Abstract
Two hundred fifty-three patients with newly diagnosed acute promyelocytic leukemia (APL) were eligible to enter the multicentric GIMEMA-AIEOP “AIDA” trial during the period July 1993 to February 1996. As a mandatory prerequisite for eligibility, all patients had genetic evidence of the specific t(15; 17) lesion in their leukemic cells confirmed by karyotyping or by reverse transcription-polymerase chain reaction (RT-PCR) of the PML/RARα fusion gene (the latter available in 247 cases). Median age was 37.8 years (range, 2.2 to 73.9). Induction treatment consisted of oral all-trans retinoic acid (ATRA), 45 mg/m2/d until complete remission (CR), given with intravenous Idarubicin, 12 mg/m2/d on days 2, 4, 6, and 8. Three polychemotherapy cycles were given as consolidation. Hematologic and molecular response by RT-PCR was assessed after induction and after consolidation. At the time of analysis, 240 of the 253 eligible patients were evaluable for induction. Of these, 11 (5%) died of early complications and 229 (95%) achieved hematologic remission. No cases of resistant leukemia were observed. Of 139 cases studied by RT-PCR after induction, 84 (60.5%) were PCR-negative and 55 (39.5%) PCR-positive. One hundred sixty-two patients were evaluable by RT-PCR at the end of consolidation. Of these, 159 (98%) tested PCR-negative and 3 (2%), PCR-positive. After a median follow up of 12 months (range, 0 to 33), the estimated actuarial event-free survival for the whole series of 253 eligible patients was 83% ± 2.6% and 79% ± 3.2% at 1 and 2 years, respectively. This study indicates that the AIDA protocol is a well-tolerated regimen that induces molecular remission in almost all patients with PML/RARα-positive APL. Preliminary survival data suggest that a remarkable cure rate can be obtained with this treatment.
ACUTE PROMYELOCYTIC leukemia (APL) is a particular type of acute myeloid leukemia (AML) with characteristic biological and clinical features, which include the presence of a specific t(15; 17) chromosome translocation in the leukemic blasts, the frequent association at diagnosis of a severe hemorrhagic diathesis, and in vitro and in vivo sensitivity to the differentiating agent all-trans retinoic acid (ATRA).1-4
The t(15; 17) involves the retinoic acid receptor α (RARα) gene on chromosome 17 and the PML (for promyelocytic) gene on chromosome 15 and generates a chimeric gene, which is transcribed into the PML/RARα fusion transcript. The resulting PML/RARα chimeric protein is crucial to the pathogenesis of the disease.3 4
The PML/RARα hybrid mRNA is readily detectable by the reverse transcription-polymerase chain reaction (RT-PCR) assay.5,6 As reported by several groups, including ours, RT-PCR studies of the PML/RARα have proved extremely useful in the clinical management of the disease.5-11 Detection of PML/RARα at diagnosis predicts response to ATRA, whereas cases with morphological features of APL, which are PML/RARα negative, are refractory to differentiative treatment.6 Moreover, when they are applied to evaluating residual disease during remission, RT-PCR tests can identify patients at risk of relapse and in need of additional therapy.7-11
Anthracycline-based chemotherapy (CHT) and ATRA are the mainstay of APL treatment.2-4 Anthracyclines alone or in association with cytosine arabinoside induce complete remission (CR) in a high percentage of cases (75% to 80%), but this approach is associated with a high mortality rate (up to 20%) during the early phases of treatment.2-4,12-17 Differentiative treatment with ATRA results in greater than 90% CRs, but patients remain invariably PCR positive after induction, and all relapse if no consolidation CHT is added.2-4,18-21 Furthermore, ATRA treatment has been associated with the occurrence, in a sizable fraction of patients, of life-threatening complications. These include a severe respiratory distress due to pulmonary infiltrates (ATRA syndrome) usually (but not uniformly) correlated to a rapid increase of white blood cell counts21,22 and pseudotumor cerebri, which is more frequently observed in younger patients.23
Preliminary clinical studies have been performed with various combinations of ATRA and CHT in an attempt to obtain more durable remissions and reduced ATRA-related toxicity. The combinations, as demonstrated in a randomized trial, seem to improve disease-free survival over that achieved with chemotherapy alone.24 Since 1983, the Italian cooperative group, Gruppo Italiano Malattie Ematologiche Maligne dell' Adulto (GIMEMA), adopted disease-tailored protocols for APL, showing that anthracyclines alone are as effective in inducing remission as polychemotherapy schemes.25 In 1993, aiming at improving the efficacy of APL-targeted treatment, a new protocol combining ATRA and Idarubicin (AIDA protocol) was designed by the GIMEMA, first as a pilot,26 then in association with the Associazione Italiana di Ematologia ed Oncologia Pediatrica (AIEOP), as a larger multicentric study. Prospective RT-PCR analyses were performed on all patients at presentation to assess study eligibility and after treatment to better evaluate response and minimal residual disease.
MATERIALS AND METHODS
Patients.Two hundred seventy-four consecutive patients with newly diagnosed APL were registered during the period July 1993 to February 1996. All cases were diagnosed as AML French-American-British (FAB) M3 or M3 variant (M3v) by conventional morphocytochemical criteria27 in 71 participating institutions (see Appendix). The majority of these centers belong to the Italian cooperative groups GIMEMA and AIEOP, while five belong to other European countries. Eligibility criteria to be enrolled in the AIDA protocol included: age between 1 and 75 years, karyotypic and/or molecular evidence of the t(15; 17) in leukemic blasts at presentation, serum creatinine <2.5 mg/dL, serum alkaline phosphatase, bilirubin, and aspartate aminotransferase (AST) <3 times the upper normal limit, no cardiac contraindications to anthracycline chemotherapy, negative pregnancy test, and informed consent. Cases presenting with hyperleukocytosis were also included in the study.
AIDA protocol.Induction therapy consisted of 45 mg/m2/d ATRA administered orally until the achievement of CR or for a maximum of 90 days, and four 12 mg/m2 doses of Idarubicin given intravenously (IV) on days 2, 4, 6, and 8. In patients less than 20 years old, ATRA doses were adjusted to 25 mg/m2. After the achievement of hematologic remission, patients received three consolidation polychemotherapy courses as reported.26 Each consolidation course was administered at recovery from the previous course, when polymorphonuclear cells and platelet counts were >1,500/μL and >100,000/μL, respectively.
At the end of consolidation, residual disease was estimated by RT-PCR before deciding further therapy. PCR-negative patients were randomized into four arms including chemotherapy alone with 6-mercaptopurine and methotrexate (arm 1), ATRA alone (arm 2), alternating chemotherapy and ATRA (arm 3), or no further therapy (arm 4). Patients who were PCR-positive underwent, if eligible, allogeneic bone marrow transplantation in first CR.
ATRA syndrome was defined as “definitely present” or “overt” in the presence of the following five signs and symptoms: fever, dyspnea, pleural and/or pericardial effusion, pulmonary infiltrates on chest radiograph, and unexplained weight gain greater than 5 kg, according to the original report by Frankel et al.22 We defined as “indeterminate” ATRA syndrome a combination of two to four signs and symptoms of the above mentioned ± lower extremities edema and hypotension. In all other patients, the ATRA syndrome was defined as “definitely absent.”28 Rules for supportive therapy, treatment of the ATRA syndrome, and prevention and control of the coagulopathy have been reported in detail elsewhere.26
Collection and preparation of samples for molecular studies. Samples for RT-PCR analyses were requested at diagnosis, at the time of CR after induction, and at the end of consolidation. With the exception of some cases with hyperleukocytosis in which peripheral blood (PB) samples were studied at presentation, all other diagnostic and all remission samples were obtained from the bone marrow (BM). Mononuclear cells, obtained by centrifuging BM or PB specimens on a Ficoll-Hypaque density gradient, were washed twice in sterile phosphate-buffered saline (PBS), suspended in a 4-mol/L guanidium thiocyanate (GTC) solution and stored at −20°C. These procedures were performed in each GIMEMA and AIEOP center using RNAase-free disposable materials. The GTC solution was previously prepared by the referral molecular biology laboratories and distributed to all peripheral centers. Samples cryopreserved in GTC were then sent in dry ice to two referral molecular biology laboratories for RT-PCR studies (Hematology, University “La Sapienza” of Rome and Clinica Pediatrica, University of Milano, Monza).
RT-PCR of PML/RARα.Total RNA was extracted by the method of Chomczynsky and Sacchi.29 The integrity of RNAs was assessed in all diagnostic and remission samples by electrophoretic run through a formaldehyde minigel. The protocol and the primers used to amplify the PML/RARα hybrid gene have been reported elsewhere.7 To assess the efficiency of the RT step and to further verify RNA integrity, a cDNA fragment containing RARα exons 2 and 3 was coamplified in each analyzed sample (internal control). A positive control (amplification of RNA extracted from the promyelocytic cell line NB4) and a negative control (all reagents plus water with no RNA) was included in each experiment. To assess the sensitivity of our RT-PCR method, total RNA isolated from a diagnostic sample with 100% blastic infiltration was serially diluted by mixing it with the t(15; 17) negative myeloid cell line GF-D8 RNA.5 Our assay allowed us to detect the PML/RARα transcript in the presence of 0.1 ng total RNA, that is a final dilution of 10−4.
Evaluation of response.CR was defined as the reconstitution of normal BM cellularity with less than 5% leukemic promyelocytes, together with PB cell counts of polymorphonuclear leukocytes (PMN) > 1,500/μL and platelets >100,000/μL. Molecular remission was defined as the disappearance, on ethidium bromide-stained electrophoresis gel, of the specific PML/RARα amplification band identified at diagnosis, in the presence of RNA integrity as evaluated by minigel visualization and successful amplification of the internal control. Overall survival (OS) and event-free survival (EFS) duration was calculated by the method of Kaplan and Meier. The median observation was 12 months (range, 0 to 33).
RESULTS
Patient characteristics at diagnosis.Of the 274 patients registered, 21 (7.7%) were considered ineligible. Exclusion criteria for these 21 patients are reported in Table 1. These 21 patients were withdrawn from the study and treated differently.
Reasons, Other Than Age, for the Exclusion From Entering the AIDA Protocol in 21 Patients Registered as AML-FAB M3
| Exclusion Criteria . | No. of Cases . |
|---|---|
| Karyotype NA*/PML-RARα-negative | 7 |
| Karyotype NA/PML-RARα NE† | 3 |
| Karyotype NE‡/PML-RARα-negative | 2 |
| Absence of t(15; 17)/PML-RARα-negative | 2 |
| Absence of t(15; 17)/PML-RARα NE | 2 |
| Karyotype NA/specimen for RT-PCR not sent | 1 |
| t(11; 17)/PML-RARα-negative | 1 |
| HIV-positive | 1 |
| Poor performance status/active serious infection | 1 |
| Other concomitant malignancy | 1 |
| Exclusion Criteria . | No. of Cases . |
|---|---|
| Karyotype NA*/PML-RARα-negative | 7 |
| Karyotype NA/PML-RARα NE† | 3 |
| Karyotype NE‡/PML-RARα-negative | 2 |
| Absence of t(15; 17)/PML-RARα-negative | 2 |
| Absence of t(15; 17)/PML-RARα NE | 2 |
| Karyotype NA/specimen for RT-PCR not sent | 1 |
| t(11; 17)/PML-RARα-negative | 1 |
| HIV-positive | 1 |
| Poor performance status/active serious infection | 1 |
| Other concomitant malignancy | 1 |
Abbreviations: NA, not available; HIV, human immunodeficiency virus.
Karyotype, not available.
PML-RARα NE, poor quality RNA.
Karyotype NE, no mitosis or poor quality metaphases.
The clinical and biological characteristics of the 253 eligible patients are shown in Table 2. Median age was 37.8 years (range, 2.2 to 73.9). Thirty-two cases (12.6%) were classified as microgranular or variant (M3v) APL on morphologic examination. The median white blood cell (WBC) count was 2.5 × 109/L (range, 0.2 to 140.0). Bleeding symptoms were present at diagnosis in 67% of patients; of these, 94% also had laboratory evidence of coagulopathy defined as fibrinogen <150 mg/dL and fibrinogen degradation products (FDP) >40 μg/mL or D-dimers (XDP) >400 μg/mL.
Clinical and Biological Features of Eligible Patients at Diagnosis
| Sex | |
| M | 138 (54.5%) |
| F | 115 (45.5%) |
| Age median | 37.8 |
| (range) | 2.2-73.9 |
| FAB M3 | 221 (86.4%) |
| M3v | 32 (12.6%) |
| WBC × 109/L median | 2.5 |
| (range) | 0.2-140.0 |
| Type of PML/RARα transcript | |
| Long (bcr 1-2) | 152 (61.5%) |
| Short (bcr 3) | 95 (38.5%) |
| NA | 6* |
| Sex | |
| M | 138 (54.5%) |
| F | 115 (45.5%) |
| Age median | 37.8 |
| (range) | 2.2-73.9 |
| FAB M3 | 221 (86.4%) |
| M3v | 32 (12.6%) |
| WBC × 109/L median | 2.5 |
| (range) | 0.2-140.0 |
| Type of PML/RARα transcript | |
| Long (bcr 1-2) | 152 (61.5%) |
| Short (bcr 3) | 95 (38.5%) |
| NA | 6* |
Abbreviation: NA, not available.
All had the t(15; 17).
The type of PML/RARα transcript as detected by RT-PCR (available in 247 cases) was bcr1-2 (or long type) in 152 (61.5%) patients, and bcr3 (or short type) in 95 (38.5%) patients. In the remaining six eligible patients, the t(15; 17) abnormality was detected by karyotypic examination only and no material was available for molecular studies at diagnosis. The type of PML breakpoint was not significantly correlated with age, sex, WBC count, or presence of coagulopathy at presentation. However, the incidence of bcr3 was significantly higher in patients with a diagnosis of M3v. In fact, a bcr3 breakpoint was observed in 20 of 32 (62%) M3v cases and in 75 of 215 (35%) hypergranular APL cases (P = .005).
Hematological and molecular response to induction therapy.Of the 253 eligible patients, three died of intracranial hemorrhage before the start treatment, one was lost to follow up, two were crossed to chemotherapy at days +16 and +20 due to ATRA toxicity and were considered as protocol violations, and it was too early for response to induction to be evaluated in seven patients.
Of the remaining 240 evaluable patients, 229 (95%) completed induction therapy and achieved hematologic CR, and 11 patients (5%) died during induction. No cases of resistant disease were documented. Of the 11 early deaths, eight were due to intracranial hemorrhage and occurred at days +1, +1, +3, +7, +8, +8, +13, and +36, respectively; one to ATRA syndrome (day +17), one to cerebral thrombosis (day +13), and one to myocardial infarction (day +20). The median time of ATRA therapy was 31 days (range, 3 to 90). The median time for polymorphonuclear cells to recover to >1.0 × 109/L and platelets to >100 × 109/L was 27 days (range, 4 to 48) and 28 days (range, 9 to 49), respectively. Table 3 shows clinical results after induction.
Response to Induction Therapy
| Eligible | 253 |
| Pretherapy deaths | 3 |
| Lost to follow-up | 1 |
| Violation | 2 |
| Too early | 7 |
| Evaluable | 240 |
| CR | 229 (95%) |
| Induction deaths | 11 (5%) |
| Resistant disease | 0 |
| Eligible | 253 |
| Pretherapy deaths | 3 |
| Lost to follow-up | 1 |
| Violation | 2 |
| Too early | 7 |
| Evaluable | 240 |
| CR | 229 (95%) |
| Induction deaths | 11 (5%) |
| Resistant disease | 0 |
RT-PCR analysis for the evaluation of the PML/RARα hybrid at the end of induction and before consolidation therapy was performed in 139 cases. As to the other patients who achieved CR, four cases were not evaluable due to poor quality RNA and no samples were sent in for the remaining cases. Fifty-five (39.5%) of the 139 patients analyzed in CR after induction were PCR-positive and 84 (60.5%) PCR-negative. There was no significant correlation between the type of PML breakpoint and molecular response to induction therapy.
Molecular response after consolidation and follow-up analysis.Of the 229 patients who entered hematologic CR, 5 died of complications during consolidation, 1 was lost to follow up, 1 relapsed after the second cycle, and 60 have yet to complete consolidation therapy. Of the remaining 162 tested by RT-PCR after consolidation, 159 (98%) were PCR-negative and three (2%) PCR-positive. The three PCR-positive patients had all been positive at the end of induction. The remaining 52 patients who had been PCR-positive at the end of induction converted to PCR negativity after consolidation.
PCR assays between cycles of consolidation were performed in a limited number of cases in this study. Of 23 patients PCR-positive postinduction, 16 (69.5%) converted to PCR-negative and 7 (30.5%) remained PCR-positive after the first consolidation. Of these seven PCR-positive, 2 were still PCR-positive and 2 converted to PCR-negative following the second consolidation (no analyses were available at this time in the other three cases. However, all three converted to PCR-negative after the third cycle). In summary, of 20 postinduction PCR-positive cases tested either after one or two consolidation cycles, 18 (90%) achieved molecular remission before receiving the third chemotherapy consolidation course.
Sixteen (9.8%) of the 162 patients evaluable after consolidation did not complete the three courses because of therapy-related toxicity. Clinical and molecular data available in this series are shown in Table 4. Median age was 61 years. In the majority of cases, treatment withdrawal was due to infection and/or prolonged BM hypoplasia. These patients were not randomized for maintenance; 7 patients received heterogeneous therapy as maintenance, whereas 9 had no further treatment. The median remission duration of patients in this group is 23 months (range, 2 to 33).
Clinical and Molecular Outcome of 16 Patients Withdrawn From Study Due To Therapy-Related Toxicity
| UPN . | Age . | No. of Consolid. Cycles Received . | Type of Toxicity . | Further Therapy Postconsolid. . | Outcome . | Follow-up RT-PCR Analyses . |
|---|---|---|---|---|---|---|
| 28024 | 65 | 04-150 | Cardiac | ATRA/MTX/6MP | CCR 14+4-151 | Negative (2 tests) |
| 28030 | 60 | 0 | Infectious | ATRA/MTX/6MP | CCR 10+ | Negative (2 tests) |
| 04003 | 63 | 1 | Infectious | IFN | CCR 33+ | Negative (3 tests) |
| 10004 | 62 | 1 | Infectious | None | Relapsed at 6 mo/dead | Negative (2 tests) |
| 35003 | 55 | 2 | Neurologic | None | CCR 31+ | Negative (5 tests) |
| 04002 | 33 | 2 | Infectious | ATRA/MTX/6MP | CCR 32+ | Negative (3 tests) |
| 36001 | 59 | 2 | Infectious | IFN | CCR 32+ | Negative (4 tests) |
| 28015 | 63 | 2 | Prolonged BM hypoplasia | MTX/6MP | CCR 33+ | Negative (6 tests) |
| D6004 | 9 | 2 | Severe mucositis | None | CCR 29+ | Negative (6 tests) |
| 43002 | 66 | 2 | Prolonged BM hypoplasia | None | CCR 29+ | Negative (4 tests) |
| 37005 | 62 | 2 | Prolonged BM hypoplasia | None | CCR 30+ | Negative (5 tests) |
| 55001 | 43 | 2 | Infectious | None | Relapsed at 7 mo/dead | Positive after ind.‡ |
| 07006 | 63 | 2 | Infectious | None | Dead at 2 moρ | Negative (1 test) |
| 43005 | 66 | 2 | Neurologic | None | CCR 16+ | Negative (3 tests) |
| 28021 | 23 | 2 | Infectious | MTX/6MP | CCR 17+ | Negative 5 tests, 1 +ve 4-155 |
| 08005 | 31 | 2 | Infectious | None | CCR 10+ | Negative (3 tests) |
| UPN . | Age . | No. of Consolid. Cycles Received . | Type of Toxicity . | Further Therapy Postconsolid. . | Outcome . | Follow-up RT-PCR Analyses . |
|---|---|---|---|---|---|---|
| 28024 | 65 | 04-150 | Cardiac | ATRA/MTX/6MP | CCR 14+4-151 | Negative (2 tests) |
| 28030 | 60 | 0 | Infectious | ATRA/MTX/6MP | CCR 10+ | Negative (2 tests) |
| 04003 | 63 | 1 | Infectious | IFN | CCR 33+ | Negative (3 tests) |
| 10004 | 62 | 1 | Infectious | None | Relapsed at 6 mo/dead | Negative (2 tests) |
| 35003 | 55 | 2 | Neurologic | None | CCR 31+ | Negative (5 tests) |
| 04002 | 33 | 2 | Infectious | ATRA/MTX/6MP | CCR 32+ | Negative (3 tests) |
| 36001 | 59 | 2 | Infectious | IFN | CCR 32+ | Negative (4 tests) |
| 28015 | 63 | 2 | Prolonged BM hypoplasia | MTX/6MP | CCR 33+ | Negative (6 tests) |
| D6004 | 9 | 2 | Severe mucositis | None | CCR 29+ | Negative (6 tests) |
| 43002 | 66 | 2 | Prolonged BM hypoplasia | None | CCR 29+ | Negative (4 tests) |
| 37005 | 62 | 2 | Prolonged BM hypoplasia | None | CCR 30+ | Negative (5 tests) |
| 55001 | 43 | 2 | Infectious | None | Relapsed at 7 mo/dead | Positive after ind.‡ |
| 07006 | 63 | 2 | Infectious | None | Dead at 2 moρ | Negative (1 test) |
| 43005 | 66 | 2 | Neurologic | None | CCR 16+ | Negative (3 tests) |
| 28021 | 23 | 2 | Infectious | MTX/6MP | CCR 17+ | Negative 5 tests, 1 +ve 4-155 |
| 08005 | 31 | 2 | Infectious | None | CCR 10+ | Negative (3 tests) |
Abbreviations: MTX, methotrexate; 6MP, 6-mercaptopurine; IFN, alpha 2b interferon.
Patients 1 and 2 received induction therapy only.
Months in continuous complete remission.
Tested after induction therapy only.
ρ Died of septic shock while in CR.
Tested PCR-positive at the last molecular follow-up analysis.
The results of the RT-PCR studies peformed at diagnosis, after induction, and after consolidation in the whole series are shown in Table 5. The three patients who tested PCR-positive at the end of consolidation underwent allogeneic BMT from an HLA-identical sibling. Of the 159 who tested PCR-negative, 14 went off the study for protocol violation (9 cases), treatment-related toxicity (4 cases), and refusal to continue the therapeutic program (1 case). The remaining 145 patients were randomized for maintenance and are currently under evaluation for the effect of the different treatment approaches on relapse-free survival.
PCR Monitoring of PML/RARα
| . | Evaluable . | PCR+(%) . | PCR−(%) . |
|---|---|---|---|
| At diagnosis | 247 | 247 (100%) | |
| After induction | 139 | 55 (39.5%) | 84 (60.5%) |
| After consolidation | 162 | 3 (2%) | 159 (98%) |
| . | Evaluable . | PCR+(%) . | PCR−(%) . |
|---|---|---|---|
| At diagnosis | 247 | 247 (100%) | |
| After induction | 139 | 55 (39.5%) | 84 (60.5%) |
| After consolidation | 162 | 3 (2%) | 159 (98%) |
As of March 1996, 17 patients relapsed at 5 to 20 months from the achievement of hematologic remission. Among these, two had not completed all cycles of consolidation chemotherapy. Fifteen had typical hypergranular morphology at presentation, whereas two were classified as M3v. The median WBC count in these series of 17 patients was 7.2 × 109/L (0.9 to 54.9). The type of breakpoint within the PML gene, available in 16 cases, was bcr 1-2 in nine patients, and bcr 3 in seven patients.
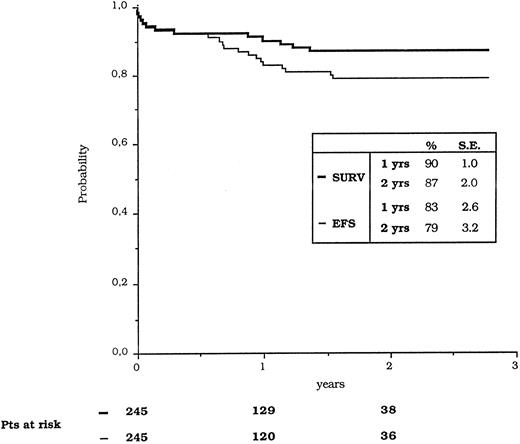
The estimated 12 and 24 months OS for all of the 253 eligible patients was 90% ± 1% and 87% ± 2%, whereas the EFS was calculated at 83% ± 2.6% and 79% ± 3.2%, respectively (Fig 1). The comparison of EFS (calculated from the achievement of CR) between patients who were PCR-positive and PCR-negative at the end of induction showed no statistically significant difference.
Kaplan-Meier estimates of overall survival and event-free survival (EFS).
ATRA syndrome and other therapy-related toxicity.Of 240 patients evaluable for induction therapy, 6 (2.5%) developed an overt or “definitely present” ATRA syndrome and one patient died of it. Clinical features including WBC initial count and peak, specific treatment for the syndrome, and outcome of these patients are reported in Table 6. In 11 additional cases, a combination of signs and symptoms was observed, which allowed us to classify them as having “indeterminate” ATRA syndrome. Finally, ATRA syndrome was considered “definitely absent” in 223 of 240 patients (93%).
Clinical Features of Patients With Overt or “Definitely Present” ATRA Syndrome
| UPN . | Age/Sex . | FAB . | WBC Count (×109/L) . | Therapy . | Outcome . | |
|---|---|---|---|---|---|---|
| . | . | . | Initial . | Peak . | . | . |
| . | . | . | . | (day of treatment) . | . | . |
| 07006 | 63/F | M3 | 0.5 | 5.9 (6) | ATRA discont./Dexamet. | CR |
| 24009 | 46/M | M3v | 54.2 | 101.0 (2) | ATRA discont./Dexamet. | CR |
| 24010 | 36/F | M3 | 7.2 | 18.2 (4) | ATRA discont./Dexamet. | CR |
| 35005 | 45/M | M3 | 11.6 | 25.8 (7) | ATRA discont./Dexamet. | CR |
| 55003 | 40/F | M3 | 0.6 | 1.6 (16) | ATRA discont./Dexamet. | CR |
| 24001 | 31/F | M3 | 1.5 | 5.1 (4) | ATRA discont./Dexamet. | Died |
| UPN . | Age/Sex . | FAB . | WBC Count (×109/L) . | Therapy . | Outcome . | |
|---|---|---|---|---|---|---|
| . | . | . | Initial . | Peak . | . | . |
| . | . | . | . | (day of treatment) . | . | . |
| 07006 | 63/F | M3 | 0.5 | 5.9 (6) | ATRA discont./Dexamet. | CR |
| 24009 | 46/M | M3v | 54.2 | 101.0 (2) | ATRA discont./Dexamet. | CR |
| 24010 | 36/F | M3 | 7.2 | 18.2 (4) | ATRA discont./Dexamet. | CR |
| 35005 | 45/M | M3 | 11.6 | 25.8 (7) | ATRA discont./Dexamet. | CR |
| 55003 | 40/F | M3 | 0.6 | 1.6 (16) | ATRA discont./Dexamet. | CR |
| 24001 | 31/F | M3 | 1.5 | 5.1 (4) | ATRA discont./Dexamet. | Died |
Abbreviation: discont./Dexamet., discontinuation/Dexamethasone.
Other ATRA-related adverse reactions included mucosal and skin dryness (29% of cases), hypotension (7%), headache (13%), severe bone pain (6%), pseudotumor cerebri (2%), and hypercholesterolemia (6%). Scrotal ulcerations were observed in two patients (1%).
As to the other therapy-related toxicity, mainly due to anthracycline treatment, these were limited to stomatitis (6%), hemorrhages (15%), nausea and vomiting (5%), diarrhea (2%), cardiac (2.5%), hepatic (3%), and renal (0.5%) toxicities considering together only World Health Organization (WHO) grades 3 and 4.
Of the 5 deaths occurring after the end of induction, 3 were recorded during the first consolidation course and 2 during the second course. Three deaths were due to bacterial infection, one to hepatitis, and one to an ATRA syndrome developed in CR.
DISCUSSION
Of all acute leukemias, APL is at present the form to which molecular studies contribute the most clinically relevant information. Three lines of evidence support this assumption, ie, (1) the PML/RARα genetic lesion is absolutely APL-specific and found in virtually 100% of cases2-4; (2) the disease responds specifically to a distinct treatment approach that is also effective against the life-threatening coagulopathy2-4; and (3) PML/RARα detection in the leukemic blasts predicts response to ATRA.6 A similar combination of circumstances is not observed in any other human leukemia.
The mandatory prerequisite for entry into this study was molecular or cytogenetic evidence of the t(15; 17) in leukemia cells at diagnosis. The characterization of the PML/RARα junction type, available in 247 of 253 (98%) eligible patients, also provided an ideal specific marker for the sensitive assessment of response to therapy. Based on past experience gained as a referral laboratory for RT-PCR analysis in a multicentric study,26 we put special emphasis on the logistic organization of the molecular studies. In particular, we recommended that rapid isolation and storage of the mononuclear (MNC) cell fraction of marrow aspirates in an RNAase inhibitor (guanidium isothiocyanate), be performed locally in each participating center before shipment. This procedure turned out to be extremely important for the extraction of good quality RNA in the vast majority of cases.
The results of induction therapy with ATRA and Idarubicin show that all PML/RARα positive patients are responsive to this treatment. As a genetic diagnosis of APL was not required in any previously published study, it might seem inappropriate to compare our data with results obtained using other protocols, whether they included ATRA or not. However, it is conceivable that the lower CR rate reported in other studies could have been due, at least in part, to PML/RARα negative patients being enrolled.12-25,28,30 Several new acute leukemia entities, which morphologically resemble typical APL but manifest distinct genetic and/or immunophenotypic features, have been described recently. They include cases with variant translocation that involve RARα on 17q- with partner chromosomes other than 15, for example t(11; 17) and t(5; 17) associated APL,31,32 as well as cases with a myeloid-natural killer (CD56+) immunophenotype.33 Because these cases do not express the PML/RARα fusion gene and fail to respond to ATRA, patients with no cytogenetic or molecular proof of the t(15; 17) in their leukemia blasts at presentation were excluded from our series (Table 1).
The AIDA induction regimen appears to be well tolerated. In fact, the incidence and the severity of complications, including hemorrhagic deaths, ATRA syndrome, and other therapy-related toxicity were lower in our series than in other induction protocols irrespective of whether or not they included ATRA.12-25,28,30 The combination of a differentiating agent and a cytotoxic drug administered in the early phase of therapy very likely provided a counteraction against either the bleeding diathesis due to the coagulopathy or the occurrence of the severe pulmonary symptoms associated with the ATRA syndrome. Several investigators have shown that ATRA treatment significantly improves the APL-associated coagulopathy,34-36 while cytotoxic chemotherapy is known to prevent the onset of the ATRA syndrome, being often anticipated in patients considered at risk because of hyperleukocytosis at presentation.21 These two severe complications, which so far represented the major causes of induction death in APL, were recorded in a minority of cases in this study. Overall, 11 of 240 parients (5%) died during induction treatment.
Following the guidelines of the New York Group (Frankel et al28 and previous personal communication by Dr R.P. Warrell Jr, April 1993), we recommended to all physicians of our Cooperative Group to promptly administer IV dexamethasone as prophylactic treatment at the earliest clinical sign of dyspnea. It is conceivable that, in conjunction with early chemotherapy administration, this policy contributed to prevent the onset of a full-blown and life-threatening ATRA syndrome in the vast majority of our patients.
Concerning consolidation, our clinical and molecular data suggest that the administration of less postremission treatment might be considered in the future, at least for elderly patients. In fact, as shown in Table 4, the median age of patients withdrawn from the study because of therapy-related toxicity was 61 years. Furthermore, the preliminary follow-up analysis in this series showed a short-term outcome comparable to that of patients receiving the three consolidation courses.
PCR results between consolidation cycles, although only available in a fraction of cases, indicate that the vast majority of patients achieve molecular remission after two cycles. A prolonged follow-up analysis is needed to establish the prognostic significance of these early achieved molecular remissions and to verify whether less postremission treatment could be effective in APL, regardless of age.
Molecular remission was achieved in 60% of our cases after induction and 98% after consolidation. We are not sure whether the PCR positivity detected at the end of induction reflects persistence of resistant blasts or delayed ATRA-induced maturation. We are also unable to assess whether the different kinetics of molecular response and leukemia-cell clearance have prognostic value. However, the attainment of a remission status corresponding to less than 10−4 PML/RARα-positive cells (according to the sensitivity of our assay) in almost all patients is further testimony for the therapeutic efficacy of the AIDA regimen.
While PCR positivity during hematologic remission in APL is generally considered a strong predictor of clinical relapse, the achievement of a PCR-negative status is no guarantee of cure, as indicated by the 17 relapses occurring in patients who had previously tested PCR-negative. This probably reflects the limited sensitivity of the RT-PCR assay for PML/RARα.4 At the time of the present analysis, due to the low number of adverse events, no predictive features could be found that were associated with a higher probability of relapse. In particular, no statistically significant association was found between treatment outcome and type of PML breakpoint, WBC count, or PCR status after induction. However, it is interesting to observe that patients who relapsed had higher median WBC counts at diagnosis. Future analysis of these parameters in a larger series and on a longer follow-up, might provide relevant information as to the possibility of identifying patients at higher risk of relapse.
Because of the uncertainty of the fate of patients who achieve PCR negativity, it is too early to predict the long-term outcome in the present series. However, the actuarial 1- and 2-year EFS estimates (83% ± 2.6% and 79% ± 3.2%, respectively) are promising figures with respect to long-term outcome. In addition, APL patients who relapse are easily rescued into second remission and become long-term survivors more often than patients with other myeloid leukemias.37
In conclusion, our results indicate that a specific treatment approach targetted to a molecular abnormality might dramatically change the natural history of APL, turning this once rapidly fatal disease into one of the most frequently curable acute leukemias. In addition, APL offers a model that should trigger interest to identify other genetico-clinical subsets of acute leukemia so that currently available therapy and future advancements can be more precisely directed at treating these diseases.
APPENDIX
The following clinical departments participated in the AIDA 0493 trial: Dipartimento di Biotecnologie Cellulari ed Ematologia, Universita' “La Sapienza,” Roma, F. Mandelli, G. Avvisati, D.Diverio, F. Lo Coco, M.C. Petti, M.L. Vegna; Divisione di Ematologia, Ospedale S. Martino, Genova, E. Damasio, R Cerri; Istituto di Ematologia L. e A.Seragnoli, Universita', Bologna, S. Tura, G. Visani, G. Martinelli; Divisione di Ematologia, Ospedale S. Bortolo, Vicenza, F. Rodeghiero, E. Di Bona; Divisione di Ematologia, Policlinico S. Matteo, Pavia, C. Bernasconi, M. Lazzarino; Divisione di Medicina E, Opedale S. Giovanni, Torino, L. Resegotti, M Falda; Divisione di Ematologia, Policlinico Careggi, Firenze, P. Rossi Ferrini, F. Leoni; Divisione di Ematologia, Ospedali Riuniti, Bergamo, T. Barbui, A. Rambaldi; Divisione di Ematologia, Ospedale Civile, Pescara, G. Fioritoni, A. Recchia; Servizio di Ematologia, Policlinico, Bari, V. Liso, G. Specchia; Divisione di Ematologia, Ospedale A. Businco, Cagliari, G. Broccia, W. Deplano; Servizio di Ematologia, Ospedale Civile, Avellino, E. Volpe, N. Cantore; Divisione di Ematologia, Ospedale A. Pugliese, Catanzaro, A. Peta, F. Iuliano; Divisione di Ematologia, Ospedale S. Gerardo, Monza, E. Pogliani, G. Corneo; Ematologia, Ospedale Generale e Regionale, Bolzano, P. Coser, P. Fabris; Sezione di Ematologia Spedali Civili, Brescia, T. Izzi, G. Rossi; Cattedra di Ematologia, Universita', Catania, E. Cacciola, F. Di Raimondo; Cattedra di Ematologia, Universita', Parma, V. Rizzoli, C. Almici; Cattedra di Ematologia, Universita', Verona, G. Perona, D. Veneri; Cattedra di Ematologia, Universita', Genova, M. Gobbi, M. Clavio; Divisione di Ematologia, Ospedale Cardarelli, Napoli, R. Cimino, F. Ferrara; Divisione di Ematologia, Osp. Nuovo Pellegrini, Napoli, R. De Biasi, E. Miraglia; Divisione di Ematologia, T.E.R.E., Napoli, L. De Rosa, V. Mettivier; Cattedra di Ematologia, Universita' Tor Vergata, Roma, S. Amadori, G. Aronica; Clinica Pediatrica, Ospedale S. Gerardo, Monza, G. Masera, A. Biondi, A. Luciano; Divisione di Ematologia, Universita' Cattolica, Roma, G. Leone, S. Sica; Divisione di Ematolgia, Ospedali Riuniti, Reggio Calabria, F. Nobile, B. Martino; Sezione di Ematolgia, Ospedale S. Croce, Cuneo, E. Gallo, A. Gallamini; Divisione di Ematologia, Ospedale S.Maria Goretti, Latina, L. Deriu, A. Cherichini; Sezione di Ematologia, CTMO, Cremona, A. Porcellini, S. Morandi; Divisione di Ematologia, Nuovo Policlinico, Napoli, B. Rotoli, C. Selleri; Cattedra di Ematologia, Università, Perugia, M.F. Martelli, A. Tabilio; Clinica Medica, Universita', Palermo, A. Cajozzo, M. Musso; Divisione di Ematologia, Ospedale V. Cervello, Palermo, F. Caronia, S. Mirto, A. Santoro; Divisione di Ematologia, Ospedale B.Gesù, Roma, G. De Rossi, M. Caniggia; Istituto di Ematologia, Nuovo Ospedale Torrette, Ancona, P. Leoni, M. Montillo; Centro di Riferimento Oncologico, Aviano, S. Monfardini, V. Zagonel; Patologia Medica, Universita', Genova, R. Ghio, E. Balleari; Clinica Medica, Policlinico S. Matteo, Pavia, E. Ascari, R. Invernizzi; Divisione di Ematologia, Universita', Pisa, B. Grassi, M. Petrini; Ematologia, Ospedale S.S. Annunziata, Taranto, P. Mazza, G. Lazzari; Cattedra di Ematologia, Universita', Udine, M. Baccarani, A. Candoni; Ematologia Pediatrica, Universita', Catania, G. Schiliro', A.M. Ippolito; Ematologia, IV Divisione Pediatrica, Genova, L. Massimo, C. Micalizzi; Cinica Pediatrica, Universita', Pavia, F. Severi, F. Locatelli; Ematologia, Ospedale Regionale A. Di Summa, Brindisi, G. Quarta, A. Melpignano; Cattedra di Ematologia, Universita', Ferrara, G. Castoldi, F. Lanza; Semeiotica Medica, Universita', Genova, F. Patrone, M. Sessarego; Divisione di Ematologia, Ospedale Niguarda, Milano, E. Morra, A.M. Nosari; Ematologia, Ospedale S. Raffaele, Milano, C. Bordignon, L. Camba; Ematologia ed Autotrapianto Ospedale S. Martino, Genova, A. M. Carella, F. Frassoni; Sezione di Ematologia, Ospedale S. Francesco, Nuoro, A. Gabbas, G. Latte; Cattedra di Ematologia, Policlinico, Palermo, P. Citarella, S. Grisanti; Divisione di Ematologia, Ospedale S. Salvatore, Pesaro, G. Lucarelli, G. Sparaventi; Sezione di Ematologia, Ospedale S. Carlo, Potenza, F. Ricciuti, M. Pizzuti; Divisione di Ematologia, Ospedale S. Camillo, Roma, A. De Laurenzi, L. Pacilli; Divisione di Ematologia, Casa Sollievo della Sofferenza, S.G. Rotondo, M. Carotenuto, S. Ladogana; Divisione di Ematologia, Ospedale A. Sclavo, Siena, E. Dispensa, A. Bucalossi; Clinica Pediatrica, Ospedale G. Salesi, Ancona, P. Giorgi, L. Felici; Clinica Pediatrica I, Policlinico, Bari, F. Schettini, N. Santoro; Onco-Ematologia Pediatrica, Ospedale Regionale, Cagliari, P. Biddau; II Divisione Pediatrica, Ospedale Pausilipon, Napoli, V. Poggi, M.F. Pintà; Clinica Pediatrica I, Universita', Napoli, M.T. Di Tullio, M. Giuliano; Clinica Pediatrica II, Universita', Padova, L. Zanesco, M. Pilon; Clinica Pediatrica III, Universita', Pisa, P. Macchia, C. Favre; Clinica Pediatrica, Universita', Torino, E. Madon, R. Miniero; Department of Hematology, University of Nijmegen (NL) T. de Witte, P. Muus; Medizinische Klinik III, University of Munich (D), U. Jehn; Department of Hematology, University of Leiden (NL), R. Willemze; Department of Hematology, University of Ankara (TK), M.Beksac; and Az Middelheim, Afdeling Hemato-Oncologie, Antwerpen (B), R. De Bock.
Supported by grants from CNR (Consiglio Nazionale delle Ricerche) contratti ACRO no. 95.00414.PF39, 95.02140, and 95.00440.PF39, ROMAIL (Associazione Italiana contro le Leucemie, Sezione di Roma), Fondazione Tettamanti, Monza, and AIRC (Associazione Italiana per la Ricerca sul Cancro), Milano.
Presented in part at the 37th Annual Meeting of the American Society of Hematology, Seattle, WA, December 1-5, 1995.
Address reprint requests to Francesco Lo Coco, MD, Hematology, Department of Human Biopathology, University La Sapienza, Via Benevento 6, 00161 Roma, Italy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal