Abstract
Acute promyelocytic leukemia (APL) is a neoplasm with the unique chromosomal translocation t(15; 17), which involves the retinoic acid receptor α gene. All-trans retinoic acid (ATRA) has been used for APL patients as a potent therapeutic agent to induce differentiation of leukemia cells. Although polymorphonuclear leukocytes (PMNs) appearing in the blood and bone marrow during ATRA treatment often possess Auer rods, indicating their neoplastic origin, other morphological abnormalities of PMNs have not been elucidated. We studied the morphological changes of APL cells during ATRA treatment at the ultrastructural level. Although most aberrant primary granules, including Auer rods, became morphologically normal in response to ATRA therapy and the nuclei showed chromatin condensation and lobulation, resulting in the emergence of PMNs, the lobulated nuclei often had nuclear filamentous connections and/or nuclear blebs, indicating some pathological process. Furthermore, PMNs, particularly early in ATRA treatment, lacked neutrophil secondary granules as did the PMNs appearing in a culture of APL cells incubated with ATRA, findings consistent with previously reported data that acute myeloid leukemia cell lines do not produce secondary granule proteins even after induction of differentiation towards mature neutrophils. The present data indicate that ATRA is incapable of inducing complete morphological maturation of APL cells and that secondary-granule deficiency may be a hallmark of aberrantly differentiated leukemic cells.
CHROMOSOMAL translocations are genetic events often found in human neoplasms, including leukemia. Acute promyelocytic leukemia (APL; or M3 in the French-American-British [FAB] classification) harbors the unique reciprocal chromosomal translocation t(15; 17),1 which involves the retinoic acid receptor α (RARα) gene on chromosome 17 and PML, the gene of a putative transcription factor on chromosome 15,2-5 and is thought to be the pathognomonic abnormality of this disorder.6 This chromosomal translocation causes maturation arrest of the leukemic cells at the promyelocyte stage by making them insensitive to retinoic acid.7,8 However, these cells are paradoxically more sensitive to high concentrations of retinoic acid than normal cells and can be successfully induced to differentiate towards mature neutrophils by all-trans retinoic acid (ATRA) both in vitro and in vivo.9-11 As the first successful model of differentiation-induction therapy for human cancer, ATRA has recently been used to induce remission of APL. However, the neutrophil polymorphonuclear leukocytes (PMNs) appearing in the blood and bone marrow during ATRA treatment occasionally have Auer rods9-11 and it is unclear whether PMNs without Auer rods are morphologically and functionally normal.
The cytoplasmic granules of neutrophils are divided into two major populations, primary (or azurophil) granules, which are myeloperoxidase (MPO)-positive, and secondary (or specific) granules, which are negative.12 It has been suggested that both types of granules are morphologically and cytochemically heterogeneous, and as yet there is no consensus as to the total number of subpopulations of neutrophil cytoplasmic granules. Nevertheless, the classification of granules into two groups according to whether they are cytochemically positive or negative for MPO is of great practical usefulness because the maturation steps of neutrophils can be clearly defined into four stages largely based on cytoplasmic granule formation and the MPO subcellular localization patterns.12 Based on this definition, the most primitive cell, corresponding to myeloblasts, is a cell that has no cytoplasmic granules and no detectable MPO reactivity. Although some investigators require the presence of a few cytoplasmic granules to establish granulocytic commitment, it is now clear on the basis of antigenic markers that myeloblasts can be totally agranular.13 The second stage of maturation, corresponding to promyelocytes, is the first cell that can be recognized as a member of the neutrophil series by the presence of primary granules and by a positive MPO reaction in the nuclear envelope, the cisternae of the rough endoplasmic reticulum (rER), Golgi apparatus, Golgi-associated small vesicles, and primary granules. Neutrophil primary granules are electron-dense large granules with or without crystalloid structures, with the former being spherical and often having a clear halo beneath the limiting membrane and the latter being ellipsoid or football-shaped.12-15 Both types of granules contain less electron-dense flocculent material in the early stages, and the latter have been shown to be MPO-negative when first produced, subsequently becoming MPO-positive in the late promyelocyte stage.13-15 Another subpopulation of primary granules is round or elongated, small granules produced in the late promyelocyte stage. They have strong MPO activity and are arranged in chains or clusters in later stages of differentiation.16,17 In this report, we refer to these three subpopulations of primary granules as types I, II, and III, respectively. The third stage of maturation, corresponding to myelocytes, is defined as a cell in which primary granules alone react positively for MPO because MPO synthesis has ceased in the preceding stage. At this stage, MPO-negative secondary granules are produced. Secondary granules are less electron-dense and smaller than primary granules, and they become elongated or dumbbell-shaped as cellular maturation proceeds.12-15 The fourth maturation stage is characterized by maturation of the nuclei, as shown by heterochromatin condensation and lobulation, involution of protein synthesis-associated cell organelles, and an increase in the number of glycogen particles, but no clearcut distinction between this and the preceding stage can be made in terms of cytoplasmic granules and MPO reactivity.
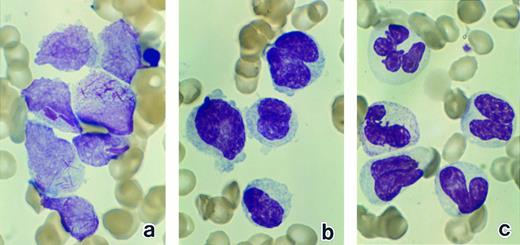
Bone marrow leukemic cells in patient no. 1 at diagnosis (a), after 1 week of ATRA treatment (b), and after 3 weeks of ATRA treatment (c). (Original magnification × 330.)
Bone marrow leukemic cells in patient no. 1 at diagnosis (a), after 1 week of ATRA treatment (b), and after 3 weeks of ATRA treatment (c). (Original magnification × 330.)
Previous electron microscopic studies with ultrastructural cytochemistry for MPO have revealed various abnormalities in acute myelogenous leukemia (AML), including the absence of secondary granules, primary granules, or MPO activity in primary granules, not only in immature leukemic cells but also in mature PMNs.18-22 However, it has not been elucidated whether these abnormal PMNs are cells of neoplastic origin. To resolve this question, the PMNs that appear during ATRA treatment of APL seem to be a good subject to study because they have been shown to be derived from leukemic clones.23 Except for the Auer rods that are occasionally observed in the PMNs of APL patients during ATRA treatment, no morphological abnormalities of mature neutrophils have been reported, because, to our knowledge, no studies have been conducted on ultrastructural aspects of differentiating APL cells. To identify morphological changes of APL cells during the differentiation process induced by ATRA and to determine whether ATRA-induced maturation of APL cells follows the normal morphological differentiation pathway, we investigated the ultrastructural characteristics of APL cells in bone marrow and in cultures treated with ATRA.
MATERIALS AND METHODS
Patients and cells.Three patients, two with a diagnosis of M3 (patients no. 1 and 2, 55- and 71-year-old men, respectively) and one with the M3 variant24 25 (patient no. 3, a 43-year-old woman), were studied. Patient no. 3 had an elevated leukocyte count of 111,100/μL with 97% of blasts and promyelocytes, whereas patients no. 1 and 2 had marked leukopenia without obvious leukemic cells in the blood, although 77% and 78%, respectively, of the cells in their bone marrow were blasts and promyelocytes. Chemotherapy, including cytosine arabinoside, was performed from the time of diagnosis in patients no. 1 and 3 for 7 and 5 days, respectively, but failed to induce remission. Then, with a 2-week interval without treatment of either patient with any antileukemic drugs, 60 mg/d ATRA was orally administered continuously to patients no. 1 and 3 for 36 and 50 days, respectively. In patient no. 2, 70 mg/d ATRA was orally administered from the time of diagnosis for 58 days continuously. Hematological abnormalities improved after ATRA treatment, and administration of ATRA was continued until complete remission was obtained in all patients (5 to 8 weeks, depending on the patient). After the patient gave informed consent, peripheral blood and/or bone marrow aspirates were obtained for light and electron microscopic examinations at the time of bone marrow evaluation at the onset and during ATRA treatment (after 1 to 8 weeks of the treatment).
Although the number of leukemia cells in the bone marrow in patients no. 1 and 3 was reduced by the initial chemotherapy, the morphology of the leukemia cells remained unchanged. In addition, as described below, similar morphological changes in APL cells in response to ATRA were observed in all three patients. These findings indicate that the chemotherapy performed before ATRA treatment in patients no. 1 and 3 did not significantly affect our morphological evaluation of APL cells.
Cell culture methods.T cells were depleted from mononuclear cell fractions obtained from heparinized peripheral blood of patient no. 3 by using the two-cycle Ficoll-Hypaque procedure as previously described, the second after the formation of E-rosettes to remove T cells.26 These cells were cultured overnight at 37°C in suspension in α minimum essential medium (αMEM; GIBCO, New York, NY) containing 20% fetal calf serum (FCS; growth medium) to remove monocytes by plastic adherence. The leukemic cell populations thus obtained were washed in αMEM and cultured at a concentration of 106 cells/mL in 24-mm Linbro tissue culture multiwell plates (Flow Laboratories, McLean, VA) in 1 mL of growth medium containing 10−6 mol/L ATRA (Sigma, St Louis, MO) or 10 ng/mL granulocyte colony-stimulating factor (G-CSF; Chugai, Tokyo, Japan). For light microscopic examination, an aliquot of the cell suspension was taken from the culture daily, and cytospin specimens were prepared. For electron microscopic examination, the cells were obtained on day 7 of culture and resuspended in FCS. They were then centrifuged, and the cell pellets were fixed by gently replacing the supernatant with the fixative as described below.
Bone marrow mononuclear cells obtained from two patients with idiopathic thrombocytopenia and one patient with nonhematologic disease were used as controls. The cell separation procedure using Ficoll-Hypaque almost completely eliminated mature neutrophils from the samples. These cell populations were cultured with 10−6 mol/L ATRA in the same manner as described above and examined for morphological changes under both light and electron microscopes.
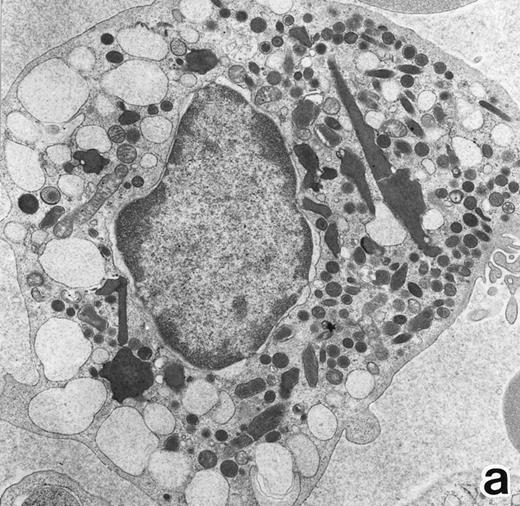
(a) A bone marrow leukemic cell in patient no. 1 at diagnosis containing abundant abnormally shaped primary granules, including Auer rods and Chediak-Higashi–type inclusions. Dilatation of rER is remarkable. (Original magnification × 10,800.) (b) Higher magnification of a cytoplasmic inclusion body of another cell in patient no. 1 that is membrane-bound and contains bundles of filamentous structures, similar to Auer rods. (Original magnification × 25,800.)
(a) A bone marrow leukemic cell in patient no. 1 at diagnosis containing abundant abnormally shaped primary granules, including Auer rods and Chediak-Higashi–type inclusions. Dilatation of rER is remarkable. (Original magnification × 10,800.) (b) Higher magnification of a cytoplasmic inclusion body of another cell in patient no. 1 that is membrane-bound and contains bundles of filamentous structures, similar to Auer rods. (Original magnification × 25,800.)
Light microscopic observation.Bone marrow and peripheral blood smears and cytospin specimens of cultured cells were stained with Wright-Giemsa, and morphological changes of leukemia cells were observed under a light microscope.
Electron microscopic observation.Electron microscopic examination was performed as previously described.26 Bone marrow aspirates and cultured cells resuspended in FCS were centrifuged at 1,000g for 10 minutes and, after removing plasma or FCS, buffy coats and cell pellets were fixed in 0.1 mol/L cacodylate buffer (pH 7.4) solution containing 1.5% glutaraldehyde for 1 hour at 4°C and washed in cacodylate buffer for 2 to 3 hours. Some of the samples were processed for enzyme cytochemistry for MPO, and the remaining samples were processed for ordinary electron microscopic examination without cytochemistry. For MPO staining, samples were embedded in agar and sliced into approximately 30-μm–thick sections using a tissue sectioner (McIlwain Tissue Chopper; The Mickle Laboratory Engineering, Gomshall, Surrey, England). They were then preincubated in 3-3′-diaminobenzidine (DAB; Wako Pure Chemical Industries, Tokyo, Japan) solution without H2O2 at room temperature for 1 hour and then exposed to DAB (0.1%) -H2O2 (0.01%) solution for 10 minutes for the peroxidase reaction. The samples, both with and without peroxidase reaction, were further fixed in 1% osmium tetroxide for 1 hour, dehydrated in alcohol and acetone at graded concentrations, and embedded in Epon. Ultrathin sections were counterstained with uranyl acetate, followed by lead citrate, and examined under an electron microscope (JEOL 1200EX; Japan Electric Optical Laboratory, Tokyo, Japan) at an accelerating voltage of 80 kV.
RESULTS
Light microscopic observation.The leukemic cells in patients no. 1 and 2 contained abundant cytoplasmic azurophilic granules, including Auer rods, and were intensely positive for peroxidase, a typical finding for the diagnosis of M3 in the FAB classification. In addition to Auer rods, the cells of patient no. 1 possessed large cytoplasmic inclusions with a crystalloid irregular shape (Fig 1a) that were positive for peroxidase. Compared with patients no. 1 and 2, the cells of patient no. 3 had a higher nuclear/cytoplasmic ratio, smaller numbers of visible azurophilic granules, and irregularly shaped nuclei with occasional deep nuclear indentation, findings consistent with the diagnosis of the M3 variant form.24 25
The morphology of the leukemic cells changed in a similar manner in response to ATRA treatment in all three patients. After 1 week of treatment, the leukemic cells in bone marrow showed chromatin condensation and nuclear lobulation and the azurophilic granules had lost their characteristic color (Fig 1b). After 2 weeks of treatment, mature neutrophils with somewhat abnormally shaped nuclei appeared (Fig 1c), and some of them contained Auer rods. After 5 to 8 weeks of treatment, when a CR had been achieved, the bone marrow consisted of normal hematopoietic cells of multiple lineages at various stages of differentiation, including neutrophils, eosinophils, monocytes, the erythroid series, and megakaryocytes. Most of the neutrophils appeared normal, but a small number of immature myeloid cells with irregular nuclei were still present.
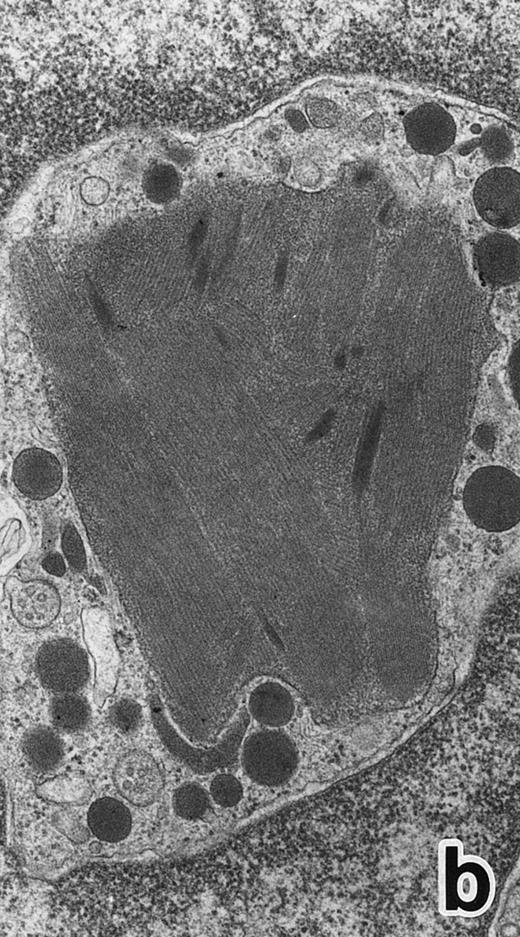
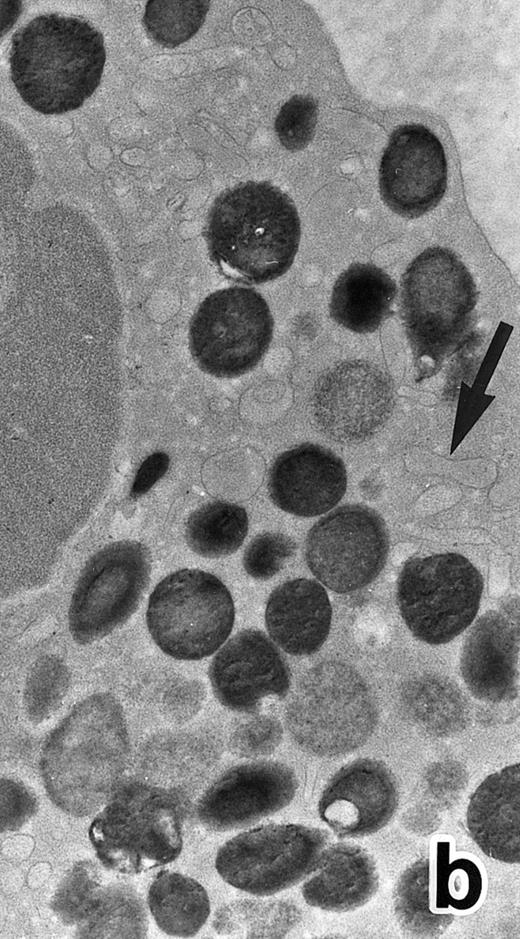
(a) A PMN in patient no. 1 after 1 week of ATRA treatment. Lobulated nuclei with a nuclear bleb (arrow) is seen. rERs are still dilated. Note that the primary granules have become normal in both size and shape and that neither Auer rods nor irregularly shaped inclusion bodies are seen in this cell. (Original magnification × 16,900.) (b) Higher magnification of the cytoplasm of another cell in patient no. 1, showing a structure in which a spherical primary granule (arrow) and an Auer rod are coupled. (Original magnification × 40,000.)
(a) A PMN in patient no. 1 after 1 week of ATRA treatment. Lobulated nuclei with a nuclear bleb (arrow) is seen. rERs are still dilated. Note that the primary granules have become normal in both size and shape and that neither Auer rods nor irregularly shaped inclusion bodies are seen in this cell. (Original magnification × 16,900.) (b) Higher magnification of the cytoplasm of another cell in patient no. 1, showing a structure in which a spherical primary granule (arrow) and an Auer rod are coupled. (Original magnification × 40,000.)
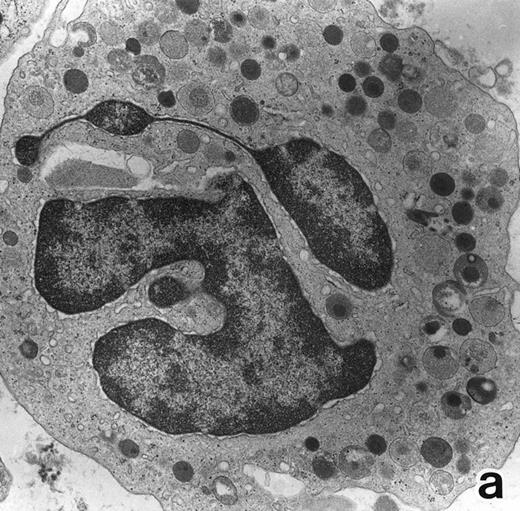
Electron microscopic observation.The leukemic cells of all three patients possessed abundant spherical (type I) primary granules containing electron-dense homogeneous material, with a peripheral halo in some granules and dilated rER (Fig 2a). Although type I primary granules predominated in all patients, small numbers of cytoplasmic granules containing crystalloid structures (type II primary granules) were also present in patient no. 1. Nuclear configurations were irregular, and the chromatin was only slightly condensed along the nuclear membrane. In addition to numerous Auer rods, the cells of patient no. 1 contained large cytoplasmic inclusion bodies also seen under the light microscope. These are the structures surrounded by a unit membrane and filled with parallel tubular material identical to that of Auer rods and belong to the psuedo–Chediak-Higashi (C-H) category of granules20,27 (Fig 2b). The cells of patient no. 3 contained smaller cytoplasmic granules than those of the other patients, and Auer rods were less frequently observed, a finding consistent with the M3 variant. Stellate rER complexes and fibrillar structures in the rER, both of which are thought to be structures specific to APL,28 were detected in this patient.
Common morphological changes of bone marrow leukemia cells were observed during ATRA treatment. After 1 week of ATRA treatment, leukemic cells showed heterochromatin condensation and nuclear lobulation (Figs 3 and 4). Although the lobulated nuclei of some cells resembled those of PMNs, these nuclei were irregular in shape and nucleoli were occasionally present. The lobulated nuclei often had nuclear blebs (Fig 3a) or were connected by thin nuclear material (Fig 4a), called filamentous connections,13 that consisted of heterochromatin delimited by the nuclear membrane. In addition to these changes, the irregularly shaped primary granules became morphologically normal. This was most evident in patient no. 1, in whom the majority of both Auer rods and irregularly shaped inclusions, as shown in Fig 2, dramatically vanished and the remaining primary granules appeared normal, being spherical in shape and relatively homogeneous in size (Fig 3a), although abnormal primary granules and/or Auer rods were infrequently observed in some cells. Rarely, a structure composed of an Auer rod coupled with a primary granule was found, appearing as though the seemingly normal primary granule had emerged from the Auer rod (Fig 3b). Type I primary granules still predominated in all patients, but type II primary granules with a nucleoid structure were rarely observed in patients no. 1 and 2. Some maturing neutrophils with abnormally shaped lobulated nuclei in patient no. 2 possessed unusual, possibly immature, primary granules containing central electron-dense material (Fig 4a and b), indicating asynchronous maturation of nuclei and cytoplasmic granules.
(a) A PMN in patient no. 2 after 10 days of ATRA treatment. The nuclei are irregularly lobulated, with some of them being connected by nuclear filamentous structures, and heterochromatin is condensed along the nuclear membrane. (Original magnification × 13,100.) (b) Higher magnification of a portion of the cell in (a). The cytoplasmic granules are large and spherical, and some of them contain flocculent material and central cores with higher electron density. (Original magnification × 20,200.)
(a) A PMN in patient no. 2 after 10 days of ATRA treatment. The nuclei are irregularly lobulated, with some of them being connected by nuclear filamentous structures, and heterochromatin is condensed along the nuclear membrane. (Original magnification × 13,100.) (b) Higher magnification of a portion of the cell in (a). The cytoplasmic granules are large and spherical, and some of them contain flocculent material and central cores with higher electron density. (Original magnification × 20,200.)
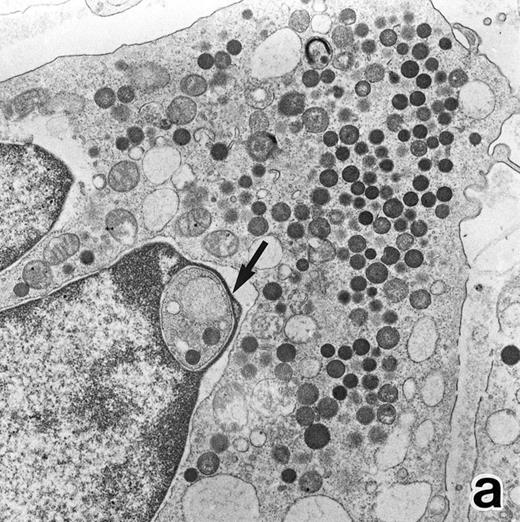
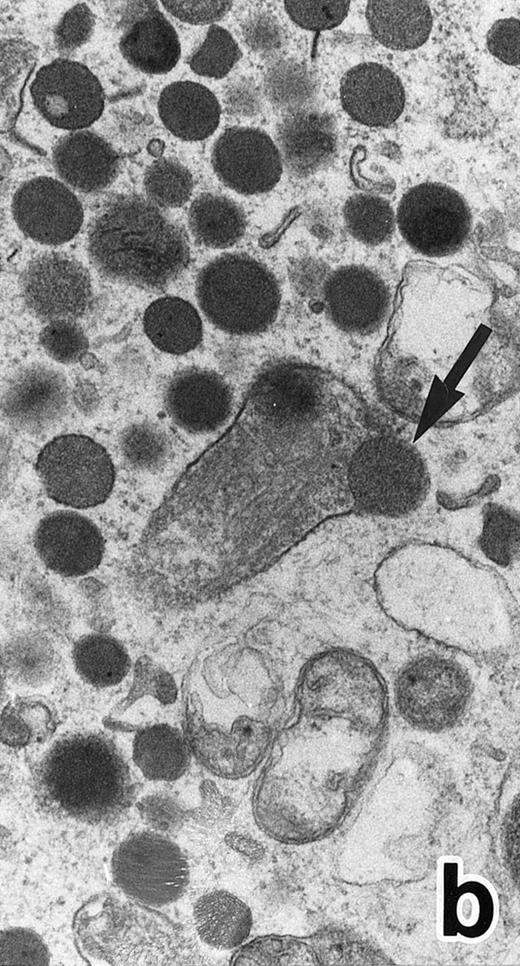
After 2 to 3 weeks of ATRA treatment, maturation of leukemia cells progressed further, and cells resembling normal PMNs appeared (Fig 5). These cells contained multilobulated nuclei with marginally condensed heterochromatin and glycogen particles, characteristic of normal mature neutrophils. rER and free ribosomes became rare as the maturation process proceeded. However, these cells were still rich in primary (type I) granules, which somewhat varied in size and shape, including small and/or elongated forms. A small number of ellipsoid type II primary granules with a nucleoid structure were occasionally observed in patients no. 1 and 2, but not in patient no. 3. Conversely, MPO-positive, small, possibly type III, primary granules were rarely observed intermingled among the larger primary granules in maturing neutrophils in patient no. 3 alone. Despite the ultrastructural features otherwise characteristic of normal mature PMNs, specific granules, which are electron-lucent, elongated or dumbbell-shaped small granules, were not present in the majority of these PMN-like cells, as clearly shown by electron-microscopic cytochemical staining for MPO (Fig 5). The cells contained only MPO-positive primary granules and no MPO-negative secondary granules. A few abnormal primary granules, including Auer rods or C-H type granules, were still present in some cells, and these cells too were consistently devoid of specific granules (Fig 6). Another unusual finding in PMN-like cells at this stage was the presence of large spherical lipid droplets (Fig 5a), which may indicate altered physiological metabolism in these cells,29 because lipid droplets are usually not seen in normal PMNs. Although few in number, PMNs having both primary and secondary granules were also observed (Fig 7), and less mature intermediate forms with indented single nuclei or irregularly lobulated nuclei and numerous primary granules were still present at this stage of ATRA treatment.
(a) A PMN in patient no. 1 that appeared in the bone marrow after 3 weeks of ATRA treatment. There are numerous granules in the cytoplasm, including spherical, elongated, and even dumbell-shaped granules, all of which are MPO-positive, ie, primary granules. A lipid droplet (L) is also present. (MPO-stained section, original magnification × 15,200.) (b) Higher magnification of a portion of the cytoplasm in (a). MPO-negative secondary granules are not seen. (MPO-stained section, original magnification × 30,900.)
(a) A PMN in patient no. 1 that appeared in the bone marrow after 3 weeks of ATRA treatment. There are numerous granules in the cytoplasm, including spherical, elongated, and even dumbell-shaped granules, all of which are MPO-positive, ie, primary granules. A lipid droplet (L) is also present. (MPO-stained section, original magnification × 15,200.) (b) Higher magnification of a portion of the cytoplasm in (a). MPO-negative secondary granules are not seen. (MPO-stained section, original magnification × 30,900.)
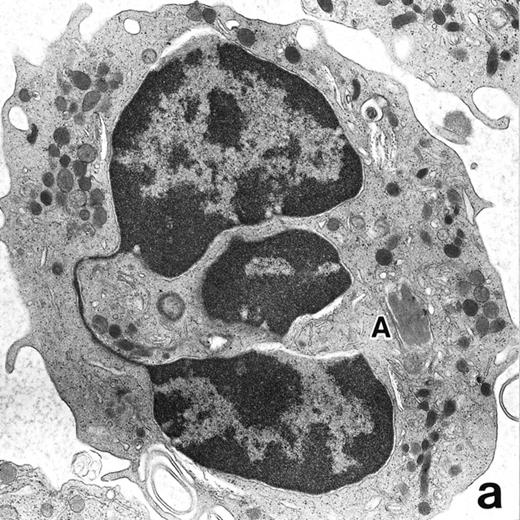
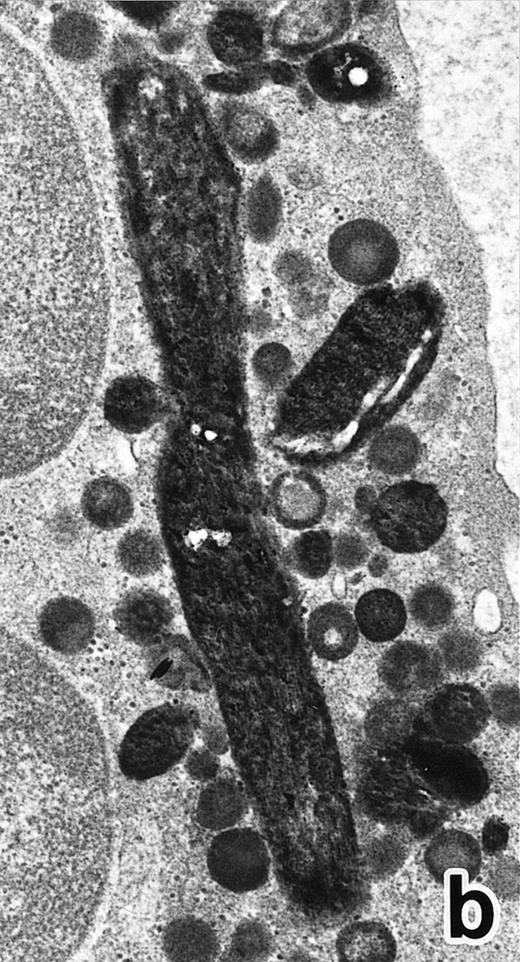
(a) A PMN in patient no. 1, seen in the bone marrow after 3 weeks of ATRA treatment. Lobulated nuclei are connected by nuclear filamentous structures and heterochromatin is highly condensed along the nuclear membrane. An Auer rod (A) is present along with primary granules. (Original magnification × 16,900.) (b) Higher magnification of the cytoplasm of a PMN in patient no. 3 after 3 weeks of ATRA treatment. Auer rods and cytoplasmic granules are all positive for MPO, and no MPO-negative secondary granules are evident. (MPO-stained section, original magnification × 41,900.)
(a) A PMN in patient no. 1, seen in the bone marrow after 3 weeks of ATRA treatment. Lobulated nuclei are connected by nuclear filamentous structures and heterochromatin is highly condensed along the nuclear membrane. An Auer rod (A) is present along with primary granules. (Original magnification × 16,900.) (b) Higher magnification of the cytoplasm of a PMN in patient no. 3 after 3 weeks of ATRA treatment. Auer rods and cytoplasmic granules are all positive for MPO, and no MPO-negative secondary granules are evident. (MPO-stained section, original magnification × 41,900.)
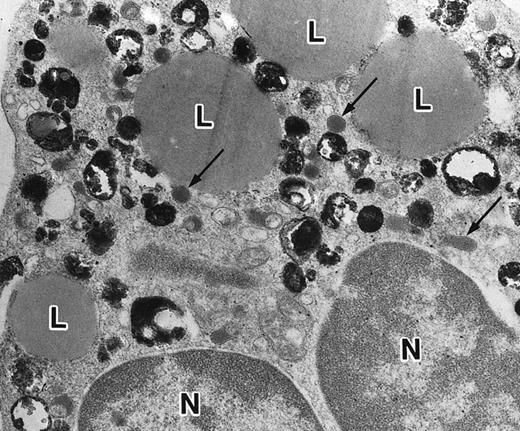
(a) A PMN in patient no. 1 that appeared after 4 weeks of ATRA treatment. (MPO-stained section, original magnification × 14,900.) (b) Higher magnification of a portion of the cytoplasm in (a). Note that, in contrast to the cells in Fig 5, this cell contains two types of cytoplasmic granules, ie, MPO-positive spherical granules and MPO-negative, elongated or dumbbell-shaped, small granules (arrow), corresponding to the primary and secondary granules of neutrophils, respectively. (MPO-stained section, original magnification × 30,000.)
(a) A PMN in patient no. 1 that appeared after 4 weeks of ATRA treatment. (MPO-stained section, original magnification × 14,900.) (b) Higher magnification of a portion of the cytoplasm in (a). Note that, in contrast to the cells in Fig 5, this cell contains two types of cytoplasmic granules, ie, MPO-positive spherical granules and MPO-negative, elongated or dumbbell-shaped, small granules (arrow), corresponding to the primary and secondary granules of neutrophils, respectively. (MPO-stained section, original magnification × 30,000.)
After 5 to 8 weeks of ATRA treatment, when the bone marrow was considered to exhibit a CR at the light microscopic level, it consisted of cells of multiple lineages at various stages of differentiation. Secondary granule-deficient PMNs, rarely containing Auer rods, still persisted in bone marrow but in very small numbers, and the majority of PMNs contained both primary and secondary granules and were morphologically normal even at the ultrastructural level. At this stage, promyelocytes with numerous primary granules and dilated rER but without definite abnormalities diagnostic of leukemic cell origin were observed.
APL cells in culture.APL cells in patient no. 3 were cultured in the presence of ATRA or G-CSF for 7 days, and their morphological changes were examined under a light microscope. After 5 days of culture with ATRA the cells displayed a tendency toward maturation, including nuclear lobulation, chromatin condensation, and a decrease or disappearance of azurophil granules, similar to that observed in bone marrow cells. The cells cultured with G-CSF for 7 days, on the other hand, retained a promyelocytic appearance with numerous azurophil granules that even seemed to have increased in size and/or number (data not shown). Electron microscopic examination showed the nuclei of the cells cultured with ATRA to be irregularly lobulated with marginally condensed heterochromatin and the lobulated nuclei to be often connected by nuclear filamentous structures, in a manner similar to the cells seen in the bone marrow (Fig 8). These cells contained numerous MPO-positive primary granules, including irregularly shaped granules, but no MPO-negative specific granules were identified.
(a) A PMN that emerged in the APL cell culture of patient no. 3 in the presence of ATRA for 1 week. From the lobulated nuclear configurations and condensed heterochromatin, this cell looks like a PMN. However, the cytoplasm contains many MPO-positive primary granules but no secondary granules. (MPO-stained section, original magnification × 14,900.) (b) Higher magnification of a portion of the cell in (a), showing that the cytoplasmic granules are all MPO-positive. (MPO-stained section, original magnification × 30,100.)
(a) A PMN that emerged in the APL cell culture of patient no. 3 in the presence of ATRA for 1 week. From the lobulated nuclear configurations and condensed heterochromatin, this cell looks like a PMN. However, the cytoplasm contains many MPO-positive primary granules but no secondary granules. (MPO-stained section, original magnification × 14,900.) (b) Higher magnification of a portion of the cell in (a), showing that the cytoplasmic granules are all MPO-positive. (MPO-stained section, original magnification × 30,100.)
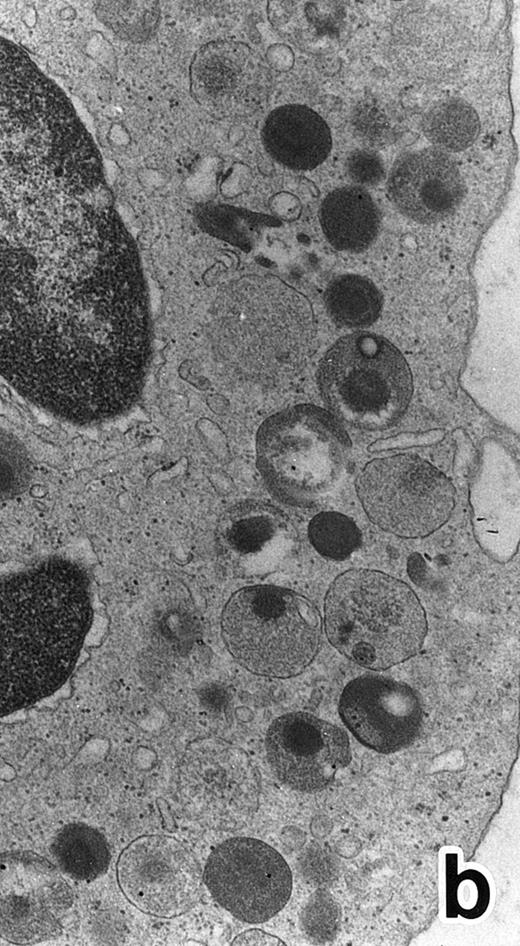
In contrast to the APL cell cultures, although only small numbers of PMNs were observed in the control cultures of nonleukemic bone marrow cells with ATRA, possibly because of the absence of added growth stimulants, they possessed both MPO-positive primary granules and MPO-negative secondary granules (Fig 9). The former outnumbered the latter, which was the reverse of what is observed in normal PMNs, and this may be because of decreased cell division of neutrophil precursors in the cultures in the absence of added growth stimulators, with resultant retention of primary granules. In addition, large lipid droplets were also present in some PMNs (Fig 9). These findings suggest that secondary granule deficiency in PMNs is a leukemia-specific phenomenon but not a general effect of ATRA to inhibit secondary granule formation and, conversely, that the accumulation of lipid droplets is caused by nonspecific effects of ATRA.
Representative appearance of a portion of a PMN that appeared in a culture of nonleukemic bone marrow mononuclear cells incubated in the presence of ATRA for 1 week. Both MPO-positive and -negative (arrow) cytoplasmic granules are present, with the former outnumbering the latter. Large spherical lipid droplets (L) are also present. N, nucleus. (MPO-stained section, original magnification × 17,700.)
Representative appearance of a portion of a PMN that appeared in a culture of nonleukemic bone marrow mononuclear cells incubated in the presence of ATRA for 1 week. Both MPO-positive and -negative (arrow) cytoplasmic granules are present, with the former outnumbering the latter. Large spherical lipid droplets (L) are also present. N, nucleus. (MPO-stained section, original magnification × 17,700.)
DISCUSSION
The key findings in the present electron microscopic study of APL cells during the process of ATRA-induced differentiation are summarized as follows. (1) ATRA induced maturation of nuclei, namely, nuclear lobulation and marginal condensation of heterochromatin, resulting in generation of nuclei of mature neutrophils, although they were irregularly shaped with occasional nuclear blebs and very often connected by nuclear filamentous structures (filamentous connections)13 that appeared to be of pathologic significance, similar to the nuclear blebs reported in AML.20 30 (2) The majority of aberrant primary granules, including Auer rods, lost their abnormal contour in response to ATRA administration, although small numbers of PMNs with Auer rods or C-H type abnormal granules persisted throughout ATRA treatment. (3) The PMNs that emerged in the bone marrow of APL patients during ATRA treatment, particularly at an early stage, and in cultures of APL cells with ATRA were devoid of neutrophil secondary granules.
Normalization of the size and shape of primary granules of APL cells in response to ATRA treatment was shown by the present electron microscopic study. This change was associated with the loss of azurophilic color in primary granules in Wright-Giemsa–stained smear specimens and, therefore, may indicate maturation of the primary granules. Sulfated glycosaminoglycans (GAGs) have been shown to be present in immature primary granules and to be responsible for their azurophilia, and the lack or masking of the sulfate group has been suspected of causing loss of azurophilic staining in mature primary granules.20,31,32 Because it has been shown that GAGs are also present in the primary granules and Auer rods of leukemic cells and that some Auer rods lack GAGs, suggesting maturational sequence of Auer rods,32 similar chemical modifications in primary granules may have occurred following the cellular maturation process of APL cells induced by ATRA. Although the process of normalization of primary granules was not clearly detected morphologically in this study, the unusual structure of a primary granule and an Auer rod connected to each other encountered in patient no. 1 (Fig 3b) suggests that normally appearing primary granules may have emerged from abnormal primary granules, including Auer rods.
A deficiency of secondary granules was noted in most of the PMNs that emerged in the bone marrow during ATRA treatment. Although there has been no direct evidence that these secondary-granule–deficient PMNs are neoplastic cells, they are likely of neoplastic origin. First, the secondary-granule–deficient PMNs decreased in number in the course of ATRA treatment and were replaced by normal PMNs when remission was obtained. This is consistent with the results of molecular studies using premature chromosome condensation and the fluorescence in situ hybridization technique,11,23 showing that a substantial proportion of PMNs during ATRA treatment had chromosome 17 translocations, whereas diploid cells without such abnormalities prevailed in the blood and bone marrow in remission. Second, PMNs with Auer rods, clear evidence of neoplastic origin, were consistently devoid of secondary granules. Third, it is known that AML cell lines that can be induced to differentiate towards mature neutrophils do not express any secondary-granule–associated proteins, including lactoferrin (LF ), even after induction of differentiation.33-35 These findings suggest that secondary-granule–deficient PMNs are aberrantly matured leukemic cells. Moreover, our observation that PMNs with secondary granules appeared in the bone marrow during ATRA treatment and in control cultures of nonleukemic bone marrow cells with ATRA indicates that normal hematopoietic precursor cells can give rise to mature neutrophils with secondary granules regardless of the presence or absence of ATRA. Therefore, secondary-granule deficiency in PMNs in APL is probably because of an intrinsic defect in leukemic cells, not because of an adverse effect of ATRA on secondary-granule formation.
Secondary-granule deficiency has been found in PMNs in AML18-21; consistent with this, we and others have shown a deficiency of LF, a constituent of neutrophil secondary granules,36,37 in the PMNs of patients with AML.21,22 As mentioned above, it has been shown that, to date, no leukemic cell lines that are chemically induced to differentiate towards mature granulocytes, including HL60, PLB-985, and NB4, express any messages for secondary-granule–associated proteins, including LF,33-35 even after induction of differentiation. These findings suggest that secondary-granule deficiency is an abnormality closely associated with and perhaps a hallmark of aberrantly differentiated leukemic blasts in AML and may not be restricted to APL alone, but may be common to all subtypes of AML. In conclusion, genetic control of the neutrophil maturation process in APL is probably defective not only at the promyelocyte stage but in later stages, in a similar manner to that in other subtypes of AML. Although ATRA is highly effective in inducing differentiation of APL cells, it is incapable of inducing complete neutrophil maturation. Because it has been reported that G-CSF, when used in combination with ATRA in vitro, induces alkaline phosphatase activity in APL cells and augments the differentiation-inducing effects of ATRA,38 other bioactive substances such as G-CSF might be required, together with ATRA for APL cells, to undergo the final neutrophil maturation steps.
Address reprint requests to Jun Miyauchi, MD, Clinical Laboratory, National Children's Hospital, 3-35-31 Taishido, Setagaya-ku, Tokyo 154, Japan.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal