by megakaryocytic colonies was seen after 11 days of culture. The peak expression of PGI2-R mRNA in normal megakaryocytes, therefore, appears to occur before terminal differentiation. The kinetics of PGI2-R expression should be studied throughout megakaryocytopoiesis, especially at the stem cell or progenitor level.
Interestingly, proinflammatory cytokines, IL-1 and TNF-α, upregulated PGI2-R expression in leukemic megakaryocytes. This upregulation was not associated with the upregulation of PF4 or GPIIb mRNA. Because IL-1 and TNF-α play a central role in inflammation, PGI2-R expression by megakaryocytes might be upregulated in the inflammatory state in vivo. IL-1 and TNF-α are known to stimulate the production of procoagulant activity34 and the plasminogen activator inhibitor35 by vascular endothelial cells and subsequently induce pre-disseminated intravascular coagulation (DIC) or DIC. A number of studies have shown that blood prostacyclin levels are elevated during endotoxemia.36 37 Taken together, IL-1– or TNF-α–induced upregulation of PGI2-R, as observed in this study, might act as a homeostatic mechanism, preventing platelet aggregation in abnormal coagulopathy. However, PGI2-R upregulation was examined only in leukemic cells, thus the effect of proinflammatory cytokines on PGI2-R expression in normal megakaryocytes or platelet awaits further clarification.
ACKNOWLEDGMENT
We are grateful to Kyoko Tanaka and Dr Hayato Shimada of the Department of Immuno-Hematology and Gynecology, Kobe City General Hospital, Kobe, Japan, for preparing cord blood.
Supported in part by a research grant from ONO Medical Research Foundation (to K.N.), by a Grant-in-Aid from the Ministry of Education, Science and Culture, Japan (to I.T., K.N.), and by Sasaki Foundation for Leukemia Research (to T.T.).
Address reprint requests to Takayuki Takahashi, MD, PhD, Department of Immuno-Hematology, Kobe City General Hospital, 4-6 Minatojima-Nakamachi, Chuo-ku, Kobe 650, Japan.



![Fig. 6. Effect of PMA and TPO on PGI2-R expression. HEL () and NS-Meg (▪) cells were preincubated for 48 hours with or without (control) either PMA or TPO. PGI2-R expression was evaluated by specific [3H]iloprost binding to the cells. Values (mean ± SE, n = 4) are expressed as a ratio of scintillation count of the control cells (nontreated HEL, 1.3 × 105 ± 5.3 × 103 dpm/1 × 106 cells, nontreated NS-Meg, 4.2 × 104 ± 3.9 × 103). Preincubation with PMA or TPO increased the binding capacity of the ligand up to sixfold and threefold in HEL cells and fivefold and twofold in NS-Meg cells, respectively, when compared with control values. The experiment is representative of three performed.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/3/10.1182_blood.v90.3.1039/4/m_bl_0006f6.jpeg?Expires=1767734203&Signature=JZDcuBeNdJ1WDjPeX-VkpxrGKlDvxL6Sr0JyMlz1y9LO0rDGZlaoMgSq7hUzePA90kBQPPwn7nQp2KO5tXtvaoYa3us0ibgIhUyz2rcViQUT9IiBpEdu13p9vzdx2xJLLV0VxKvJ5mYgCdK9tMAx--h99tabtyWOZOc4Eg6tJIC01dqdU7dGHUiBGGuhhdcZcAd-lqxieE3vJg4idI5~2weWaGbjK3DblDTb~18VqoCn6WKJJyR0MOFJU8VLlMsPTz5cS~jOfbHgf4RJC7wM5FtjHs23anINgEbiwB8LofcFBgQ7b9bQ3wssHEmvhtGpOK22F9AqumcYsiznqD31nw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal