Abstract
Recent reports have described families in whom a combination of elevated serum ferritin not related to iron overload and congenital nuclear cataract is transmitted as an autosomal dominant trait. We have studied the molecular pathogenesis of hyperferritinemia in two families showing different phenotypic expression of this new genetic disorder. Serum ferritin levels ranged from 950 to 1,890 μg/L in affected individuals from family 1, and from 366 to 635 μg/L in those from family 2. Cataract was clinically manifested in family 1 and asymptomatic in family 2. By using monoclonal antibodies specific for the H and L ferritin subunits, serum ferritin was found to be essentially L type in both normal and affected individuals. The latter also showed normal amounts of H-type ferritin in circulating mononuclear cells; on the contrary, L-type ferritin contents were 13 times normal in family 1 and five times normal in family 2 on average. Serum ferritin was glycosylated in both normal and affected individuals. There was a close relationship between mononuclear cell L-type ferritin content and serum ferritin concentration (r = 0.95, P < .00001), suggesting that the excess production of ferritin in cells was directly responsible for the hyperferritinemia. The dysregulated L-subunit synthesis was found to result from different point mutations in a noncoding sequence of genomic L-subunit DNA, which behaves as an mRNA cis-acting element known as iron regulatory element (IRE). Affected individuals from family 1 were heterozygous for a point mutation (a single G to A change) in the highly conserved, three-nucleotide motif forming the IRE bulge. Affected members from family 2 were heterozygous for a double point mutation in the IRE lower stem. Using a gel retardation assay, the observed molecular lesions were shown to variably reduce the IRE affinity for an iron regulatory protein (IRP), which inhibits ferritin mRNA translation. The direct relationship between the degree of hyperferritinemia and severity of cataract suggests that this latter is the consequence of excessive ferritin production within the lens fibers. These findings provide strong evidence that serum ferritin is a byproduct of intracellular ferritin synthesis and that the L-subunit gene on chromosome 19 is the source of glycosylated serum ferritin. From a practical standpoint, this new genetic disorder should be taken into account by clinicians when facing a high serum ferritin in an apparently healthy person.
ALTHOUGH THE physiological function of serum ferritin is unknown, its measurement is clinically useful representing the simplest noninvasive assay of iron stores.1 The circulating protein, in fact, is believed to be a byproduct of intracellular ferritin synthesis, although it partly differs from the protein found in cells. Ferritin is a protein shell with a molecular weight of about 500,000 Mr made up of 24 subunits.2 The multiple forms, or isoferritins, that occur in human tissues appear to originate by the presence of three subunit types: L (light, 19 kD, 174 residues), H (heavy, 21 kD, 182 amino acids), and G (glycosylated, 23 kD).3 Tissue ferritins consist of variable proportions of H and L subunit types, whereas serum ferritin contains almost only L and G subunits, which are immunologically similar. The H and L chains are encoded in chromosomes 11 and 19, respectively, and have large immunological differences. The G subunit, which is present only in the extracellular ferritins, likely derives from a posttranslational modification of the L chain.3
A large body of evidence indicates that the level of serum ferritin parallels the concentration of storage iron within the body, regardless of the cell type in which it is stored.4 This relationship of serum ferritin to body iron stores, however, is altered in inflammatory states and liver disease, conditions that can disproportionately elevate the circulating protein.1
Serum ferritin concentrations above 300 μg/L in men and 200 μg/L in women, in the absence of inflammation and liver disease, are currently taken to indicate increased iron stores and require further investigation to determine the site of iron overload.4 Until recently, the only genetic disorder with elevated serum ferritin levels known in Western countries was hereditary HLA-related hemochromatosis, and a high serum ferritin in apparently healthy persons is presently considered suggestive of this disease.5 In Africa, a non-HLA–linked iron overload syndrome exists that also shows a hereditary nature.6
Ferritin synthesis is regulated by cellular iron both at a translational and posttranslational level,7 the former control being of crucial importance for gene expression,8-10 as schematically summarized in Fig 1.
Translational regulation of ferritin synthesis, which applies to both the H and L subunit. (A) A single functional IRE is found in the 5′ UTR of the genes encoding ferritin H and L subunits, and this noncoding sequence behaves as an mRNA cis-acting element. The other component of this regulatory system is a specific trans-acting cytoplasmic binding protein, called iron regulatory protein (IRP; two IRPs, IRP-1 and IRP-2, have been identified so far). When cellular iron is scarce, IRP binds to IRE, where it inhibits ferritin translation. When cellular iron is abundant, formation of [4Fe-4S] clusters within IRP prevents binding to IRE; as a consequence, ferritin synthesis is allowed. The reader is referred to the comprehensive review by Klausner et al8 for details. (B) Sequence and proposed structure of ferritin L-subunit IRE. The reader is referred to the comprehensive review by Theil9 for details. The IRE bulge has been depicted according to Butt et al.10
Translational regulation of ferritin synthesis, which applies to both the H and L subunit. (A) A single functional IRE is found in the 5′ UTR of the genes encoding ferritin H and L subunits, and this noncoding sequence behaves as an mRNA cis-acting element. The other component of this regulatory system is a specific trans-acting cytoplasmic binding protein, called iron regulatory protein (IRP; two IRPs, IRP-1 and IRP-2, have been identified so far). When cellular iron is scarce, IRP binds to IRE, where it inhibits ferritin translation. When cellular iron is abundant, formation of [4Fe-4S] clusters within IRP prevents binding to IRE; as a consequence, ferritin synthesis is allowed. The reader is referred to the comprehensive review by Klausner et al8 for details. (B) Sequence and proposed structure of ferritin L-subunit IRE. The reader is referred to the comprehensive review by Theil9 for details. The IRE bulge has been depicted according to Butt et al.10
Recent reports have described families in whom a combination of elevated serum ferritin not related to iron overload and congenital nuclear cataract is transmitted as an autosomal dominant trait.11 12 In the present work, we have studied the molecular pathogenesis of hyperferritinemia in two families showing different phenotypic expressions of this new genetic disorder.
MATERIALS AND METHODS
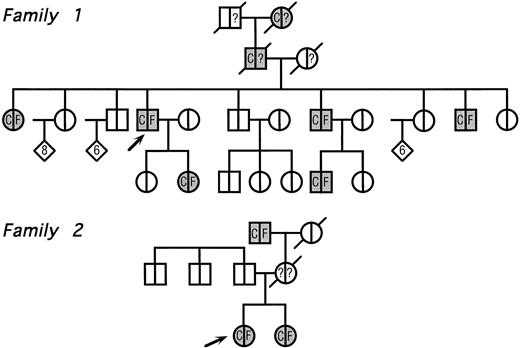
The pedigrees of the two families with congenital cataract and hyperferritinemia are reported in Fig 2. The clinical features of family 1 have already been reported elsewhere,11 whereas the second one has never been described.
Pedigree of two families with hyperferritinemia and congenital cataract. Circles denote female family members; squares, male family members; and diamonds, additional members of either sex (the number of additional members is shown in the diamonds); arrows indicate the two probands and symbols with diagonal lines indicate deceased members. Within each symbol, the left side indicates the lens status and the right side, the serum ferritin level. Blank symbols indicate normal lens and normal serum ferritin levels, respectively. C denotes congenital cataract; F, hyperferritinemia; and question marks indicate unknown serum ferritin levels. No member indicated within diamonds had evidence of cataract and/or hyperferritinemia.
Pedigree of two families with hyperferritinemia and congenital cataract. Circles denote female family members; squares, male family members; and diamonds, additional members of either sex (the number of additional members is shown in the diamonds); arrows indicate the two probands and symbols with diagonal lines indicate deceased members. Within each symbol, the left side indicates the lens status and the right side, the serum ferritin level. Blank symbols indicate normal lens and normal serum ferritin levels, respectively. C denotes congenital cataract; F, hyperferritinemia; and question marks indicate unknown serum ferritin levels. No member indicated within diamonds had evidence of cataract and/or hyperferritinemia.
Patients
Family 1.Several members were known to have congenital cataract, with visual symptoms appearing during childhood and consisting of glare and impaired visual acuity. A few years ago, a routine blood examination showed a high serum ferritin (1,650 μg/L) in the proband. Serum iron and total iron-binding capacity (TIBC) were normal and a liver biopsy showed normal amounts of stainable iron. A proband's daughter also showed isolated hyperferritinemia. Although the diagnostic criteria of hereditary hemochromatosis were not met, physicians decided to start venesection therapy in the proband and her daughter. Both developed microcytic anemia and hypoferremia after 7 to 14 venesections, whereas serum ferritin levels did not change significantly.
Clinical evaluation of body iron status was performed in all available family members. As shown in Fig 2, all patients with congenital cataract also showed high serum ferritin with normal values for serum iron and TIBC.
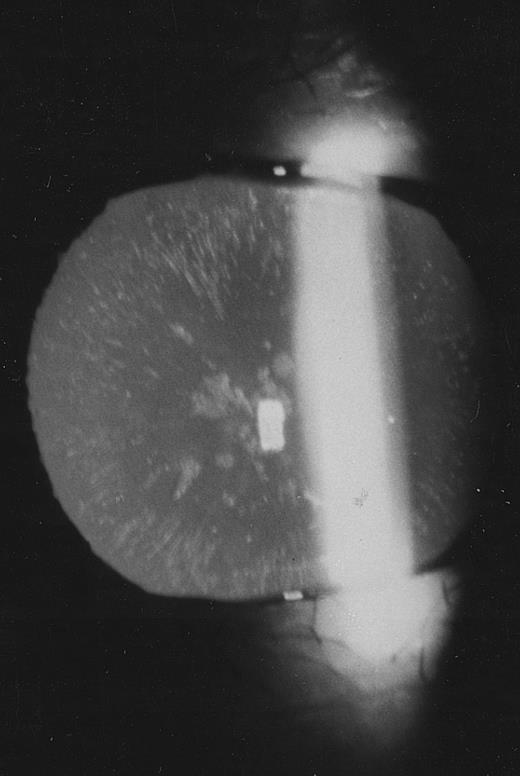
Family 2.The proband, a 26-year old woman, had a routine examination of body iron status that showed a high serum ferritin (366 and 448 μg/L on two different occasions) with normal blood cell counts, normal serum iron and transferrin saturation. She was referred to one of us, who suspected a new case of hereditary hyperferritinemia with congenital cataract. Although this woman had never complained of visual symptoms, a congenital nuclear cataract was found at slit-lamp lens examination (Fig 3). In addition, the combination of hyperferritinemia not due to iron overload and congenital cataract was transmitted as an autosomal dominant trait also in this family (Fig 2). It should be noted that cataract was asymptomatic also in the other two affected individuals, ie, the sister and the maternal grandfather of the proband.
Slit-lamp examination of the lens in the proband of family 2. Multiple spots are visible in the lens core.
Slit-lamp examination of the lens in the proband of family 2. Multiple spots are visible in the lens core.
Methods
Body iron status and proteins of iron metabolism.Body iron status was evaluated by measuring serum iron, TIBC, and serum ferritin.13 The amount of serum transferrin receptor (TfR) was estimated by an enzyme-linked polyclonal antibody assay, as described in detail elsewhere.14 TfR levels in 165 normal control subjects were 5.0 ± 1.1 mg/L, 95% confidence limits ranging from 2.9 to 7.1 mg/L. The available evidence suggests that circulating TfR is derived primarily from erythroid precursors in the bone marrow, and that elevated levels may reflect either increased erythroid mass or iron deficient erythropoiesis, this latter as a consequence of an increased transferrin receptor number on individual erythroblasts.15
Subunit composition of serum and cellular ferritin was studied using monoclonal antibodies specific for the L and H subunit, as reported in detail in previous works.16-18
Ferritin glycosylation was studied through evaluation of binding to concanavalin A as previously described.3 19 Concanavalin A-Sepharose and Sepharose 4B were purchased from Pharmacia Biotech, Uppsala, Sweden.
DNA sequence analysis.Genomic DNA was isolated from peripheral-blood samples in selected normal and affected members of both families by means of proteinase K digestion and phenol/chloroform extraction.20 The entire IRE sequence of the L-subunit gene was amplified by the polymerase chain reaction (PCR) with the use of flanking primers.
Amplified DNA fragments were electrophoresed on 2% agarose gel and then purified with the Qiaquick Gel Extraction Kit (Qiagen, Chatsworth, CA). Purified DNA fragments were used as a template for fluorescence-based DNA sequence analysis using Taq Dye Primer Cycle Sequencing following conditions specified by the supplier (Appied Biosystems Inc, Foster City, CA). One μg DNA template was added to 3.2 pmol of adequate primer and 9.5 μL of terminator premix containing 4 U of AmpliTaq DNA polymerase, and the final volume was adjusted to 20 μL. The reaction was performed for 25 cycles and each cycle was at 96°C for 30 seconds, 50°C for 15 seconds, and 60°C for 4 minutes. Then PCR products were electrophoresed on an automated DNA sequencer (Model 373A, which uses version 3.01 software, Applied Biosystems Inc) fitted with 6% (wt/vol) polyacrylamide gel. Both sense and antisense strands were analyzed.
Detection of Ferritin in Serum and Peripheral Blood Cells Using Monoclonal Antibodies Specific for the H and L Subunit in Normal and Affected Individuals of Family 1 and 2
| Subjects . | Serum Ferritin (μg/L)* . | Mononuclear Cell Ferritin (fg/cell) . | Red Cell Ferritin (ag/cell) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | H-Type . | L-Type . | % Glycosylation . | H-Type . | L-Type . | L/H Ratio . | H-Type . | L-Type . | L/H Ratio . |
| Normal subjects (n = 5) | <30 | 133 | 60 | 16 | 8 | 0.5 | 18 | 7 | 0.4 |
| (45-169) | (50-66) | (10-22) | (7-15) | (0.4-0.9) | (12-32) | (5-8) | (0.2-0.5) | ||
| Affected individuals of family 1 (n = 4) | <30 | 1,385 | 42 | 8 | 106 | 16.5 | 22 | 114 | 5.3 |
| (1,320-1,890) | (36-45) | (4-12) | (87-119) | (8.9-21.8) | (16-36) | (88-195) | (5.1-5.5) | ||
| Affected individuals of family 2 (n = 3) | < 30 | 556 | 40 | 12 | 44 | 3.6 | ND | ND | ND |
| (470-635) | (31-55) | (11-14) | (40-58) | (3.1-4.8) | |||||
| Subjects . | Serum Ferritin (μg/L)* . | Mononuclear Cell Ferritin (fg/cell) . | Red Cell Ferritin (ag/cell) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | H-Type . | L-Type . | % Glycosylation . | H-Type . | L-Type . | L/H Ratio . | H-Type . | L-Type . | L/H Ratio . |
| Normal subjects (n = 5) | <30 | 133 | 60 | 16 | 8 | 0.5 | 18 | 7 | 0.4 |
| (45-169) | (50-66) | (10-22) | (7-15) | (0.4-0.9) | (12-32) | (5-8) | (0.2-0.5) | ||
| Affected individuals of family 1 (n = 4) | <30 | 1,385 | 42 | 8 | 106 | 16.5 | 22 | 114 | 5.3 |
| (1,320-1,890) | (36-45) | (4-12) | (87-119) | (8.9-21.8) | (16-36) | (88-195) | (5.1-5.5) | ||
| Affected individuals of family 2 (n = 3) | < 30 | 556 | 40 | 12 | 44 | 3.6 | ND | ND | ND |
| (470-635) | (31-55) | (11-14) | (40-58) | (3.1-4.8) | |||||
Abbreviation: ND, not done.
Data are median values and range.
Gel retardation assays.Labeled RNAs were synthesized using T7 RNA polymerase and template oligonucleotides corresponding to the wild type and mutated sequences of L-ferritin IRE. The oligonucleotide used to synthesize the normal IRE probe was: 5′-TGTTCCGTCCAAACACTGTTGAAGCAAGAGACAGACCCGCTATAGTGAGTCGTATTA-3′ where the underlined sequence represents the T7 RNA polymerase promoter. The sequences of the oligonucleotides used to synthesize the mutated IRE probes were as follows: 5′-TGTTCCGTCCAAACACTGTTGAAGTAAGAGACAGACCCGCTATAGTGAGTCGTATTA-3′ (family 1) and 5′-T G T T C C G T C C A A A C A C T G T T G A A G C A A G A G A C A G C C C C A -CTATAGTGAGTCGTATTA-3′ (family 2) where the bold letters indicate the complementary mutated base.
32P-uridine triphosphate (UTP) labeled RNAs were incubated with cytoplasmic extracts from the K652 cell line, and both high-affinity (that is, in the presence of RNAse T1 and/or heparin) and low-affinity binding of RNAs to IRP were evaluated. RNA protein complexes were analyzed by electrophoresis through a 6% polyacrylamide gel.
RESULTS
Serum ferritin levels ranged from 950 to 1,890 μg/L in affected individuals from family 1 and from 366 to 635 μg/L in affected individuals from family 2.
Subunit composition of both serum and cell ferritin was studied with monoclonal antibodies specific for the H and L ferritin chains. As shown in Table 1, serum ferritin was essentially L type in both normal and affected individuals, these latter showing on average 10 and four times higher levels in family 1 and 2, respectively. Serum ferritin binding to concanavalin A was slightly higher in normal individuals (50% to 66%) than in those with hyperferritinemia (31% to 55%).
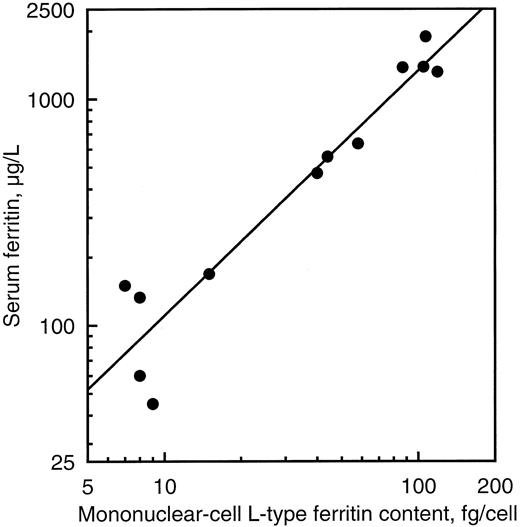
Peripheral blood cell ferritin was studied as an example of tissue ferritin. There was no significant difference between normal and affected individuals with respect to either red cell or mononuclear cell H-type ferritin content (Table 1). On the contrary, L-type ferritin contents of mononuclear cells were 13 times normal in affected individuals of family 1 and five times normal in those of family 2 on average. There was a close relationship between serum ferritin concentration and mononuclear cell L-type ferritin content (r = 0.96, P < .00001, Fig 4), suggesting that the excess production of ferritin in cells was directly responsible for the hyperferritinemia. A similar relationship was found between glycosylated serum ferritin and mononuclear cell L-type ferritin content (r = 0.90, P < .00001).
Relationship between peripheral blood mononuclear cell L-type ferritin content and serum ferritin concentration in five normal subjects and seven affected individuals of the two families studied.
Relationship between peripheral blood mononuclear cell L-type ferritin content and serum ferritin concentration in five normal subjects and seven affected individuals of the two families studied.
Normal levels of circulating transferrin receptor were found in both normal individuals (4.6 to 6.9 mg/L) and affected members (4.8 to 6.9 mg/L) of both families. This indicated that affected individuals did not have any abnormality in either erythroid marrow activity or iron supply for erythropoiesis.
Overall, the above findings suggested that ferritin L-subunit synthesis was dysregulated in affected individuals. We, therefore, postulated that the L-subunit gene IRE of affected individuals had a molecular lesion preventing high-affinity IRP binding (Fig 1) and leading to overproduction of L subunits. To verify this hypothesis, we decided to sequence the IRE in both families.
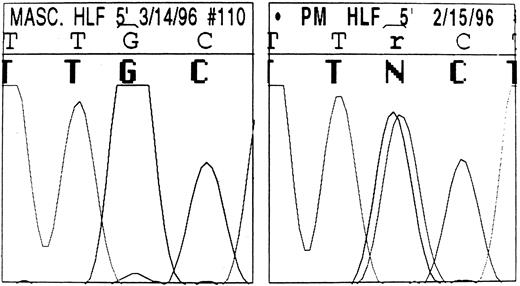
The sequence of the L-ferritin gene 5′ UTR we found in normal individuals is reported in Table 2: it differs slightly from that previously reported by Santoro et al.21 The proband and three other affected individuals from family 1 were heterozygous for a point mutation in the IRE of the L-subunit DNA (Fig 5): a single G32 to A change in the highly conserved, three-nucleotide motif forming the IRE bulge (Fig 6). Four unaffected family members had the normal IRE sequence.
Sequence of Human L-Ferritin Gene 5′UTR (001-199) Found in Normal Subjects
| 001 | gcagttcggc | ggtcccgcgg | gtctgtctct | tgcttcaaca | gtgtttggac | ggaacagatc |
| 061 | cggggactct | cttccagcct | ccgaccgccc | tccgatttcc | tctccgcttg | caacctccgg |
| 121 | gaccatcttc | cggccatct | cctgcttctg | ggacctgcca | gcaccgtttt | tgtggttagc |
| 181 | tccttcttgc | caaccaacca | tgagctccca | gattcgtcag | aattattcca | ccgacgtgga |
| 241 | ggcagccgtc | aacagcctgg | tcaatttgta | cctgcaggcc | tcctacacct | acctctctct |
| 301 | gggcttctat | ttcgaccgcg | atgatgtggc | tctggaaggc | gtgagccact | tcttccgcga |
| 361 | actggccgag | gagaagcgcg | agggctacga | gcgtctcctg | aagatgcaaa | accagcgtgg |
| 421 | cggccgcgct | ctcttccagg | acatcaag |
| 001 | gcagttcggc | ggtcccgcgg | gtctgtctct | tgcttcaaca | gtgtttggac | ggaacagatc |
| 061 | cggggactct | cttccagcct | ccgaccgccc | tccgatttcc | tctccgcttg | caacctccgg |
| 121 | gaccatcttc | cggccatct | cctgcttctg | ggacctgcca | gcaccgtttt | tgtggttagc |
| 181 | tccttcttgc | caaccaacca | tgagctccca | gattcgtcag | aattattcca | ccgacgtgga |
| 241 | ggcagccgtc | aacagcctgg | tcaatttgta | cctgcaggcc | tcctacacct | acctctctct |
| 301 | gggcttctat | ttcgaccgcg | atgatgtggc | tctggaaggc | gtgagccact | tcttccgcga |
| 361 | actggccgag | gagaagcgcg | agggctacga | gcgtctcctg | aagatgcaaa | accagcgtgg |
| 421 | cggccgcgct | ctcttccagg | acatcaag |
Data on exon 1 and 2 (200-448) are derived from Santoro et al.21 Position 001, start of transcription; bold, IRE loop; underlined, 3′ primer used to amplify the 5′UTR (the 5′ primer in not reported); double underlined, first codon.
DNA automatic sequence of the L-subunit IRE in a normal member (left) and the proband (right) of family 1. The highly conserved 5′-TGC-3′ motif is shown. In the affected member, an ambiguity can be seen (r/N) resulting from a G to A change present in the heterozygous state: overlapping signals corresponding to both guanine and adenine are visible. By contrast, only the guanine signal is visible in the normal DNA.
DNA automatic sequence of the L-subunit IRE in a normal member (left) and the proband (right) of family 1. The highly conserved 5′-TGC-3′ motif is shown. In the affected member, an ambiguity can be seen (r/N) resulting from a G to A change present in the heterozygous state: overlapping signals corresponding to both guanine and adenine are visible. By contrast, only the guanine signal is visible in the normal DNA.
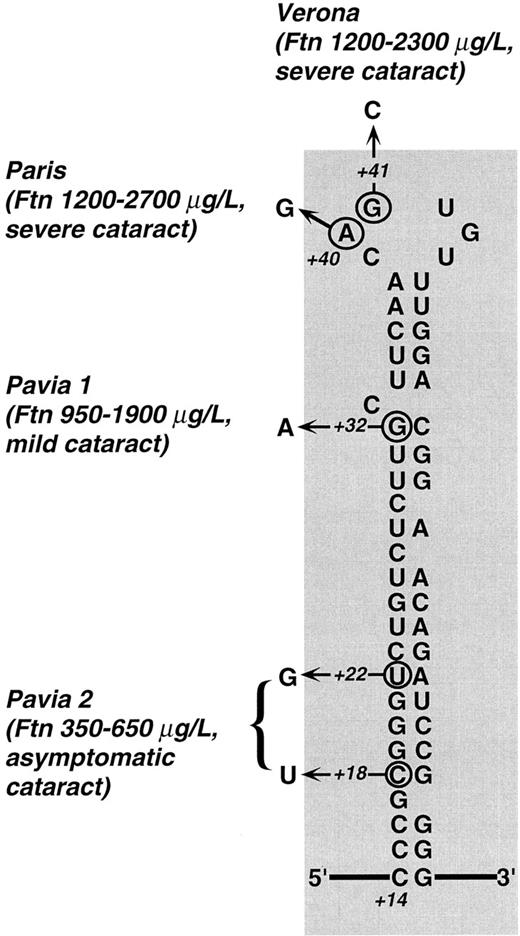
Predicted secondary structure of L-subunit IRE and mutations described so far in families with hereditary hyperferritinemia. Town names denote different mutations: Paris,26,27 Verona,27 and Pavia 1 and 2 (present work, family 1 and 2). For each mutation, the observed range of hyperferritinemia and the severity of cataract are reported. Clearly, the highest ferritin levels were found in patients with mutations within the CAGUG sequence of the loop, whereas the lowest ones were those of the family with the double mutation in the lower stem. Cataract was judged severe when there was a marked loss in visual acuity in the first decades (with some individuals requiring surgery), mild when the defect in visual acuity could be corrected with the use of appropriate eyeglasses, and asymptomatic when it did not impair visual acuity. It is apparent that a relationship between the degree of hyperferritinemia and severity of cataract exists within the families described so far. Numbers (+18, +22, +32, +40, +41) indicate the nucleotide position according to Table 2 with position +1 being the start of transcription.
Predicted secondary structure of L-subunit IRE and mutations described so far in families with hereditary hyperferritinemia. Town names denote different mutations: Paris,26,27 Verona,27 and Pavia 1 and 2 (present work, family 1 and 2). For each mutation, the observed range of hyperferritinemia and the severity of cataract are reported. Clearly, the highest ferritin levels were found in patients with mutations within the CAGUG sequence of the loop, whereas the lowest ones were those of the family with the double mutation in the lower stem. Cataract was judged severe when there was a marked loss in visual acuity in the first decades (with some individuals requiring surgery), mild when the defect in visual acuity could be corrected with the use of appropriate eyeglasses, and asymptomatic when it did not impair visual acuity. It is apparent that a relationship between the degree of hyperferritinemia and severity of cataract exists within the families described so far. Numbers (+18, +22, +32, +40, +41) indicate the nucleotide position according to Table 2 with position +1 being the start of transcription.
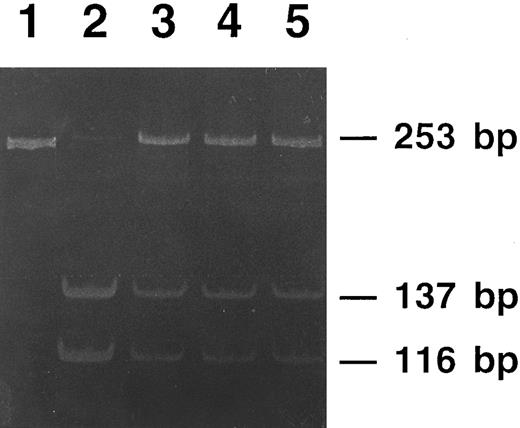
The three affected members from family 2 were heterozygous for a double point mutation in the IRE stem, as schematically shown in Fig 6: a C18 to U change and a U22 to G change in the lower stem. Three unaffected members of this family had normal IRE sequence, normal iron status, and normal serum ferritin levels. Interestingly, the first of the two above mutations modifies a restriction site recognized by Sac II, an endonuclease that cuts within the sequence CCGC/GG. As shown in Fig 7, Sac II digestion of amplified DNA allowed us to track the mutation within the family.
Polyacrylamide gel electrophoresis of PCR products from members of family 2 digested with the restriction endonuclease Sac II. The undigested PCR product (lane 1) gives a single 253-bp band. DNA from a nonaffected member is completely digested (lane 2), showing two bands of 137-bp and 116-bp, respectively. DNAs from the three affected members of the family (lanes 3 to 5) show digestion of the normal allele (the 137-bp and the 116-bp bands), whereas the mutated allele is not digested and appears as the original 253-bp band.
Polyacrylamide gel electrophoresis of PCR products from members of family 2 digested with the restriction endonuclease Sac II. The undigested PCR product (lane 1) gives a single 253-bp band. DNA from a nonaffected member is completely digested (lane 2), showing two bands of 137-bp and 116-bp, respectively. DNAs from the three affected members of the family (lanes 3 to 5) show digestion of the normal allele (the 137-bp and the 116-bp bands), whereas the mutated allele is not digested and appears as the original 253-bp band.
To prove that the above mutations could be responsible for L-subunit overexpression, we studied the relative binding affinities of the normal and mutated IRE RNA for the binding protein by gel retardation assay. In three experiments with the G32 to A bulge mutation, both wild type and mutated IRE sequences appeared to associate with a protein in the gel-retardation assay. Binding of wild type RNA was not influenced by the presence of RNase T1 and/or heparin. On the contrary, the mutated sequences were displaced by incubation with heparin and RNase, suggesting a low-affinity interaction with IRP: quantitation of changes in band intensity by densitometric scanning showed a reduction > 80% in the IRE-protein interaction in the presence of RNase T1 and/or heparin.
Gel retardation assays with the IRE corresponding to the double lower stem mutation of family 2 also showed a reduced interaction between mutated IRE and binding protein in the presence of heparin and RNase. However, the reduction in band intensity was lower than that found with the G32 to A bulge mutation, ranging from 25% to 45% in three experiments.
To investigate whether the double lower stem mutation of family 2 might affect the IRE structure, we made computer folding simulations at the web site www.ibc.wustl.edu/∼zuker/mfold Computer folds of nucleotides 1-52 showed that the double mutation markedly alters the IRE stem, possibly generating an alternative secondary structure.
DISCUSSION
Iron regulatory elements are the only family of cis-acting noncoding regulatory sequences characterized so far in eukaryotic mRNA.9 Cellular iron metabolism needs to be highly regulated, and IREs are crucial in coordinating the synthesis of three metabolically related proteins: transferrin receptor for cellular iron uptake, ferritin for intracellular iron storage, and erythroid-specific δ-aminolevulinate synthase (δ-ALAS) for iron use for hemoglobin synthesis. Single functional IREs are found in the 5′ untranslated region (UTR) of the genes encoding ferritin H and L subunits and erythroid δ-ALAS, while multiple IREs are present in the 3′ UTR of the gene encoding transferrin receptor.8,22 The other component of this regulatory system are specific trans-acting cytoplasmic binding proteins, called iron regulatory proteins (IRP). There are two identified IRPs in humans, IRP-1 and IRP-2.10 23 IRP-1 has approximately 30% sequence homology to mitochondrial aconitase and may contain a [4Fe-4S] cluster, thus behaving as a cytosolic aconitase. IRP-2 lacks aconitase activity. When cellular iron is scarce, IRP-1 binds to IRE inhibiting translation (ferritin and erythroid δ-ALAS) or mRNA degradation (transferrin receptor). As a result of these interactions, iron deficiency induces the expression of transferrin receptors, thereby increasing iron uptake and represses the synthesis of proteins involved in iron storage and use, ie, ferritin and δ-ALAS. When cellular iron is abundant, formation of [4Fe-4S] clusters within IRP-1 prevents binding to IREs. As a consequence, transferrin receptor expression and iron uptake are lowered, while ferritin synthesis and iron storage are increased.
A large body of evidence suggests that the IRPs recognize the IRE as a sequence/structure motif.8,9 Studies of site-directed mutagenesis have shown that integrity of the CAGUG sequence in the loop, integrity of the bulge structure, and base pairing along the upper stem are all critical for the IRE/IRP high-affinity interaction.24 25 Most likely, each IRP forms multiple contacts with its cognate RNA, and these in concert, provide high-affinity binding.
The present work, along with three recent reports,26-28 clarifies the molecular pathogenesis of hereditary hyperferritinemia. The IRE mutations described so far are summarized in Fig 6. In the case of the loop mutations, in vitro studies of site-directed mutagenesis29 or gel retardation assays26 have shown a profound reduction of the IRE affinity for IRP. The fact that the G32 to A bulge mutation found in family 1 markedly affects the IRE/IRP high-affinity interaction is also not surprising. In fact, a SELEX study10 has suggested that the G32 residue is involved in a G-C base below the bulge C. Thus, the G32 to A bulge mutation may affect the IRE/IRP high-affinity binding either by removing an interaction with the protein or by abolishing base pair formation with C50 and affecting the stucture of the stem loop.
The lesion found in family 2 (Fig 6) is more difficult to interpret. It carries two substitutions in the lower stem, which probably do not directly affect contact sites for IRP binding. As suggested by computer fold simulations, these mutations may determine an alternative secondary structure of the stem, which reduces the access to the protein binding pocket. An alternative fold, for example, has been suggested to play a role in the decreased function of the IRE of human mitochondrial aconitase.30 In summary, different mutations in the IRE may variably prevent IRP binding and lead to different degrees of hyperferritinemia.
Normal serum ferritin differs from cellular ferritin in that it is partly glycosylated and almost free of iron. It is believed to be synthesized by the rough endoplasmic reticulum, where it is partly glycosylated before being secreted from the cell.31 The close relationship we found between cellular ferritin and serum ferritin (in a range from 45 to 1,890 μg/L) (Fig 4) provides strong evidence that the circulating protein is a byproduct of intracellular ferritin synthesis. In addition, the observation that the elevated serum ferritin was glycosylated is consistent with a conclusion that the ferritin L chain gene on chromosome 19 is the source of glycosylated serum ferritin. Indeed, the L chain has a glycosylation consensus sequence NYS at the very end of the N-terminal helix in a position easily accessible. However, the presence of a hydrophobic stretch near the N-terminus of the L-chain from residue 14 to 38 might function as an inefficient signal peptide and may explain the low proportion of ferritin found in circulation (less than 0.1% of the estimated total body ferritin) and the difficulty to detect active secretion in in vitro studies.32 The homologous sequence in the H-chain carries a substitution Tyr → Glu, which makes the stretch less hydrophobic and probably less efficient, in keeping with the finding that the H-chain is virtually absent in circulation.
Our findings and recent observations clearly indicate that serum ferritin concentration is closely related to tissue ferritin. Thus, the elevated serum ferritin levels seen in hyperthyroidism reflect a thyroid-hormone–mediated reduction in IRP binding to IRE with upregulated hepatic ferritin synthesis.33 It can be concluded that serum ferritin concentration reflects the degree of iron stores only to the extent that intracellular iron determines cell ferritin synthesis.
Apart from hyperferritinemia, the only consistent abnormality in our patients was congenital nuclear cataract. Because different IRE mutations have been found so far in the families studied (Fig 6), it is very improbable that cataract is due to a separate, cosegregating molecular lesion; more likely, lens opacity is a consequence of excessive L-type ferritin production. Cultured canine lens epithelial cells synthesize ferritin, and that synthesis is normally influenced by iron, ascorbic acid, or desferrioxamine.34 Thus, an increased L-rich ferritin content is easily predictable in lens cells of individuals with hereditary hyperferritinemia. Various observations suggest that L-subunit rich isoferritins are mainly involved in long-term iron storage, whereas H-subunit rich isoferritins have more active functions and may contribute to prevent oxidative damage.35 Subtle disturbances in ferritin metabolism, iron, and reactive oxygen species generation could account for the pathology that occurs in the lens.
From a practical standpoint, this new genetic disorder should be taken into account by clinicians when facing a high serum ferritin in an apparently healthy person. As the serum ferritin determination is becoming a routine examination, physicians are expected to face differential diagnosis of hyperferritinemia more frequently, and cases of hereditary hyperferritinemia might be more frequent than believed until now. Because four different mutations have been found in the four families studied so far, it may be anticipated that additional molecular lesions, including IRE interstitial deletions, can be found in other cases of hereditary hyperferritinemia. Indeed, a recent report36 has described a family with hereditary hyperferritinemia-cataract syndrome due to a 29-bp deletion in the IRE of the L-ferritin gene.
Supported in part by grants from AIRC (Associazione Italiana per la Ricerca sul Cancro), IRCCS Policlinico S. Matteo, and Fondazione Ferrata Storti to M.C.
Address reprint requests to Prof Mario Cazzola, Internal Medicine and Medical Oncology, IRCCS Policlinico S. Matteo, 27100 Pavia, Italy.

![Fig. 1. Translational regulation of ferritin synthesis, which applies to both the H and L subunit. (A) A single functional IRE is found in the 5′ UTR of the genes encoding ferritin H and L subunits, and this noncoding sequence behaves as an mRNA cis-acting element. The other component of this regulatory system is a specific trans-acting cytoplasmic binding protein, called iron regulatory protein (IRP; two IRPs, IRP-1 and IRP-2, have been identified so far). When cellular iron is scarce, IRP binds to IRE, where it inhibits ferritin translation. When cellular iron is abundant, formation of [4Fe-4S] clusters within IRP prevents binding to IRE; as a consequence, ferritin synthesis is allowed. The reader is referred to the comprehensive review by Klausner et al8 for details. (B) Sequence and proposed structure of ferritin L-subunit IRE. The reader is referred to the comprehensive review by Theil9 for details. The IRE bulge has been depicted according to Butt et al.10](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/2/10.1182_blood.v90.2.814/4/m_bl_0036f1.jpeg?Expires=1765929748&Signature=iZ91bUFJKASqxAbqqUV5XescFHoPg6pOblP2kZhzaOfGXlYGbfHZYpuyTkJyep1eLGbXsuG25Xxtn5vaETMY3OqnDNKU0IrnHd1oQAimtVfxHi37HGkJD6fDfqE4iSd0kTZyXKLAttLr0SXF3i7DTkuZcIQ48~uOJR5khKYWR2vk8TQo72hcT8qsrkT3ubbrfizfxigdmRLVKriOSQKmFkAx0y7HVe2~5th5D7YPUpsRw7fzzg7BpUe4P4-CCcSwRmOF--NNxIdgVekw~dJKNj6DLKdS40joENE~iSdG38btGXfeo9st4ipxX4BLvF5MN7aKtDz5tbpzFAytfjFDPA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal