Abstract
The improved outcome of acquired aplastic anemia (AA) has revealed later complications, such as myelodysplastic syndrome (MDS) and acute myelogenous leukemia (AML). We retrospectively analyzed 167 children with severe acquired AA. Eleven of 50 children treated with cyclosporin (CSA) and recombinant human granulocyte colony-stimulating factor (rhG-CSF ) developed MDS/AML; 8 of these were within 36 months of the diagnosis of AA, much earlier than previous reports. Six of the 11 children received rhG-CSF exceeding 10 μg/kg/d, and 9 received rhG-CSF therapy for over 1 year. Ten children showed monosomy 7 at diagnosis of MDS. All of the 11 children were administered both CSA and rhG-CSF. There was no development of MDS/AML among 41 children treated with either CSA or rhG-CSF or among 48 children who underwent bone marrow transplantation. A well-controlled clinical trial is warranted to determine whether therapeutic modalities affect the development of MDS/AML in children with severe acquired AA.
OVER THE PAST 20 years, the results of treatment for severe acquired aplastic anemia (SAA) have greatly improved.1-3 A recent study found that simultaneous administration of recombinant human granulocyte colony-stimulating factor (rhG-CSF ) in combination with immunosuppressive (IS) therapy produced a very favorable outcome.4 Although the number of survivors has been increasing, the development of clonal diseases, such as paroxysmal nocturnal hemoglobinuria and myelodysplastic syndrome/acute myelogenous leukemia (MDS/AML), has been observed in the long-term follow-up of patients with aplastic anemia (AA) who had received IS therapy.5-8 We have reported that children with typical acquired SAA developed MDS/AML after treatment with rhG-CSF and IS therapy.9 10
MATERIALS AND METHODS
All patients were under 16 years of age and were diagnosed with untreated severe idiopathic or posthepatitis AA between April 1988 and March 1993. The diagnosis of AA and the assessment of the severity of the disease were based on established criteria.12-14 All clinical findings were examined by a committee member of the Japanese Society of Pediatric Hematology (Toho University School of Medicine, Tokyo, Japan) to assess their validity for study registration. Fanconi's anemia and other congenital anemias were excluded.
Recorded data included age; gender; hematologic findings at the time of diagnosis; bone marrow (BM) findings, including chromosome examination; treatments; blood transfusions; and outcome. These were noted retrospectively from a questionnaire. Because the choice of treatment depended on each institution, the data showed a variety of treatments performed in various combinations. Therefore, any drug administered during the treatment, even if only once, was recorded. Another questionnaire was mailed to all children who developed MDS/AML to investigate the therapeutic condition and presence or absence of chromosome aberration. Review by the committee was performed on all BM samples at the time of diagnosis of AA and MDS/AML. French-American-British (FAB) classification was used for the diagnosis of MDS. Actuarial survival rates and incidences of MDS/AML were determined according to the method of Kaplan-Meier, using the JMP statistical program (SAS Institute Inc, Cary, NC).
RESULTS
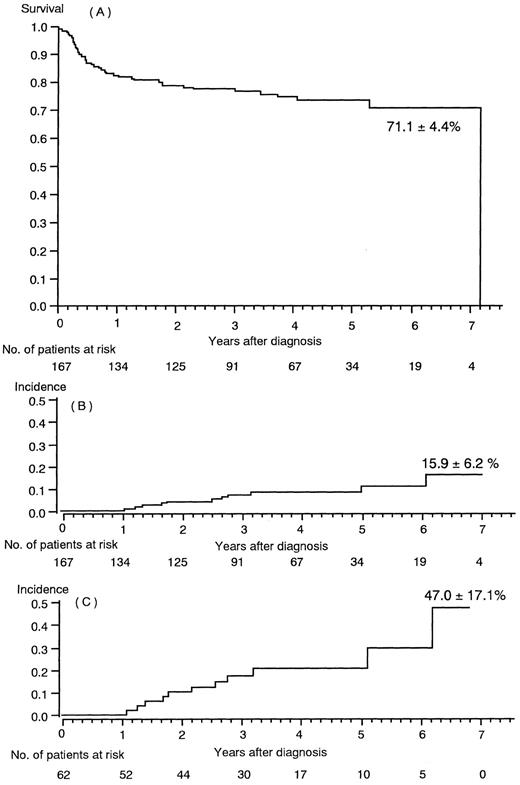
The clinical features of 167 children with SAA are summarized in Table 1. The estimated survival rate at a 7-year follow-up (median 1,294 days) was 71.1% ± 4.4% (SE); this included 48 transplanted patients (Fig 1A). Eleven of these 167 patients developed MDS/AML (Tables 2 and 3). Two of the 11 children had posthepatitis AA and 6 were assessed as having severe AA at the time of diagnosis. MDS/AML developed between 17 and 36 months following the time of diagnosis of AA in 8 of the 11 children. The actuarial incidence of MDS/AML was 15.9% ± 6.2% for all 167 children with SAA (Fig 1B). If the estimate was restricted to the 62 children who received both IS and rhG-CSF therapy (Table 4, categories 1, 2, and 3), the incidence was 47.0% ± 17.1% (±SE) of 6 years and 3 months (Fig 1C). The 119 children who did not undergo a BM transplantation had an estimated 22.5% ± 8.3% actuarial incidence of MDS/AML.
Clinical Features of 167 Children With Severe Aplastic Anemia
| No. of patients | 167 | |
| Age (yr) | Median (range) | 8 (6 mo-16 yr) |
| Gender | M/F | 78/89 |
| Etiology | Idiopathic/hepatitis | 146/21 |
| Disease severity | Very severe/severe | 76/91 |
| Presentation | Median (range) | 0.2 (0-2.7) |
| ANC (×109/L) | ||
| <0.1 × 109/L | 52 patients | |
| Plt (×109/L) | Median (range) | 11 (1-141) |
| <10 × 109/L | 68 patients | |
| Ret (×109/L) | Median (range) | 9 (0-113) |
| <20 × 109/L | 132 patients |
| No. of patients | 167 | |
| Age (yr) | Median (range) | 8 (6 mo-16 yr) |
| Gender | M/F | 78/89 |
| Etiology | Idiopathic/hepatitis | 146/21 |
| Disease severity | Very severe/severe | 76/91 |
| Presentation | Median (range) | 0.2 (0-2.7) |
| ANC (×109/L) | ||
| <0.1 × 109/L | 52 patients | |
| Plt (×109/L) | Median (range) | 11 (1-141) |
| <10 × 109/L | 68 patients | |
| Ret (×109/L) | Median (range) | 9 (0-113) |
| <20 × 109/L | 132 patients |
Abbreviations: M, male; F, female; ANC, absolute neutrophil count; Plt, platelet count; Ret, reticulocyte count.
(A) Actuarial survival rate in 167 children with severe aplastic anemia. (B) MDS/AML incidence among 167 children with severe aplastic anemia. (C) MDS/AML incidence in the 62 children who were treated with immunosuppressive therapy and rhG-CSF.
(A) Actuarial survival rate in 167 children with severe aplastic anemia. (B) MDS/AML incidence among 167 children with severe aplastic anemia. (C) MDS/AML incidence in the 62 children who were treated with immunosuppressive therapy and rhG-CSF.
Characteristics of 11 Children Who Developed MDS/AML
| Patients . | Age . | Gender . | Etiology . | Severity . | Interval AA to MDS/AML (mo) . | FAB Classification MDS/AML . | Cytogenetic Study . | Treatment for MDS/AML and Outcome . | |
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | . | . | . |
| 1 | 13 | M | idiop | vS | 74 | RA | −7,+21 | U-BMT | Dead |
| 2 | 14 | M | idiop | vS | 61 | RA | −7 | Alive | |
| 3 | 16 | M | hepat | S | 38 | M4 | −7 | Dead | |
| 4 | 5 | M | idiop | S | 17 | RA/M4 | −7 | U-BMT | Dead |
| 5 | 3 | F | idiop | S | 30 | RAEB | +21 | BMT* | Dead |
| 6 | 3 | F | idiop | S | 32 | RA | −7 | Alive | |
| 7 | 5 | F | idiop | vS | 12 | RAEB | −7 | Dead | |
| 8 | 9 | F | idiop | vS | 15 | RAEBin T/M4 | −7 | U-BMT | Dead |
| 9 | 10 | M | idiop | vS | 23 | RAEBin T/M4 | −7 | Dead | |
| 10 | 2 | M | idiop | vS | 22 | RA | −7 | U-BMT | Alive |
| 11 | 7 | M | hepat | S | 25 | RAEBin T/M4 | −7/−7,+21 | U-BMT | Dead |
| Patients . | Age . | Gender . | Etiology . | Severity . | Interval AA to MDS/AML (mo) . | FAB Classification MDS/AML . | Cytogenetic Study . | Treatment for MDS/AML and Outcome . | |
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | . | . | . |
| 1 | 13 | M | idiop | vS | 74 | RA | −7,+21 | U-BMT | Dead |
| 2 | 14 | M | idiop | vS | 61 | RA | −7 | Alive | |
| 3 | 16 | M | hepat | S | 38 | M4 | −7 | Dead | |
| 4 | 5 | M | idiop | S | 17 | RA/M4 | −7 | U-BMT | Dead |
| 5 | 3 | F | idiop | S | 30 | RAEB | +21 | BMT* | Dead |
| 6 | 3 | F | idiop | S | 32 | RA | −7 | Alive | |
| 7 | 5 | F | idiop | vS | 12 | RAEB | −7 | Dead | |
| 8 | 9 | F | idiop | vS | 15 | RAEBin T/M4 | −7 | U-BMT | Dead |
| 9 | 10 | M | idiop | vS | 23 | RAEBin T/M4 | −7 | Dead | |
| 10 | 2 | M | idiop | vS | 22 | RA | −7 | U-BMT | Alive |
| 11 | 7 | M | hepat | S | 25 | RAEBin T/M4 | −7/−7,+21 | U-BMT | Dead |
Abbreviations: BMT, bone marrow transplantation; idiop, idiopathic AA; hepat, posthepatitis AA; vS, very severe; S, severe; RA, refractory anemia; RAEB, refractory anemia with excess blasts; RAEBin T; refractory anemia with excess of blasts in transformation; M4, AML M4(FAB classification, Acute Myelomonocytic Leukemia); U-BMT, unrelated donor BMT; BMT*, 2 locus mismatch BMT from family donor.
Detail of Therapies in 11 Children Who Developed MDS/AML
| Patient . | rhG-CSF . | CSA . | ALG . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | Duration of Use (mo) . | Relation to the Onset of MDS/AML . | Cumulative Dose (mg/kg) . | Average Dose (μg/kg/d) . | Duration of Use (mo) . | Interval to the Onset of MDS/AML (mo) . | Relation to the Onset of MDS/AML . | Cumulative Dose (g) . | Interval to the Onset of MDS/AML (mo) . |
| 1 | 73 | In use | 2.4 | 1.1 | 48 | 70 | 19 mo later | 246 | 74 |
| 2 | 32 | In use | 2.4 | 2.5 | 46 | 57 | 10 mo later | 241 | 60 |
| 3 | 26 | In use | 10.0 | 12.8 | 6 | 15 | 9 mo later | 25 | |
| 4 | 11 | In use | 7.9 | 23.9 | 13 | 13 | In use | 4 | 14 |
| 5 | 25 | 5 mo later | 8.6 | 11.5 | 25 | 25 | In use | 543 | |
| 6 | 32 | In use | 9.7 | 9.0 | 3 | 25 | 22 mo later | 15 | 34 |
| 7 | 12 | In use | 8.2 | 17.0 | 4 | 8 | 4 mo later | 8 | |
| 8 | 15 | In use | 3.0 | 4.8 | 4 | 14 | 10 mo later | 19 | 4 |
| 9 | 22 | In use | 8.7 | 13.2 | 5 | 16 | 11 mo later | 13 | 14 |
| 10 | 22 | In use | 8.7 | 17.5 | 6 | 16 | 10 mo later | 17 | |
| 11 | 25 | In use | 4.7 | 6.3 | 6 | 24 | 18 mo later | 16 | |
| Patient . | rhG-CSF . | CSA . | ALG . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | Duration of Use (mo) . | Relation to the Onset of MDS/AML . | Cumulative Dose (mg/kg) . | Average Dose (μg/kg/d) . | Duration of Use (mo) . | Interval to the Onset of MDS/AML (mo) . | Relation to the Onset of MDS/AML . | Cumulative Dose (g) . | Interval to the Onset of MDS/AML (mo) . |
| 1 | 73 | In use | 2.4 | 1.1 | 48 | 70 | 19 mo later | 246 | 74 |
| 2 | 32 | In use | 2.4 | 2.5 | 46 | 57 | 10 mo later | 241 | 60 |
| 3 | 26 | In use | 10.0 | 12.8 | 6 | 15 | 9 mo later | 25 | |
| 4 | 11 | In use | 7.9 | 23.9 | 13 | 13 | In use | 4 | 14 |
| 5 | 25 | 5 mo later | 8.6 | 11.5 | 25 | 25 | In use | 543 | |
| 6 | 32 | In use | 9.7 | 9.0 | 3 | 25 | 22 mo later | 15 | 34 |
| 7 | 12 | In use | 8.2 | 17.0 | 4 | 8 | 4 mo later | 8 | |
| 8 | 15 | In use | 3.0 | 4.8 | 4 | 14 | 10 mo later | 19 | 4 |
| 9 | 22 | In use | 8.7 | 13.2 | 5 | 16 | 11 mo later | 13 | 14 |
| 10 | 22 | In use | 8.7 | 17.5 | 6 | 16 | 10 mo later | 17 | |
| 11 | 25 | In use | 4.7 | 6.3 | 6 | 24 | 18 mo later | 16 | |
Therapy and MDS/AML Development in 167 Children With SAA
| Category . | ALG . | CSA . | G-CSF . | Steroids . | BMT . | No. of Patients . | No. of Patients Who Developed MDS/AML . |
|---|---|---|---|---|---|---|---|
| 1 | + | + | + | + | − | 24 | 6 |
| 2 | − | + | + | + | − | 26 | 5 |
| 3 | + | − | + | + | − | 12 | 0 |
| 4 | − | − | + | + | − | 20 | 0 |
| 5 | + | + | − | + | − | 5 | 0 |
| 6 | − | + | − | + | − | 4 | 0 |
| 7 | + | − | − | + | − | 15 | 0 |
| 8 | − | − | − | + | − | 12 | 0 |
| 9 | − | − | − | − | − | 1 | 0 |
| 10 | 4-150 | 4-151 | ‡ | + | + | 48 | 0 |
| 69 | 74 | 105 | 166 | 48 | 167 | 11 |
| Category . | ALG . | CSA . | G-CSF . | Steroids . | BMT . | No. of Patients . | No. of Patients Who Developed MDS/AML . |
|---|---|---|---|---|---|---|---|
| 1 | + | + | + | + | − | 24 | 6 |
| 2 | − | + | + | + | − | 26 | 5 |
| 3 | + | − | + | + | − | 12 | 0 |
| 4 | − | − | + | + | − | 20 | 0 |
| 5 | + | + | − | + | − | 5 | 0 |
| 6 | − | + | − | + | − | 4 | 0 |
| 7 | + | − | − | + | − | 15 | 0 |
| 8 | − | − | − | + | − | 12 | 0 |
| 9 | − | − | − | − | − | 1 | 0 |
| 10 | 4-150 | 4-151 | ‡ | + | + | 48 | 0 |
| 69 | 74 | 105 | 166 | 48 | 167 | 11 |
Abbreviations: ALG, anti lymphocyte globuline; BMT, bone marrow transplantation.
7 of 48 patients received ALG.
15 of 48 patients received CSA.
23 of 48 patients received rhG-CSF.
The FAB classification at the time of diagnosis of MDS/AML was refractory anemia in 5, refractory anemia with excess of blasts in 2, refractory anemia with excess of blasts in transformation in 3, and AML (M4) in 1. Cytogenetic analysis of BM cells at the time of diagnosis of MDS/AML revealed monosomy 7 in 10 children and trisomy 21 in 1 child. Previous BM cytogenetic studies showed a normal karyotype in 5 children, but cytogenetic studies could not be performed in the other 6 children because of low mitotic rates at the time of AA diagnosis. Six of 167 children died in the 3 years after the diagnosis of AA; 5 of these died because of MDS/AML.
All 11 of the 11 children with MDS/AML received rhG-CSF and cyclosporin (CSA). rhG-CSF was given for 11 to 73 months (median 25 months). The actuarial total dosage of rhG-CSF ranged from 2.4 to 10 mg/kg (median 8.6). The mean dose per day, which was determined by dividing the total dosage by the number of days of administration, ranged from 1.1 to 23.9 μg/kg/d (median 11.5). CSA was given for 3 to 48 months (median 6 months), and the median cumulative dose of CSA was 17 g, ranging from 8 g to 543 g. MDS/AML was found only among children who had received both rhG-CSF and CSA (Table 4, categories 1 and 2). None of the 32 children who were administered rhG-CSF but not CSA developed MDS/AML (Table 4, categories 3 and 4). Similarly, MDS/AML was not present in any of the 9 children treated with CSA but not rhG-CSF (Table 4, categories 5 and 6). Regardless of the type of therapy administered before transplantation, MDS/AML was not seen in the 48 children who underwent BM transplantation (Table 4, category 10).
DISCUSSION
The therapeutic outcome for acquired SAA has improved markedly with the introduction of IS therapy and hematopoietic growth factors.1-4 As the therapeutic outcome has improved, long-term problems have become apparent among patients who might have previously died of acute AA complications. Of these long-term complications, the most important issue is the development of clonal hematopoietic disorders such as paroxysmal nocturnal hemoglobinuria and MDS/AML.5-8
In this study, we identified 11 children who developed MDS/AML, 8 of whom developed MDS/AML within 36 months of AA diagnosis. These children developed MDS/AML relatively earlier than those in previous reports.6 7 A normal karyotype was confirmed in 5 of the 11 children at the time of diagnosis of AA. Our results of chromosomal analysis do not necessarily preclude the presence of a minor malignant clone at the time of AA diagnosis. Nevertheless, all the children who developed MDS/AML had typical features of SAA, which satisfied the diagnostic criteria for this study.
Fifty of the 167 children received both rhG-CSF and CSA, and all 11 children with MDS/AML were in this group. Among 32 children treated with rhG-CSF but not CSA (Table 4, categories 3 and 4) and 9 children treated with CSA but not rhG-CSF (Table 4, categories 5 and 6), the development of MDS/AML had not been observed at the time of the survey. This finding suggests the possible involvement of the combination of these two drugs in the development of MDS/AML from AA. Most of the children in our study who developed MDS/AML had been treated with rhG-CSF for periods of over 1 year; 6 of the 11 children had received doses of rhG-CSF exceeding 10 μg/kg/d. In children treated with a low daily dose, the duration of administration was long (up to 73 months). These findings suggest a dose-response relationship of the total dosage and period of administration of rhG-CSF with the development of MDS/AML. There are reports of rhG-CSF–related MDS/AML in patients with severe chronic neutropenia.15-17 In these patients,17 the dosage and duration of rhG-CSF administration were massive and lengthy, similar to the present study. In contrast, the children who were not treated with both rhG-CSF and CSA did not develop MDS/AML. It is possible that MDS/AML will appear in some children who did not receive both rhG-CSF and CSA with continued follow-up. Nevertheless, our data suggest the need for confirmatory retrospective or, ideally, prospective studies.
Clinical data to date suggest that development of MDS/AML occurs in about 5% to 15% of long-term survivors with AA.5-8,11 In these patients, the primary issue is whether or not the patients already had a MDS clone at the time of AA diagnosis.18 Typical AA is usually distinct from typical MDS with respect to dysplastic morphology and BM cellularity. Detection of chromosomal aberration provides definite evidence of MDS. However, some researchers have proposed the concept of hypoplastic MDS,19 and others have argued that AA is a clonal hematopoietic disorder.20,21 Thus, it may not be easy to differentiate MDS from AA, even in patients with typical features of SAA22 in whom an adequate number of mitose for cytogenetic study is not available.23 24 Therefore, taking this unresolved issue into account, some patients with primary MDS may be inadvertently included among patients with the diagnosis of AA.
Treatment with rhG-CSF has proven effective for infection in SAA.25 Prevention of severe infection after IS therapy may result in increasing numbers of long-term SAA survivors. However, rhG-CSF administration also has a theoretical risk for stimulating leukemic clones,26 leading to the development of MDS/AML. Thus, it is possible that rhG-CSF therapy may prolong survival, unmasking the true evolution of MDS/AML, but may stimulate and accelerate the growth of a MDS clone which might have otherwise remained dormant.
Further studies are needed to identify the risk factors in SAA for developing MDS/AML. In patients receiving immunosuppressants in combination with rhG-CSF, it may be necessary to survey for chromosomal changes at regular intervals soon after beginning therapy, especially in patients receiving prolonged or high-dose rhG-CSF.
APPENDIX
The following institutions and investigators participated in this study: Hokkaido University (J. Ishikawa); Hirosaki University (Y. Sato); Institute of Development, Aging and Cancer, Touhoku University (S. Tsuchiya); Ibaraki Children Hospital (M. Tsuchida); Dokkyou University (T. Furukawa, K. Sugita); Saitama Children Hospital (R. Hanada); Tokyo University (F. Bessho); Toho University (I. Tsukimoto [study organizer], A. Ohara); Nihon University (H. Mugishima); Tokyo Medical Dental University (H. Ohkawa); Chiba University (T. Sato); Chiba Children Hospital (Y. Okimoto); Jikei-Kai University School of Medicine (J. Akatsuka [chairman]); Kanagawa Children Medical Center (H. Kigasawa); Yokohama City University, School of Medicine (H. Sasaki); Institute of Medical Science, Tokyo University (T. Nakahata); Yamanashi Medical University (K. Sugita, M. Saito); Kanazawa University (S. Koizumi); Shizuoka Children Hospital (J. Mimaya); Japanese Red Cross Nagoya First Hospital (S. Kojima); Aichi Cancer Center Research Institute (N. Hamajima); Shiga University Medical School (S. Ohta); Kyoto University (Y. Akiyama); Children's Research Hospital, Kyoto Prefectural University of Medicine (S. Imashuku, S. Hibi); Child Health Center, Osaka City General Hospital (S. Konishi); Kobe City Hospital (Y. Kiriyama); Hyogo College of Medicine (M. Yamamoto); Hiroshima University (M. Kobayashi); Japan Red Cross Hiroshima Hospital and Atomic Bomb Survivors Hospital (K. Hamamoto); Okayama University (A. Akazai); Saga Medical School (S. Miyazaki); Kyushu University (S. Ohga); and Kyushu Cancer Center Hospital (J. Okamura).
Supported by Intractable Hemopoietic Disease Research from the Ministry of Health and Welfare of Japan.
Address reprint requests to Akira Ohara, MD, Toho University School of Medicine, First Department of Pediatrics, 6-11-1, Omori-Nishi, Ota-ku, Tokyo, 143 Japan.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal