Abstract
The occurrence of non-Hodgkin's lymphoma (NHL) is the most serious complication of Sjögren's syndrome (SS). We performed a study of 16 NHLs occurring in patients with an underlying SS. These lymphomas arose not only in salivary glands (7 cases) but also in other mucosal extranodal sites (the stomach [4 cases], the lung [3 cases], the skin [3 cases], the buccal mucosa [1 case], the thymus [1 case]) and in nodal sites (8 cases). Low-grade marginal zone lymphomas (MZL) were diagnosed in 12 of the 16 patients, 9 of mucosa-associated lymphoid tissues (MALT) type in mucosal sites and 3 exclusively nodal. The 4 other patients presented with a high-grade B-cell lymphoma that was probably a histological transformation of an underlying low-grade MZL at least in 3 of the cases involving skin, stomach, and parotid, respectively. A t(14; 18) translocation was detected in 1 of 8 lymphomas tested. We detected serum anti-p53 antibodies in 2 of the 14 studied patients. p53 protein was detected in 1 of 11 lymphomas tested. LMP protein and Eber RNAs of Epstein-Barr virus (EBV) were not detected in the 16 NHL biopsies. Using polymerase chain reaction, EBV was never detected except in 1 of 4 parotid lymphomas. No human T-lymphotropic virus 1 or human herpes virus 8 DNAs were detected in NHL biopsies. None of the patients had hepatitis C virus infection found using serological methods. Chemotherapy was usually efficient. In conclusion, lymphomas occurring in patients with an underlying SS are in most cases MZL. These lymphomas are not associated with viruses known to be present in other types of lymphomas. Some of the translocations or mutations of oncogenes or antioncogenes described in other lymphomas are detected in SS-associated lymphomas.
SJÖGREN'S SYNDROME (SS) is an autoimmune disease characterized by a lymphocytic infiltration of salivary and lacrimal glands leading to a progressive destruction of these glands and by production of autoantibodies.1 This disorder is either isolated (primary SS) or associated with other systemic diseases (secondary SS). The occurrence of B-cell non-Hodgkin's lymphoma (NHL) represents the major complication in the evolution of SS patients.2 The risk of developing NHL, which is equivalent for both primary and secondary SS, was estimated to be 44 times greater than that observed in a comparable normal population.3 NHLs in SS patients occur preferentially in salivary glands and in other mucosa-associated lymphoid tissues (MALT) but also in lymph nodes and bone marrow. The evolution from benign lymphocytic infiltration characteristic of SS to malignant NHL is probably a multistep process, the underlying molecular events of which are still unknown.4 5
We present here the clinical, biological, histological, and phenotypical characteristics of 16 NHLs occurring in patients with an underlying SS. Because viruses are believed to play a role in the natural history of SS either as a triggering event or as a complication,6 we looked for an association between these NHLs and viruses such as Epstein-Barr virus (EBV), human herpes virus 8 (HHV8), hepatitis C virus (HCV), or human T-cell lymphocytotropic virus 1 (HTLV-1). In addition, we investigated whether translocations or mutations of oncogenes or antioncogenes such as bcl-2 or p53 may represent one step of the malignant transformation in these patients.
PATIENTS AND METHODS
Patients.Sixteen consecutive patients with NHL complicating SS were studied in two French hospitals (Hôpital Saint-Louis [Paris, France] and Hôpital de Hautepierre [Strasbourg, France]). All patients had a diagnosis of SS according to the criteria of Fox et al.7
Histology and immunohistochemistry.Tissue was fixed in 10% formalin or in Bouin's fixative. After being embedded in paraffin, the sections were stained with hemotoxylin and eosin and Giemsa. No frozen material was available. The lymphomas were classified according to the Kiel classification8 and according to the newly proposed REAL classification from the International Lymphoma Study Group.9 Immunohistochemistry was performed on a representative section to determine the phenotype of malignant cells and the isotype of the lymphoid infiltrate when a plasmacytoid differentiation was present and to study the expression of the bcl-2 protein and of the EBV latent membrane protein (LMP). We used antibodies to CD20 (L26), CD3, κ, and λ Ig light chains, bcl-2 and LMP (Dako SA, Glostrup, Denmark) according to a three-step immunoperoxidase method.10
p53 expression was tested on fixed sections with the monoclonal antibody DO7 (from T. Soussi, Institut Curie, Paris, France) using a microwave-based procedure as already described11 in 11 cases. The sections were cut immediately before testing; indeed, it has been recently shown that a marked decrease of p53 immunostaining occurs over time if sections on glass slides were stored more than 3 months before testing.12 Appropriate positive and negative controls were also tested.
Detection of EBV Eber RNAs by in situ hybridization.The presence of the EBV genome was detected using the fluorescein-conjugated EBV oligonucleotides Eber 1 and 2 (Dako SA), complementary to the nuclear RNAs portions of the Eber genes, actively transcribed in latently infected cells. Deparaffinized sections were rehydrated and pretreated with proteinase K, dehydrated, air-dried, and hybridized for 2 hours at 37°C with the fluorescein isothiocyanate (FITC)-conjugated Eber probes in hybridization solution (0.1% Triton X-100). After washing in TBS, pH 7.6, containing 0.1% Triton X-100, the following immunohistochemical detection system was used: mouse anti-FITC, rabbit antimouse Ig, and APAAP complexes (Dako SA). Appropriate controls were studied in parallel.
Detection of EBV DNA by polymerase chain reaction (PCR).Genomic DNA was extracted from paraffin-embedded LNH sections from biopsy samples of each patient as described elsewhere13 and was subjected to PCR amplification using uracil-N-glucosylase (UNG).14 PCR was performed with oligonucleotide primers localized in the BamHI-W fragment of the virus at positions 967-992 and 1121-1096, as described elsewhere.15 After transfer, amplification products were detected with a specific oligosynthetic probe localized at position 1055-1085, labeled with the digoxygenin (DIG)-oligonucleotide tailing kit (Boehringer Mannheim, Mannheim, Germany).15 Positive and negative controls were performed with DNA extracts from EBV-infected and -uninfected tissue samples.
Detection of the pX gene of HTLV-1 by PCR.Genomic DNA extracted from paraffin-embedded LNH sections from biopsy samples of each patient was subjected to PCR amplification using UNG.14 The primer pair SK 43/44 used by Kwok et al16 amplified the tax region of the HTLV-I genome. The amplified products were tested under conditions described elsewhere.17 Positive and negative controls included an HTLV-1–positive cell line (SLB1) and lymphoid blood cells of a normal donor, respectively.
Detection of HHV8 by PCR.Amplification conditions used HHV8 primers, as described elsewhere.18 After transfer, amplification products were detected with a 25-bp internal oligomer probe18 end-labeled with the DIG-oligonucleotide tailing kit (Boehringer Mannheim). Positive and negative controls were performed with DNA extracts from patients with and without Kaposi's sarcoma. The absence of PCR inhibitors in DNA samples was checked by amplification in the same conditions of HHV8 DNA from a mix of the DNA sample tested and DNA extracts from BC-1 cells obtained from American Type Culture Collection (Rockville, MD).19
Detection of bcl-2 rearrangement by PCR.PCR amplification at the major breakpoint region (Mbr) of the bcl-2/IgH translocation was performed using primers for the Mbr and for the JH consensus region as described elsewhere.20 After transfer, PCR products were hybridized with an internal radiolabeled oligonucleotide probe described by Crescenzi et al.21 Sensitivity of the assay assessed by amplification of 10-fold serial dilutions of DNA from t(14; 18)-carrying tumor cells in DNA from peripheral blood mononuclear cells was approximately 1 tumor cell in 105 normal cells. The quality of DNA samples was checked by amplification of β-actin genes.
Detection of anti-p53 antibodies by enzyme-linked immunosorbent assay (ELISA).Circulating anti-p53 antibodies were detected using an ELISA as previously described.22 Previous studies have shown that anti-p53 antibodies are specifically found in patients with various types of neoplasia and are not present in the normal population.22
RESULTS
Patients' characteristics.Clinical characteristics of the 16 patients are shown in Table 1. The mean age was 57 years (range, 18 to 80 years). They were 11 women and 5 men. SS was primary in 15 cases and secondary to rheumatoid arthritis in 1 case. All patients were diagnosed with SS according to the criteria of Fox et al.7 In 14 patients, the diagnosis of SS was established 2 to 25 years before the diagnosis of NHL. In 2 patients, SS and NHL were diagnosed simultaneously. Although a lymphoid infiltration of salivary glands may occur in NHL,23 these 2 patients were considered to have an underlying SS because sicca symptoms were present for more than 2 years before NHL. Furthermore, 1 patient's serum contained anti–SS-A antibodies and labial salivary gland biopsies did not disclose any monotypic B-cell (data not shown). For the 16 patients, the mean duration between the diagnosis of SS and NHL was 11 years (range, 0 to 25 years).
Clinical Characteristics of Patients
| . | Age/Sex . | Duration SS . | Anti–SS-A/SS-B . | RF . | Cryo . | Mono Ig . | Hypo Ig . | Localization . | Treatment . | Response (yr) . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 80/F | 25 | Yes | Yes | Yes | Mk | Yes | Buccal mucosa | CLB | CR (2) |
| Lymph node | ||||||||||
| Lung | ||||||||||
| 2 | 63/M | 12 | Yes | No | No | No | No | Stomach | ACVBP | CR (1) |
| MTX | ||||||||||
| 3 | 57/F | 12 | Yes | Yes | No | No | No | Parotid | CPM | CR (5) |
| Peripheral nerve | ||||||||||
| 4 | 18/F | 4 | Yes | Yes | No | No | No | Parotid | CLB | CR (5) |
| Skin | ||||||||||
| 5 | 60/M | 18 | No | No | No | No | Yes | Bronchus | CLB | |
| Lung | CHOP | PR (2) | ||||||||
| Lymph node | ACVBP | Relapse | ||||||||
| 6 | 38/F | 2 | Yes | No | No | No | No | Parotid | MTX | CR (1) |
| 7 | 71/M | 15 | Yes | Yes | No | No | Yes | Skin | CHOP | CR (0.5) |
| Muscle | COP | Dead | ||||||||
| 8 | 66/F | 0 | No | No | No | No | No | Parotid | mBACOD | CR (7) |
| Lymph node | ||||||||||
| 9 | 64/M | 2 | Yes | No | No | No | No | Lymph node | ACVBP | CR (8) |
| 10 | 62/M | 10 | No | Yes | No | No | No | Lymph node | COP | CR (9) |
| Radiotherapy | ||||||||||
| 11 | 38/F | 10 | Yes | Yes | No | Mk | No | Parotid | ||
| Thymus | CHOP | PR (3) | ||||||||
| Stomach | CPM | |||||||||
| 12 | 60/F | 4 | Yes | Yes | Yes | Mk | No | Lymph node | CHOP | PR (1.5) |
| Stomach | CLB | |||||||||
| 13 | 73/F | 0 | No | No | No | No | No | Stomach | CLB/IFN | PR (0.5) |
| Skin | Dead | |||||||||
| 14 | 56/F | 2 | Lymph node | COP | CR (12) | |||||
| 15 | 48/F | 11 | Yes | Yes | No | No | No | Parotid | CLB | CR (2) |
| 16 | 71/F | 22 | Yes | Yes | No | No | No | Parotid | CHOP | CR (0.5) |
| Lung |
| . | Age/Sex . | Duration SS . | Anti–SS-A/SS-B . | RF . | Cryo . | Mono Ig . | Hypo Ig . | Localization . | Treatment . | Response (yr) . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 80/F | 25 | Yes | Yes | Yes | Mk | Yes | Buccal mucosa | CLB | CR (2) |
| Lymph node | ||||||||||
| Lung | ||||||||||
| 2 | 63/M | 12 | Yes | No | No | No | No | Stomach | ACVBP | CR (1) |
| MTX | ||||||||||
| 3 | 57/F | 12 | Yes | Yes | No | No | No | Parotid | CPM | CR (5) |
| Peripheral nerve | ||||||||||
| 4 | 18/F | 4 | Yes | Yes | No | No | No | Parotid | CLB | CR (5) |
| Skin | ||||||||||
| 5 | 60/M | 18 | No | No | No | No | Yes | Bronchus | CLB | |
| Lung | CHOP | PR (2) | ||||||||
| Lymph node | ACVBP | Relapse | ||||||||
| 6 | 38/F | 2 | Yes | No | No | No | No | Parotid | MTX | CR (1) |
| 7 | 71/M | 15 | Yes | Yes | No | No | Yes | Skin | CHOP | CR (0.5) |
| Muscle | COP | Dead | ||||||||
| 8 | 66/F | 0 | No | No | No | No | No | Parotid | mBACOD | CR (7) |
| Lymph node | ||||||||||
| 9 | 64/M | 2 | Yes | No | No | No | No | Lymph node | ACVBP | CR (8) |
| 10 | 62/M | 10 | No | Yes | No | No | No | Lymph node | COP | CR (9) |
| Radiotherapy | ||||||||||
| 11 | 38/F | 10 | Yes | Yes | No | Mk | No | Parotid | ||
| Thymus | CHOP | PR (3) | ||||||||
| Stomach | CPM | |||||||||
| 12 | 60/F | 4 | Yes | Yes | Yes | Mk | No | Lymph node | CHOP | PR (1.5) |
| Stomach | CLB | |||||||||
| 13 | 73/F | 0 | No | No | No | No | No | Stomach | CLB/IFN | PR (0.5) |
| Skin | Dead | |||||||||
| 14 | 56/F | 2 | Lymph node | COP | CR (12) | |||||
| 15 | 48/F | 11 | Yes | Yes | No | No | No | Parotid | CLB | CR (2) |
| 16 | 71/F | 22 | Yes | Yes | No | No | No | Parotid | CHOP | CR (0.5) |
| Lung |
Abbreviations: RF, rheumatoid factor; mono Ig, monoclonal Ig; hypo Ig, hypogammaglobulinemia; CR, complete remission; PR, partial remission; CLB, chlorambucil; CPM, cyclophosphamide; MTX, methotrexate; IFN, α interferon; COP, cyclophosphamide + vincristine + prednisone; CHOP, cyclophosphamide + doxorubicine + vincristine + prednisone; CVB, cyclophosphamide + vindesine + bleomycine; ACVBP, doxorubicine + cyclophosphamide + vindesine + bleomycine + prednisone; MBACOD, methotrexate + bleomycine + doxorubicine + cyclophosphamide + vincristine + dexamethasone.
Four patients received an immunosuppressive therapy before the onset of lymphoma: prednisone for more than 1 year (cases no. 3, 6, and 13), azathioprine for 1 year (case no. 3), or low-dose methotrexate for 3 years (case no. 7). Another patient (case no. 2) received radiotherapy on the parotid 5 years before the onset of gastric lymphoma.
Extranodal mucosal localization of the lymphoma was observed in 13 of the 16 cases. The lymphoma was exclusively nodal in only 3 cases; of note, 5 of the 13 patients with extranodal lymphomas also had a nodal involvement. The mucosal localizations were the parotid (7 cases), the lung (3 cases), the stomach (4 cases), the skin (3 cases), the buccal mucosa (1 case), and the thymus (1 case).
Treatment and outcome.All patients were treated with chemotherapy (Table 1), sometimes in conjunction with surgery (cases no. 4, 6, and 8) or radiotherapy (case no. 11). Complete remission (CR) was obtained in 12 patients. Eleven of these 12 patients are still alive in CR with a follow-up from 0.5 to 12 years and 1 died suddenly in CR 5 months after the end of the chemotherapy. A partial remission (PR) was obtained in 3 patients; it was stable in 2 of them (18 months and 3 years after the end of the treatment), while the third patient experienced a relapse after 2 years. The last patient, who presented with a gastric MALT lymphoma, was apparently cured with antibiotics administered for Helicobacter pylori infection and died from an aggressive cutaneous and nodal T-cell lymphoma that was refractory to chemotherapy.
Serological data.Autoantibodies associated with SS were detectable in the majority of patients' sera (Table 1): rheumatoid factor (9 of 15), antinuclear antibodies (12 of 15), and anti–SS-A and/or anti–SS-B antibodies (11 of 15). Serum from three patients featured a decrease in the level of the 3 main classes of Igs when the diagnosis of NHL was made. A monoclonal IgM κ was detected in the serum of 3 patients and was associated with a type II mixed cryoglobulinemia in 2 instances. Antibodies to human immunodeficiency virus, HTLV-1, and HCV were not detected in patients' sera.
Histology, immunohistochemistry, and in situ hybridization.Table 2 shows the histological types and the immunohistochemical results of the 16 cases of lymphoma reviewed.
Histological, Phenotypical, Virological, and Molecular Data of SS-Associated Lymphomas
| . | Tissue Specimen . | Histological Pattern . | VL Exp . | LMP Exp . | Eber RNA . | EBV DNA . | HHV8 DNA . | HTLV-1 . | trans 14,18 . | bcl-2 . | p53 Exp . | Anti-p53 Ab . | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Kiel . | REAL . | . | . | . | . | . | DNA . | . | Exp . | . | . |
| 1 | Buccal | — | MALT | κ | No | No | Yes | No | |||||
| Mucosa | |||||||||||||
| 2 | Stomach | Centroblastic | Diffuse large B-cell | κ | No | No | No | No | No | No | No | No | No |
| 3 | Parotid | — | MALT | κ | No | No | No | Yes | No | No | |||
| 4 | Skin | — | MALT | κ | No | No | No | No | Yes | No | No | ||
| 5 | Bronchus | — | MALT | Neg | No | No | No | No | No | Yes | Yes | No | No |
| Lymph node | Centroblastic | Diffuse large B-cell | No | No | No | No | Yes | ||||||
| 6 | Parotid | — | MALT | Neg | No | No | No | No | No | Yes | No | No | |
| 7 | Skin | Centroblastic | Diffuse large B-cell | κ | No | No | No | No | No | No | No | No | |
| 8 | Lymph node | Immunocytoma | Marginal zone B-cell | Neg | No | No | No | No | No | No | Yes | Yes | Yes |
| 9 | Lymph node | Centroblastic | Diffuse large B-cell | No | No | No | No | Yes | No | ||||
| 10 | Lymph node | Immunocytoma | Marginal zone B-cell | No | No | No | No | Yes | |||||
| 11 | Stomach | — | MALT | No | No | No | No | Yes | No | No | |||
| 12 | Stomach | — | MALT | κ | No | No | No | Yes | No | ||||
| 13 | Stomach | — | MALT | Neg | No | No | No | No | No | Yes | No | ||
| Skin | Pleiomorphic T-cell | Peripheral T-cell | No | ||||||||||
| 14 | Lymph node | Immunocytoma | Marginal zone B-cell | Neg | No | No | Yes | Yes | |||||
| 15 | Parotid | — | MALT | κ | No | No | No | No | No | No | Yes | No | No |
| 16 | Parotid | Centroblastic | Diffuse large B-cell | Neg | No | No | Yes | No | No | No | Yes | No | No |
| . | Tissue Specimen . | Histological Pattern . | VL Exp . | LMP Exp . | Eber RNA . | EBV DNA . | HHV8 DNA . | HTLV-1 . | trans 14,18 . | bcl-2 . | p53 Exp . | Anti-p53 Ab . | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Kiel . | REAL . | . | . | . | . | . | DNA . | . | Exp . | . | . |
| 1 | Buccal | — | MALT | κ | No | No | Yes | No | |||||
| Mucosa | |||||||||||||
| 2 | Stomach | Centroblastic | Diffuse large B-cell | κ | No | No | No | No | No | No | No | No | No |
| 3 | Parotid | — | MALT | κ | No | No | No | Yes | No | No | |||
| 4 | Skin | — | MALT | κ | No | No | No | No | Yes | No | No | ||
| 5 | Bronchus | — | MALT | Neg | No | No | No | No | No | Yes | Yes | No | No |
| Lymph node | Centroblastic | Diffuse large B-cell | No | No | No | No | Yes | ||||||
| 6 | Parotid | — | MALT | Neg | No | No | No | No | No | Yes | No | No | |
| 7 | Skin | Centroblastic | Diffuse large B-cell | κ | No | No | No | No | No | No | No | No | |
| 8 | Lymph node | Immunocytoma | Marginal zone B-cell | Neg | No | No | No | No | No | No | Yes | Yes | Yes |
| 9 | Lymph node | Centroblastic | Diffuse large B-cell | No | No | No | No | Yes | No | ||||
| 10 | Lymph node | Immunocytoma | Marginal zone B-cell | No | No | No | No | Yes | |||||
| 11 | Stomach | — | MALT | No | No | No | No | Yes | No | No | |||
| 12 | Stomach | — | MALT | κ | No | No | No | Yes | No | ||||
| 13 | Stomach | — | MALT | Neg | No | No | No | No | No | Yes | No | ||
| Skin | Pleiomorphic T-cell | Peripheral T-cell | No | ||||||||||
| 14 | Lymph node | Immunocytoma | Marginal zone B-cell | Neg | No | No | Yes | Yes | |||||
| 15 | Parotid | — | MALT | κ | No | No | No | No | No | No | Yes | No | No |
| 16 | Parotid | Centroblastic | Diffuse large B-cell | Neg | No | No | Yes | No | No | No | Yes | No | No |
Abbreviations: VL, variable light chain; Exp, expression; trans, translocation; LMP, latent membrane protein.
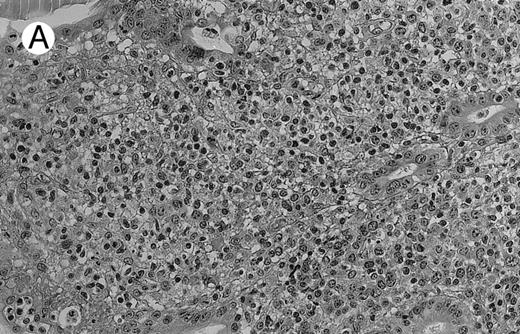
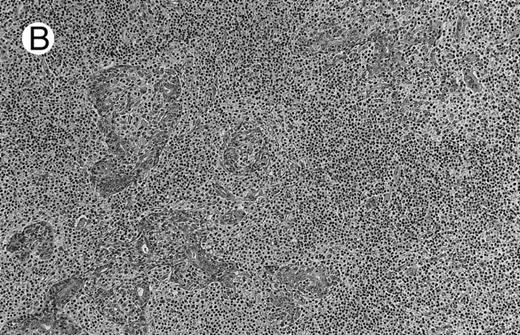
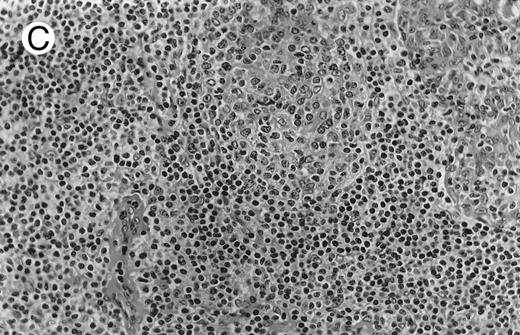
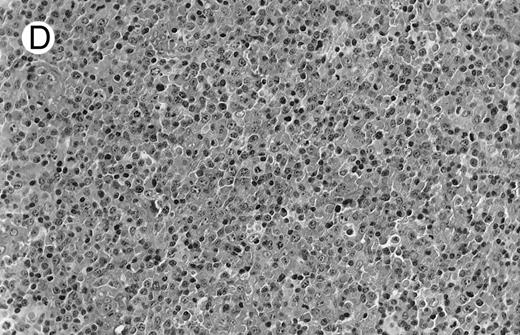
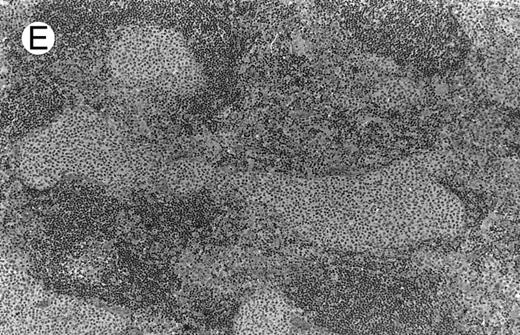
Low-grade marginal zone B-cell lymphoma (MZL; REAL classification)9 comprised the largest number of cases (n = 12). Nine of them were low-grade MALT type lymphomas involving parotid, stomach, bronchus, buccal mucosa, and skin. The histological pattern was relatively homogeneous, showing diffuse lymphoid infiltrates that included small lymphocytes with round nuclei or with plasma-cell differentiation and/or centrocyte-like cells with indented nuclei (Fig 1A). Lymphoepithelial lesions were observed in each case, regardless of the tissue involved (Fig 1B). Monocytoid cells characterized by a larger cytoplasm and a slightly irregular nucleus were mixed with centrocyte-like cells in 2 cases of MALT lymphoma arising in the parotid (cases no. 3 and 15). There were also scattered occasional large cells (centroblast or immunoblast). In cases no. 3, 6, and 15, remnants of germinal centers with partial follicular colonization were observed (Fig 1C). Beside these 9 low-grade-type mucosal lymphomas, 3 nodal lymphomas (cases no. 8, 10, and 14) had histological features similar to that of low-grade MALT type lymphoma because they showed a typical lymphoplasmacytoid infiltration with a variable proportion of blasts. In 2 cases, the presence of numerous immunoblasts or centroblasts, mixed with small lymphoplasmacytoid cells or plasma cells, indicated an evolution to a more aggressive lymphoma (Fig 1D). The third nodal lymphoma showed a small lymphocytic or lymphoplasmacytoid diffuse infiltrate associated with intrasinusal large nests of monocytoid cells. This histological picture is characteristic of monocytoid B-cell lymphoma (MBLC; Fig 1E).
NHL associated with SS (hematoxylin-eosin stains). (A) Case no. 13. Low-grade B-cell lymphoma of MALT type in the stomach, composed mainly of centrocyte-like cells with a few small lymphocytes and plasma cells. (B) Case no. 15. Low-grade B-cell lymphoma of MALT type in the parotid. Lymphoepithelial lesions are numerous, surrounded by centrocyte-like cells. (C) Case no. 6. Remnants of a germinal center in a low-grade B-cell lymphoma of MALT type in the parotid.
(D) Case no. 10. Immunocytoma in lymph node. The infiltrate is composed of small lymphocytes, plasma cells and blasts. (E) Case no. 14. Monocytoid B-cell lymphoma in a cervical lymph node with a monocytoid component located in dilated sinuses.
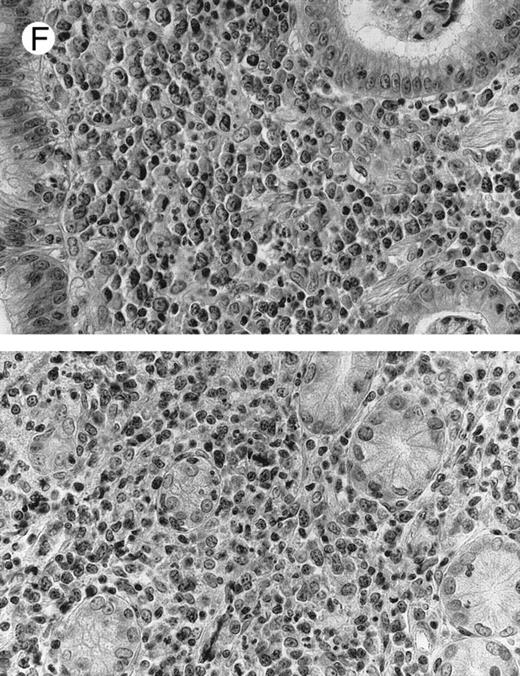
(F ) Case no. 2. High-grade B-cell lymphoma in the stomach (centroblastic lymphoma; upper), with remnants of low-grade lymphoma of MALT type (lower).
NHL associated with SS (hematoxylin-eosin stains). (A) Case no. 13. Low-grade B-cell lymphoma of MALT type in the stomach, composed mainly of centrocyte-like cells with a few small lymphocytes and plasma cells. (B) Case no. 15. Low-grade B-cell lymphoma of MALT type in the parotid. Lymphoepithelial lesions are numerous, surrounded by centrocyte-like cells. (C) Case no. 6. Remnants of a germinal center in a low-grade B-cell lymphoma of MALT type in the parotid.
(D) Case no. 10. Immunocytoma in lymph node. The infiltrate is composed of small lymphocytes, plasma cells and blasts. (E) Case no. 14. Monocytoid B-cell lymphoma in a cervical lymph node with a monocytoid component located in dilated sinuses.
(F ) Case no. 2. High-grade B-cell lymphoma in the stomach (centroblastic lymphoma; upper), with remnants of low-grade lymphoma of MALT type (lower).
Diffuse large B-cell lymphomas (REAL classification)9 or centroblastic lymphoma (Kiel classification)8 were observed in the last 4 cases, 1 nodal (case no. 9) and 3 extranodal, involving stomach, skin, and parotid (cases no. 2, 7, and 16). The gastric (case no. 2) and the skin (case no. 7) lymphomas were considered as a blastic transformation of a low-grade MALT-type lymphoma because large tumoral cells were mixed with a monoclonal plasmacytic cell component (Fig 1F ). The pathological features of these high-grade lymphomas did not differ from conventional centroblastic lymphoma. A high-grade diffuse large B-cell lymphoma was also subsequently diagnosed in the retroperitoneal area 10 months after the initial bronchial low-grade MALT type lymphoma in 1 patient (case no. 5). Lastly, 1 patient (case no. 13) with a gastric low-grade MALT-type lymphoma subsequently developed a nonepidermotropic cutaneous CD4 T-cell lymphoma.
Aside from this latter T-cell lymphoma, the B phenotype of each lymphoma was confirmed by immunoreactivity with the pan-B antibody CD20. Most specimens contained variable mixed T-cell reactive with CD3 monoclonal antibody. Monotypic Ig κ light chains were noted on fixed section in 7 MALT lymphomas (cases no. 1, 2, 3, 4, 7, 12, and 15). Bcl-2 expression was found in 14 of the 16 lymphomas tested. p 53 expression was found in 1 of the 11 lymphomas tested (case no. 8). This patient had a nodal immunocytoma with approximately 10% of large cells, which were precisely the cells positive for the p53 protein.
H pylori was present in 1 of the 4 gastric lymphomas (case no. 13); EBV LMP or EBV RNAs were not detected by immunohistochemistry and hybridization techniques, respectively, in the 16 cases.
Detection of viral genes by PCR.PCR to detect EBV was performed in 12 NHL biopsies, with 4 of them being parotid samples; EBV DNA was detected in 1 parotid lymphoma (case no. 16).
The pX gene of HTLV-1 was looked for by PCR in 12 NHL biopsies but was always absent. Lip biopsies were also tested in 3 of these 12 patients; 1 was positive (the gastric lymphoma of this patient was negative, however, for pX DNA).
HHV8 DNA was looked for by PCR in 8 NHL biopsies but was not detected.
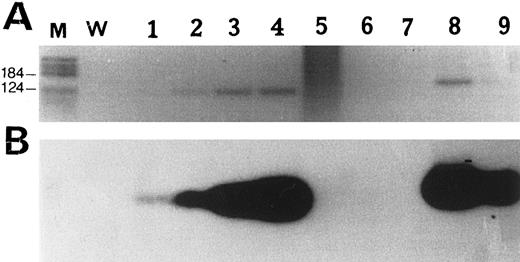
Detection of bcl-2 rearrangement by PCR.We looked for bcl-2 rearrangements at the Mbr in 7 patients. Results are shown in Fig 2. Bcl-2 rearrangement was detected in two samples from patient no. 5. This patient had a bronchial MALT lymphoma that progressed 10 months later into a retroperitoneal nodal large B-cell lymphoma. The t(14; 18) translocation was detected in both the low-grade and the high-grade lymphoma.
PCR amplification of Bcl-2 rearrangement at the Mbr. (A) Reverse view of an ethidium bromide-stained agarose gel. (B) Southern blot analysis of PCR products hybridized with an internal Mbr oligoprobe (see Materials and Methods). M, molecular weight marker V (Boehringer Mannheim, Meylan, France); W, negative control (water). Lanes 1 through 4, positive control diluted 10−5 to 10−2; lane 5, case no. 2; lane 6, case no. 13; lane 7, case no. 16; lanes 8 and 9, case no. 5, detection of a Bcl-2 rearrangement both in the bronchic MALT lymphoma (lane 8) and in the nodal large B-cell lymphoma (lane 9) occurring 2 years later.
PCR amplification of Bcl-2 rearrangement at the Mbr. (A) Reverse view of an ethidium bromide-stained agarose gel. (B) Southern blot analysis of PCR products hybridized with an internal Mbr oligoprobe (see Materials and Methods). M, molecular weight marker V (Boehringer Mannheim, Meylan, France); W, negative control (water). Lanes 1 through 4, positive control diluted 10−5 to 10−2; lane 5, case no. 2; lane 6, case no. 13; lane 7, case no. 16; lanes 8 and 9, case no. 5, detection of a Bcl-2 rearrangement both in the bronchic MALT lymphoma (lane 8) and in the nodal large B-cell lymphoma (lane 9) occurring 2 years later.
Detection of anti-p53 antibodies by ELISA.p53 mutations are known to induce an increase in the half-life of the mutant p53, leading to the accumulation of the p53 protein in the tumor cells. Such accumulation can lead to a self-immunization process and the apparition of high levels of anti-p53 antibodies in the sera of patients that harbor a mutant p53 in their tumor.24 The dosage of anti-p53 antibodies is a good alternative to assess the p53 status in a tumor. Anti-p53 antibodies were detected at a high titer in the sera of 2 of 14 tested patients (cases no. 8 and 14; Table 2). These 2 patients had a nodal immunocytoma and a lymphoplasmacytoid lymphoma, respectively. One of these patients (case no. 8) was also positive with p53 immunohistochemical assay; for the other patient, no material was available for p53 immunohistochemical assay.
DISCUSSION
The occurrence of NHL is the most serious complication of SS.2 The risk of lymphoma in these patients reached 6.4 cases per 1,000 per year (44 times greater than in a normal population) in 136 women with SS observed for an average of 8.1 years.3 Comparable results were obtained in several reports including a small number of patients with NHL.25-29 No specific histological features or unique pathogenesis could be established in these studies. We therefore performed an extensive study with clinical, histological, phenotypical, virological, and molecular data of 16 NHL occurring in patients with an underlying SS.
Previous studies identified predisposing factors for the occurrence of NHL in SS patients: parotidomegaly, splenomegaly, lymphadenopathy, low-dose parotid irradiation, cytotoxic therapy,3 the presence of a serum or urinary monoclonal component30 or mixed cryoglobulinemia,29 and a decrease in serum polyclonal Igs.31 Our study was not a prospective analysis of SS patients and, therefore, we could not establish definite conclusions on the value of these predisposing factors. However, a history of parotid enlargement and irradiation was present in 1 patient and previous cytotoxic therapy had been administered in 4 patients (prednisone in 3 cases, methotrexate in 1 case, and azathioprine in 1 case). Three patients had a serum monoclonal Ig, 2 of them having a mixed type II cryoglobulin and 2 others having a decrease in serum polyclonal Igs occurring at the time of lymphoma diagnosis.
Various histological subtypes of NHL have been described in the literature of patients with SS: Waldenström's macroglobulinemia,31 immunocytomas,27 follicular lymphomas,32 diffuse large B-cell lymphomas,3 immunoblastic lymphomas,33 and especially MALT lymphomas.34 The recent description of monocytoid B-cell lymphomas (MBCL),35-37 which appears as the nodal counterpart of MALT lymphomas38 (the term marginal zone B-cell lymphoma [MZL] has been proposed to encompass both entities39 ), allows a reinterpretation of the various histological subtypes of NHL described in patients with SS. Both variants (MALT and MBCL lymphomas) involve the marginal B-cell compartment of lymphoid tissue outside the follicular mantle zone, share the same propensity of plasmacytic differentiation and a distinctive immunological phenotype, and often exhibit a similar chromosomal abnormality (trisomy 3 in 50% of the cases).39 Furthermore, MZL can progress to high-grade large B-cell lymphomas.40,41 In the light of these data, a large number of NHLs in SS patients reported in the literature can be reclassified as MZL either of low-grade or of low-grade transformed into high-grade lymphomas. Our study provides arguments in favor of this interpretation. Indeed, low-grade MZL were diagnosed in 12 of the 16 patients, 9 of MALT type in mucosal sites and 3 exclusively nodal. The 4 other patients presented with a high-grade B-cell lymphoma that was probably a histological transformation of an underlying low-grade MZL at least in 3 of the cases involving skin, stomach, and parotid, respectively, and in which large tumoral cells were mixed with a monoclonal plasmacytic component. Lastly, T-cell lymphomas, as observed in case no. 13, have been described only rarely in patients with SS.42
In nearly half of the cases (7 cases), lymphomas arise in a main target of the sicca syndrome, ie, the parotid gland. The benign lympho-epithelial lesions characteristic of SS are composed by a majority of CD4 T-cell lymphocytes43 that secrete interleukin 2, γ-interferon, and interleukin-1044 and by a minority of B-cell lymphocytes that are often oligoclonal.45,46 A similar T-cell CD4 lymphoid hyperplasia may be present in lacrimal glands, lungs, and kidneys of SS patients.47 These T cells may contribute significantly to B-lymphocyte hyperactivity.48 Aside from this characteristic T-cell CD4 hyperplasia, the term pseudolymphoma has been used to coin an unusual presumably benign parotid lymphoid infiltration. In the original description,31 the infiltrate was described as being composed of small lymphocytes, plasma cells, immunoblasts, and a distinct mononuclear cell population that most likely represents monocytoid B lymphocytes. Thus, what was called pseudolymphoma in the literature corresponds probably in most cases to slowly progressive MALT/MBCL. The possibility that detection of clonal B cells by PCR may help to distinguish MALT/MBCL from benign lympho-epithelial lesions remains controversial. In one study, 14 of 14 (100%) labial salivary gland (LSG) specimens of SS patients exhibited oligoclonal or monoclonal Ig gene rearrangements by PCR45; in 1 patient with lymphoma, tumor and LSG specimens obtained at the same time displayed different Ig gene rearrangements. However, in another study, monoclonal B cells were detected by PCR in only 11 of 76 (15%) LSG specimens of SS patients49; 4 of these 11 patients subsequently developed extrasalivary lymphoma and in each case the rearranged bands in the lip biopsy and the lymphoma were of the same size. Likewise, in another study, the use of a VH CDR3 allele-specific probe showed that benign lymphoepithelial lesions of stomach and salivary glands present 13 and 2 years, respectively, before the onset of a gastric lymphoma contained B cells with a rearranged heavy chain identical to that found in the late lymphoma.50 Therefore, one of the B cells present in benign lymphoepithelial lesions evolved to malignant lymphoma probably because of additional genetic events.
The data given above suggest that the development of lymphoma in SS involves a multistep process, possibly the sequential activation of proto-oncogenes by translocations or mutations and/or viral infection. Using a PCR detecting translocations focused in the major breakpoint region,51 we detected a t(14; 18) translocation in 1 of 8 lymphomas tested. Interestingly, this patient had a bronchial MALT lymphoma that progressed 10 months later into a nodal large B-cell lymphoma. The t(14; 18) translocation was detected in both the low-grade and the high-grade lymphoma. The t(14; 18) translocation is usually not present in MZL.39 However, it was already detected by two groups in a subset of Sjögren's lymphomas.26,52 Pisa et al26 found the t(14; 18) translocation in 5 of 7 SS-associated salivary lymphomas. It was not present, either in prelymphoma biopsies from these patients, even though they exhibited oligoclonal B-cell rearrangements, or in 50 salivary gland biopsies of SS patients. We studied the expression of the bcl-2 protein in the 16 lymphoma specimens. The bcl-2 protein was expressed in 14 of these 16 lymphomas, as expected in low-grade MZL, which are usually considered to overexpress the bcl-2 protein53 without rearrangement of the bcl-2 gene.39
Mutations of the tumor-suppressor activity gene p53 have been observed in lymphomas and have been associated with progression of low-grade MALT lymphoma to high-grade.54 This inactivation of p53 activity is accompanied by an overexpression of the p53 protein and in half of the cases by detection of serum anti-p53 antibodies.24 We detected serum anti-p53 antibodies in 2 of the 14 patients studied (cases no. 8 and 14); these 2 patients had a nodal MZL. One of these 2 patients could be tested with p53 immunohistochemically assay and was positive in a small component of large cells that represented approximately 10% of lymphoma cells. Despite this minor component of large cells, this patient could not be considered to have a transformation of low-grade MZL into large B-cell lymphoma.
We also looked for the presence of viruses in these lymphomas because they have been implicated in the etiology of some sicca syndromes.6 We focused on the potential role of EBV in the pathogenesis of SS-associated lymphomas because the frequency of EBV genes or protein expression is increased in labial salivary gland biopsies from patients with SS in comparison with control subjects.15,55 However, LMP protein and Eber RNAs were not detected in the 16 SS-associated NHL biopsies. Using PCR, EBV was detected in only 1 of 4 parotid lymphomas, as could be expected in normal salivary glands. Likewise, Fox et al56 found EBV DNA in only 1 of 14 NHL in SS patients. It is not so surprising that Sjögren's lymphomas are not associated with EBV because the virus, when present in LSG of SS patients, is detected in epithelial cells by in situ hybridization.15 Moreover, EBV is present in lymphomas occurring in immunosuppressed patients57; that is not the case in Sjögren's patients. Interestingly, patients with connective tissue diseases treated with low-dose methotrexate may develop EBV-associated lymphoproliferative disorders that usually achieve CR after methotrexate withdrawal.58 One of our patients (case no. 7) was treated with methotrexate for 3 years before diffuse large-cell lymphoma occurred. However, it is unlikely that methotrexate might have a role in the development of the lymphoma of this patient because it had none of the above-mentioned characteristics of methotrexate-associated lymphomas.
Recently, another herpes virus termed HHV8 has been found to be associated not only with Kaposi's sarcoma18 but also with body cavity lymphomas, Castleman disease, and some lymphoid hyperplasia occurring in autoimmune diseases.59 Therefore, we looked for HHV8 by PCR in SS-associated lymphomas. However, this study was negative. None of our patients had HCV infection, which may be associated with some sicca syndrome60 and in some countries with lymphomas.61 Lastly, because the pX gene of the retrovirus HTLV-1 has been detected in labial salivary glands from some Japanese and French SS patients seronegative for HTLV-1,17 62 we looked for the presence of this gene in SS-associated lymphomas, but it was not detectable by PCR. Three of the 16 SS patients with lymphoma were also tested for the presence of HTLV-1 pX sequences in LSG. One of them was positive; however, the gastric lymphoma of this patient did not contain the pX sequence.
In conclusion, lymphomas occurring in patients with an underlying SS are in most cases MZL. Chemotherapy is usually efficient and long-term survival in CR or PR is common in these patients. These lymphomas are not associated with viruses known to be present in other types of lymphomas. Some of the translocations or mutations of oncogenes or antioncogenes described in other lymphomas are detected in SS-associated lymphomas. However, we could not detect a recurrent molecular abnormality that could have given new insights into a common pathogenesis for these lymphomas.
ACKNOWLEDGMENT
The authors are indebted to Dr Luc Marcellin and Dr Bernard Gasser for providing samples from Strasbourg; to Prof Frédéric Morinet, Prof François Sigaux, and Dr Patrick Cherot for help and advice; and to Martine Brunet and Laurence Grollet for technical assistance.
Supported by a grant from the Délégation de la Recherche Clinique (Assistance Publique-Hôpitaux de Paris).
Address reprint requests to Xavier Mariette, MD, Service d'Immuno-Hématologie-Hôpital Saint-Louis, 1, avenue Claude Vellefaux, 75475 Paris cédex 10, France.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal