Abstract
Prior in vitro studies have suggested a role of adhesion molecules, bone marrow stromal cells (BMSCs), and cytokines in the regulation of human multiple myeloma (MM) cell growth and survival. Although in vivo models have been developed in severe combined immunodeficient (SCID) mice that support the growth of human MM within the murine BM microenvironment, these xenograft models do not permit a study of the role of adhesion proteins in human MM cell-human BMSC interactions. We therefore established an in vivo model of human MM using SCID mice implanted with bilateral human fetal bone grafts (SCID-hu mice). For the initial tumor innoculum, human MM derived cell lines (1 × 104 or 5 × 104 ARH-77, OCI-My5, U-266, or RPMI-8226 cells) were injected directly into the BM cavity of the left bone implants in irradiated SCID-hu mice. MM cells engrafted and proliferated in the left human fetal bone implants within SCID-hu mice as early as 4 weeks after injection of as few as 1 × 104 MM cells. To determine whether homing of tumor cells occurred, animals were observed for up to 12 weeks after injection and killed to examine for tumor in the right bone implants. Of great interest, metastases to the right bone implants were observed at 12 weeks after the injection of 5 × 104 MM cells, without spread of human MM cells to murine BM. Human MM cells were identified on the basis of characteristic histology and monoclonal human Ig. Importantly, monoclonal human Ig and human interleukin-6 (IL-6), but not human IL-1β or tumor necrosis factor-α, were detectable in sera of SCID-hu mice injected with MM cells. In addition, specific monoclonal Ig light chain deposition was evident within renal tubules. This in vivo model of human MM provides for the first time a means for identifying adhesion molecules that are responsible for specific homing of human MM cells to the human, as opposed to murine, BM microenvironment. Moreover, induction of human IL-6 suggests the possibility that regulation of MM cell growth by this cytokine might also be investigated using this in vivo model.
ADHESION MOLECULES on multiple myeloma (MM) cells play a role in the homing of malignant plasma cells to the bone marrow (BM) after Ig class switching within the lymph node; regulation of MM cell growth and survival in the BM microenvironment; tumor cell egress from the BM with the development of plasma cell leukemia (PCL); and, finally, metastatic seeding at extramedullary sites. However, to date, the majority of studies examining the role of adhesion molecules, BM stromal cells (BMSCs), and cytokines in regulating MM cell localization, growth, and apoptosis within the BM microenvironment have been performed in vitro1-18 and may not accurately identify factors important for the pathophysiology of MM in vivo. For example, in vitro studies suggest that localization of tumor cells in the BM is related to MM cell binding to fibronectin and collagen I via very late antigen-4 (VLA-4) and syndecan, respectively,3,13 as well as tumor cell binding to BMSCs by VLA-4 to vascular cell adhesion molecule-1 (VCAM-1) and LFA-1 to intercellular adhesion molecule-1 (ICAM-1) interactions.1,4 Moreover, changes in cell surface phenotype of tumor cells have been described with disease progression, ie, acquisition of CD11b and LFA-1 with loss of CD56, VLA-5, MPC-1, and syndecan-1 on PCL cells.12,17,18 However, in vitro adhesion assays are dependent on the ability of BMSCs to be grown to confluence under the influence of serum and growth factors, and it is therefore possible that their adhesion molecule profile may have been altered. In addition, cells may also be selected for outgrowth in vitro that do not reflect BMSCs in vivo. Delineation of the role of adhesion molecules in disease pathogenesis requires in vivo models. Available models in severe combined immunodeficient (SCID) mice have shown that intraperitoneal injection of MM cells permits tumor cell growth within the peritoneal cavity19 and that intravenous injection of ARH-77 MM cells results in disseminated tumor cell growth both in the murine BM and other organs.20-23 However, to date, an in vivo model to assess the role of adhesion molecules in homing of human MM cells to human BMSCs has not been described.
Once MM cells are localized in the BM microenvironment, BMSCs and cytokines regulate their growth and survival. Interleukin-6 (IL-6) is the major autocrine and paracrine growth factor for human MM24 and can inhibit apoptosis of tumor cells induced by corticosteroids, serum starvation, and anti-Fas in vitro.25-28 Importantly, recent studies show that adhesion of MM cells to BMSCs, in addition to localizing tumor cells within the BM microenvironment, also upregulates IL-6 secretion by BMSCs.1,2,15 However, in vitro studies again may not accurately reflect the biologic significance of BMSCs and cytokines in vivo. For example, most freshly isolated MM patient cells do not produce IL-6 or demonstrate autocrine IL-6–mediated growth in vitro; however, triggering tumor cells via their cell surface CD40, as may occur in vivo, induces IL-6–mediated autocrine growth.29 In vitro studies also show that MM cells produce transforming growth factor-β1 (TGF-β1), but lack TGF-β1 responsiveness.30 However, TGF-β1 stimulates IL-6 production by BMSCs and may therefore indirectly regulate paracrine IL-6–mediated MM cell growth in vivo. Previously described in vivo murine models have already shed considerable insight into the role of cytokines, ie, IL-6, in the pathogenesis of human MM. For example, either mice injected with pristane or IL-6 transgenic mice develop massive plasmacytosis and/or transplantable monoclonal plasmacytomas31-33; in contrast, pristane injection in IL-6–deficient mice induces lymphocytosis without either plasmacytosis or the development of mouse plasmacytomas.34 35 However, these models do not allow for the characterization of the role of human BMSCs and cytokines in the regulation of human MM cell growth and survival in vivo.
The SCID-hu mouse, developed by surgical implantation of fetal hematolymphoid organs (eg, bone grafts, lymph node, and thymus) into SCID mice, has been used to study normal hematopoiesis and malignant cell growth as well as cytokine and gene therapies.36-42 In this study, we have established an in vivo model for the study of MM localization and growth within the human BM microenvironment using SCID-hu mice implanted with bilateral human fetal bone grafts. Human MM-derived cell lines injected directly into the marrow cavity of the left bone implant of irradiated SCID-hu mice spread to the right bone implant, but did not home to murine BM. Immunoperoxidase staining confirmed monoclonal tumor cell growth, which was coupled with secretion of monoclonal human Ig and human IL-6 in sera of tumor-bearing mice. This in vivo model may therefore facilitate studies of homing of human MM cells to the human BM microenvironment as well of as the role of BMSCs and cytokines in regulation of tumor cell growth and survival.
MATERIALS AND METHODS
MM-derived cell lines.The ARH-77, U-266, and RPMI-8226 human MM-derived cell lines were obtained from American Type Culture Collection (Rockville, MD) and cultured in RPMI-1640 medium with 10% fetal bovine serum.43 The OCI-My5 MM cell line was kindly provided by Dr H.A. Messner (Ontario Cancer Institute, Toronto, Ontario, Canada), and cultured in Iscove's media (Sigma, St Louis, MO) with 10% fetal bovine serum.44
SCID-hu mice and cell innoculation.Homozygous C.B-17 scid/scid mice (SCID) were bred, treated with antibiotics, and used when 6 to 8 weeks old. The collection of fetal tissues and implantation of human fetal long bone grafts into SCID mice to produce SCID-hu mice has been previously described.36-42 In brief, the femurs and tibias of 19 to 23 gestational week fetuses were cut into fragments (approximately 5 × 5 × 10 mm) and implanted subcutaneously into both sides of SCID mice. The human fetal tissues were obtained with appropriate informed consent and in compliance with regulations issued by the state and the federal governments. SCID-hu mice were exposed to 400 cGy γ irradiation from a 137Cs γ irradiator at a dose rate of 75 cGy/min. Either 1 × 104 or 5 × 104 ARH-77, U-266, OCI-My5, or RPMI-8226 MM cells or phosphate-buffered saline (PBS) alone in 10 μL was then injected directly into the marrow cavity of the left fetal bone implant in SCID-hu mice or was injected subcutaneously into SCID mice without human fetal bone implants.
Histopathological analysis.Mice were killed at 4, 8, and 12 weeks after MM cell injection. Excised organs included the left- and right-sided human fetal bone grafts, spleen, liver, thymus, kidney, lung, vertebrae, spinal cord, and brain. Tissues were fixed in Bouin's solution, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E) to perform histopathological examination. Immunoperoxidase studies were performed on paraffin sections using an indirect technique as previously described, with modifications.45 Rabbit antibodies (Abs) to human Ig light (κ and λ) and heavy (IgG, IgA, IgM, and IgE) chains, as well as swine antirabbit Ig conjugated to horseradish peroxidase, were obtained from Dako Corp (Carpenteria, CA). Antibody localization was effected using a peroxidase reaction with 3,3-diaminobenzidine tetrahydrochloride as chromogen.
Measurement of human IL-6, IL-1β, and human tumor necrosis factor-α (TNF-α) secretion.IL-6 levels in the sera of SCID and SCID-hu mice, in sera of SCID-hu mice injected with human MM cells, and in the supernatants of human MM-derived cell lines cultured in media were measured using an enzyme-linked immunosorbent assay (ELISA), as previously described.29 46 Briefly, (1) 96-well plates (Costar, Cambridge, MA) were coated with anti–IL-6 MoAb (murine IgG1; Toray, Ohtsu-shi, Shiga, Japan); (2) wells were saturated with calfskin gelatin (BioRad, Melville, NY)-PBS for 1 hour; (3) serial dilutions (100 μL) of test sample supernatants were added in duplicate to plates; and (4) biotinylated detector anti–IL-6 MoAb (Genetics Institute, Cambridge, MA) was added and developed with avidin-peroxidase (Amersham, Arlington Heights, IL), tetramethylbenzidine, and 30% peroxide (Sigma). The minimal detectable level of IL-6 (Kirin-Brewery Co Ltd, Minato-ku, Tokyo, Japan) was 10 pg/mL. Human IL-1β and TNF-α levels in sera of mice were measured using ELISA kits (R&D Systems [Minneapolis, MN] and Endogen [Cambridge, MA], respectively). The minimal detection levels were 4 pg/mL IL-1β and 5 pg/mL TNF-α. These ELISA systems do not detect mouse cytokines.
Solid-phase ELISA for IgG and IgA.Quantitative ELISA was used to measure levels of human IgG and IgA in the mouse sera in a manner similar to that described for IL-6 above.30 Goat antihuman polyvalent Ig Ab (Sigma) was used as the coating Ab, and biotin-conjugated goat antihuman IgG Ab (Sigma) or antihuman IgA Ab (Sigma) was used as detection Ab. The levels of detection of both IgG and IgA were 1 ng/mL.
RESULTS
Human MM cells grow in fetal human bone implants within SCID-hu mice.To study homing of human MM cells to human BMSCs, we first attempted to establish MM cell growth in SCID-hu mice. After irradiation (400 cGy) of SCID-hu mice, 1 × 104 or 5 × 104 ARH-77, OCI-My5, U-266, or RPMI-8226 MM cells or PBS was injected directly into the left human bone implant. Mice were killed 4, 8, and 12 weeks later, and the left fetal bone implant was examined histologically by H&E staining and also for monoclonality by Ig heavy and light chain immunoperoxidase staining. As early as 4 weeks after injection of as few as 1 × 104 MM cells, the left human bone implant specimens in all SCID-hu mice injected with MM cells showed infiltration of the human BM by tumor cells characteristic of the innoculated MM cell line, with only rare normal BM elements. By 12 weeks after injection of tumor cells, the surrounding tissue was largely replaced by neoplastic plasma cells. Histologic findings in left implants from ARH-77 injected animals (Fig 1A) are illustrative of findings in animals injected with OCI-My5, U-266, or RPMI-8226 MM cells. In contrast, BM within fetal bone implants from the SCID-hu mice injected with PBS alone (control) was hypoplastic with increased fatty deposits, without any signs of inflammation, granulation, or tumor either in the human BM or surrounding tissue (Fig 1B). Finally, no tumor growth was observed in SCID mice without human bone implants that were similarly treated with irradiation (400 cGy), injected with the same MM cell innocula subcutaneously, and killed at these intervals (data not shown).
Human MM cells grow in fetal human bone implants within SCID-hu mice. SCID-hu mice were treated with irradiation (400 cGy), injected directly into the marrow cavity of the left fetal human bone implant with 5 × 104 ARH-77 MM cells or with PBS, and killed 12 weeks later. H&E-stained tissue sections of the left human fetal bone marrow implant from SCID-hu mice injected with ARH-77 MM cells (original magnification × 100 [A]) or PBS (original magnification × 40 [B]) are shown.
Immunoperoxidase staining of the same human fetal bone marrow implant from SCID-hu mice injected with ARH-77 MM cells was also performed using antihuman κ (original magnification × 100 [C]) and antihuman λ (original magnification × 100 [D]) Ig light chain Abs.
Human MM cells grow in fetal human bone implants within SCID-hu mice. SCID-hu mice were treated with irradiation (400 cGy), injected directly into the marrow cavity of the left fetal human bone implant with 5 × 104 ARH-77 MM cells or with PBS, and killed 12 weeks later. H&E-stained tissue sections of the left human fetal bone marrow implant from SCID-hu mice injected with ARH-77 MM cells (original magnification × 100 [A]) or PBS (original magnification × 40 [B]) are shown.
Immunoperoxidase staining of the same human fetal bone marrow implant from SCID-hu mice injected with ARH-77 MM cells was also performed using antihuman κ (original magnification × 100 [C]) and antihuman λ (original magnification × 100 [D]) Ig light chain Abs.
To confirm that the tumor cells infiltrating the left bone implant were monoclonal, immunoperoxidase staining was performed using antihuman κ or λ Ig light chain Abs and antihuman IgG, IgA, IgM, or IgE heavy chain Abs. For ARH-77 MM cell line, tumor cells infiltrating the bone implants stained with antihuman IgG heavy chain Ab and antihuman κ Ig light chain Ab (Fig 1C), but not with antihuman IgA, IgM, or IgE heavy chain Abs or with antihuman λ Ig light chain Ab (Fig 1D), consistent with monoclonal ARH-77 cells that secrete IgG κ. These findings are illustrative of the characteristic monoclonal specificity observed in tumor cells within bone implants of SCID-hu mice injected with other MM cells: IgA λ, λ, and IgE λ for OCI-My5, RPMI-8226, and U-266 MM cells, respectively. Control murine BM and splenic cells were not stained with these Abs, confirming that they are not crossreactive with murine cells.
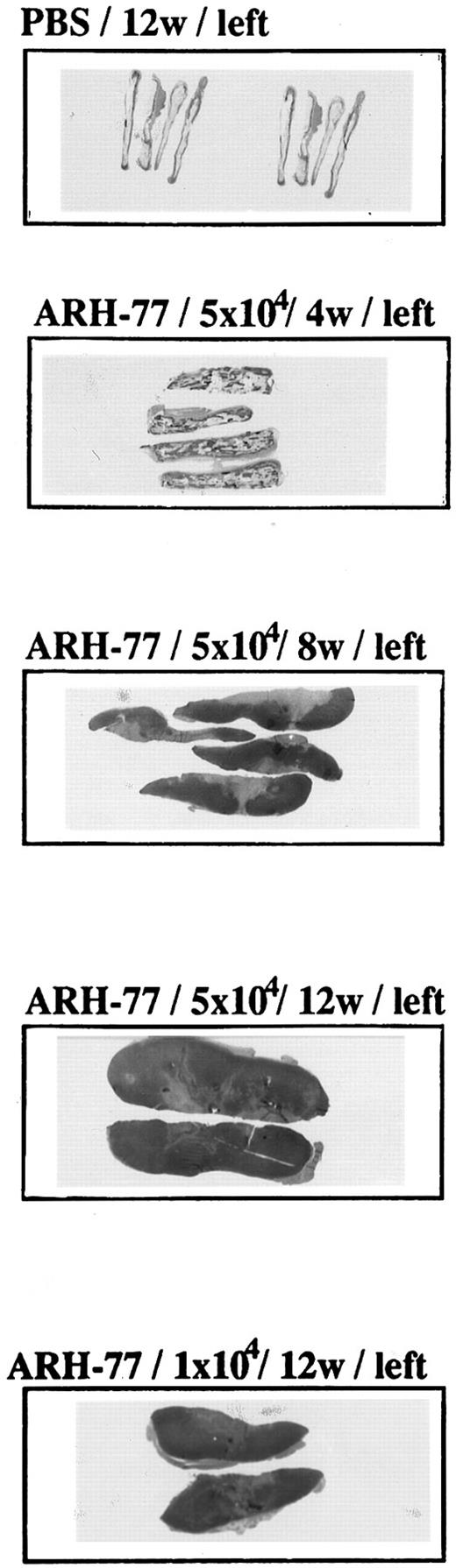
Time- and dose-dependent growth of tumor cells in fetal bone implants.The size of tumor within the left bone implants, as well as the extent of local infiltration, correlated with number of MM cells in the innoculum (5 × 104 > 1 × 104) and with time between injection of MM cells and killing of the animals (12 weeks > 8 weeks > 4 weeks; Fig 2).
Time- and dose-dependent growth of tumor cells in fetal bone implants. ARH-77 MM cells (1 × 104 or 5 × 104) or PBS was injected directly into the marrow cavity of the left fetal human bone implant of previously irradiated (400 cGy) SCID-hu mice. Mice were killed 4, 8, and 12 weeks later, and macroscopic sections of left human bone implants were stained with H&E.
Time- and dose-dependent growth of tumor cells in fetal bone implants. ARH-77 MM cells (1 × 104 or 5 × 104) or PBS was injected directly into the marrow cavity of the left fetal human bone implant of previously irradiated (400 cGy) SCID-hu mice. Mice were killed 4, 8, and 12 weeks later, and macroscopic sections of left human bone implants were stained with H&E.
Metastasis of MM cells to the right bone implant within SCID-hu mice.We next examined for homing of MM cells to the right bone implant in SCID-hu mice that had been treated with irradiation (400 cGy); injected directly into the left bone implant with ARH-77, OCI-My5, U-266, or RPMI-8226 MM cells (1 × 104 to 5 × 104) or PBS; and killed at 4, 8, and 12 weeks. Sites examined for tumor spread included not only the right bone implant, but also murine BM within vertebra and long bones, spleen, liver, thymus, kidney, lung, vertebrae, spinal cord, and brain. At intervals of 4 to 8 weeks after MM cell injection, no spread of tumor beyond the left bone implant was observed (data not shown). However, at 12 weeks after the injection of each of these MM cell lines (5 × 104 cells), characteristic tumor cell infiltration within the BM of the right bone implant was observed. Histologic findings in right-sided implants 12 weeks after innoculation of 5 × 104 ARH-77 cells into the left bone implants of SCID-hu mice (Fig 3A and B) are illustrative of findings in animals injected with OCI-My5, U-266, or RPMI-8226 cells. Immunoperoxidase staining of the right bone implants with antihuman IgG, IgA, and IgE heavy chain Abs, as well as antihuman κ or λ Ig light chain Abs, confirmed monoclonality of tumor cells. For example, predominant IgG κ staining was noted in right bone implants from animals injected in the left implant with ARH-77 MM cells (Fig 3C). Tumor cells were rarely observed in blood vessels (Fig 3D). Additional spread of focal tumor formation was observed at 12 weeks: ARH-77 MM cells within the mediastinum (Fig 3E), peritoneum, and epidural space; and OCI-My5 MM cells within the peritoneum. Importantly, no spread of ARH-77, U-266, and RPMI-8226 MM cells to murine BM was observed at any time (Fig 3F ), except for focal spread of tumor cells to vertebral BM of SCID-hu mice 12 weeks after injection with 5 × 104 OCI-My5 MM cells. Although scattered isolated MM cells were present in lung, kidney, liver, and heart, there was no evidence of tumor formation at these sites. All metastatic lesions were shown to be monoclonal, based on pattern of immunoperoxidase staining using antihuman IgG, IgM, IgA, IgE, κ and λ Abs, and were identical to the tumor cells previously injected into the left bone implants.
Metastasis of MM cells within SCID-hu mice. SCID-hu mice were treated with irradiation (400 cGy), injected directly into the marrow cavity of the left fetal human bone implant with 5 × 104 ARH-77 MM cells, and killed 12 weeks later. H&E (original magnification × 100 [A] and × 400 [B]) and immunoperoxidase staining with anti-κ Ig Ab (original magnification × 100 [C] and × 1,000 [D]) of the right-sided human fetal bone marrow implant is shown.
Mediastinum (original magnification × 40 [E]) and murine vertebral BM (original magnification × 400 [F ]) were also stained with H&E.
Metastasis of MM cells within SCID-hu mice. SCID-hu mice were treated with irradiation (400 cGy), injected directly into the marrow cavity of the left fetal human bone implant with 5 × 104 ARH-77 MM cells, and killed 12 weeks later. H&E (original magnification × 100 [A] and × 400 [B]) and immunoperoxidase staining with anti-κ Ig Ab (original magnification × 100 [C] and × 1,000 [D]) of the right-sided human fetal bone marrow implant is shown.
Mediastinum (original magnification × 40 [E]) and murine vertebral BM (original magnification × 400 [F ]) were also stained with H&E.
Growth of human MM cells within SCID-hu mice triggers human monoclonal Ig secretion.To further confirm the growth of human MM cells within the SCID-hu mice, we next examined for human monoclonal proteins in the sera of mice at similar intervals after the injection of MM cells into the left bone implants. We therefore next measured levels of human IgG and IgA in sera of SCID-hu mice by ELISA (Fig 4). Although human IgG or IgA were undetectable in sera of SCID-hu mice injected with PBS, U-266 MM cells (IgE secreting), or RPMI-8226 MM cells (λ secreting), those mice injected with ARH-77 MM cells had low levels of human IgG but not IgA at 4 weeks, which increased at 8 and 12 weeks. In a parallel fashion, mice injected with OCI-My5 MM cells had detectable human IgA but not IgG in sera at 4 weeks, which increased at 8 and 12 weeks.
Growth of human MM cells within SCID-hu mice triggers human monoclonal Ig secretion. ARH-77 and OCI-My5 MM cells were injected directly into the left human fetal bone implant in SCID-hu mice, and sera samples were taken from these mice 4, 8, and 12 weeks later. Human IgG (▪) in sera from mice injected with ARH-77 MM cells and human IgA (▧) in sera from mice injected with OCI-My5 MM cells were quantitated by ELISA.
Growth of human MM cells within SCID-hu mice triggers human monoclonal Ig secretion. ARH-77 and OCI-My5 MM cells were injected directly into the left human fetal bone implant in SCID-hu mice, and sera samples were taken from these mice 4, 8, and 12 weeks later. Human IgG (▪) in sera from mice injected with ARH-77 MM cells and human IgA (▧) in sera from mice injected with OCI-My5 MM cells were quantitated by ELISA.
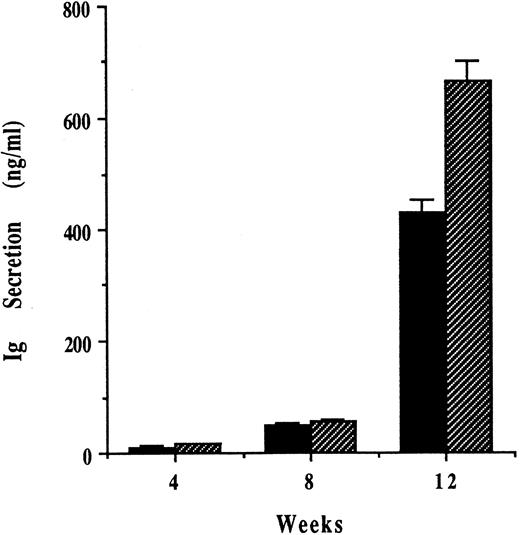
Growth of human MM cells within SCID-hu mice triggers human IL-6, but not human IL-1β and TNF-α secretion.The levels of human IL-6, IL-1β, and TNF-α in mouse serum were next examined by ELISA. Human IL-6 remained undetectable in SCID-hu mice before and at 4, 8, and 12 weeks after injection with PBS. In contrast, low levels of human IL-6 were present in sera of SCID-hu mice at 4 weeks after injection of RPMI-8226 MM cells and increased at 8 and 12 weeks after tumor cell injection, correlating with spread of disease beyond the left bone implant (Fig 5A). For each of the 4 MM cell lines studied, human IL-6 secretion into mouse sera was greater in animals injected with 5 × 104 than with 1 × 104 MM cells (Fig 5B). Neither human IL-1β nor human TNF-α were detectable in the sera of mice under any conditions. Finally, IL-6 was undetectable in day-1, -2, -3, and -7 supernatants of these 4 cell lines (data not shown).
Growth of human MM cells within SCID-hu mice triggers human IL-6 secretion. RPMI-8226 MM cells (1 × 104) were injected directly into the marrow cavity of the left human fetal bone implant in SCID-hu mice, and secretion of human IL-6 in mouse sera at 4, 8, and 12 weeks was determined by ELISA (A). ARH-77, OCI-My5, U-266, and RPMI-8226 MM cells ([▧] 1 × 104 MM cells; and [] 5 × 104 MM cells) were also injected directly into the marrow cavity of the left human fetal bone implant in SCID-hu mice, and the levels of human IL-6 in sera samples taken from these mice 12 weeks later were measured by ELISA (B).
Growth of human MM cells within SCID-hu mice triggers human IL-6 secretion. RPMI-8226 MM cells (1 × 104) were injected directly into the marrow cavity of the left human fetal bone implant in SCID-hu mice, and secretion of human IL-6 in mouse sera at 4, 8, and 12 weeks was determined by ELISA (A). ARH-77, OCI-My5, U-266, and RPMI-8226 MM cells ([▧] 1 × 104 MM cells; and [] 5 × 104 MM cells) were also injected directly into the marrow cavity of the left human fetal bone implant in SCID-hu mice, and the levels of human IL-6 in sera samples taken from these mice 12 weeks later were measured by ELISA (B).
Deposition of human Ig light chain in kidney of SCID-hu mice.We next examined the kidneys of SCID-hu mice injected with MM cells (5 × 104 cells) and killed at 12 weeks. Although no MM cells were noted in the kidneys of these animals, characteristic specific renal tubular staining for human κ or λ light chain was observed in mice injected with ARH-77 or U-266, RPMI-8226, and OCI-My5 MM cells, respectively. For example, λ and κ tubular staining in animals injected with U-266 cells is shown in Fig 6A and B, respectively. Staining was predominant in the proximal tubules as reabsorption droplets, with less staining in collecting tubules and ducts. Glomerular staining or tumor formation within the renal parenchyma was not observed.
Growth of human MM cells within SCID-hu mice triggers monoclonal Ig light chain renal tubular staining. U-266 MM cells (5 × 104) were injected directly into the marrow cavity of the left human fetal bone implant in SCID-hu mice. Mice were killed 12 weeks later and renal tissue was stained with rabbit antihuman λ (original magnification × 100 [A]) and κ (original magnification × 100 [B]) immunoperoxidase Abs.
Growth of human MM cells within SCID-hu mice triggers monoclonal Ig light chain renal tubular staining. U-266 MM cells (5 × 104) were injected directly into the marrow cavity of the left human fetal bone implant in SCID-hu mice. Mice were killed 12 weeks later and renal tissue was stained with rabbit antihuman λ (original magnification × 100 [A]) and κ (original magnification × 100 [B]) immunoperoxidase Abs.
DISCUSSION
MM remains incurable, with a median survival at best of only 48 months regardless of whether a single agent or combination chemotherapy is used, and there is a great need for novel treatment approaches. The establishment of a murine model reflecting the pathophysiology of human MM in vivo is required not only for enhanced understanding of the mechansims of tumor cell homing and localization to the BM and disease progression, but also for the development and testing of potential novel treatment approaches. To date, SCID mice have been used to establish a human MM mouse model. Feo-Zuppardi et al19 first reported growth of human MM cells in SCID mice after intraperitoneal injection of 2 to 10 × 106 patient MM cells, evidenced morphologically and by secretion of monoclonal Ig. In this model, most human plasmacytes were localized in the peritoneal cavity, without metastasis to murine BM. Others have shown sustained production of human antibodies by B cells after intraperitoneal transfer of normal human peripheral blood mononuclear cells into SCID mice.47 Therefore, growth within the peritoneal cavity of SCID mice may not require a malignant phenotype. Huang et al20 next showed the disseminated growth of ARH-77 MM cells after intravenous injection of 107 ARH-77 MM cells into irradiated SCID mice. Disease manifestations included hind limb paralysis due to infiltration of tumor cells into the thoracolumbar vertebrae. Subsequently, these investigators evaluated the impact of Ab directed against intercellular adhesion molecule-1 (anti-CD54) on tumor cell growth in this model.21 More recently, the SCID mouse model of MM has proven useful for characterizing MM bone disease.22 After injection of 106 ARH-77 MM cells intravenously, hind limb paralysis was observed, as noted previously20,21; however, of great interest was the development of bone disease manifest by lytic bone lesions and hypercalcemia. In this study,22 there was diffuse spread of tumor to murine BM and other organs, but no increase in either murine or human cytokines, such as IL-6, IL-1, or TNF-α. Most recent reports suggest the utility of the MM SCID mouse model for evaluation of therapies for drug resistant MM48 and of gene therapy approaches for MM.49
Multiple prior studies have defined mechanisms of MM cell binding to BMSCs in vitro,1-18 but these studies depend on the growth of BMSC cultures, which may not reflect the cellular composition or adhesion molecule profile of BMSCs in vivo. In the current study, we used the SCID-hu mouse model, which has been used to evaluate human infectious diseases, hematopoiesis, malignant cell growth, and gene therapies.36-42 In contrast to all prior studies, the present model allows for the evaluation of the homing and adhesion of human MM cells to human BMSCs, because it is the only model with human fetal bone grafts in SCID mice. In our study, as few as 1 × 104 MM cells injected directly into the fetal bone implant grew in SCID-hu mice. Importantly, after MM cells were established in the left human bone implant, as evidenced by marked infiltration of neoplastic plasma cells, tumor cells subsequently homed and spread to human BM within the right-sided human bone implants. In our study, immunoperoxidase staining of tumor cells, growing initially within left bone implants and subsequently in right bone implants, confirmed monoclonality consistent with tumor cells in the innoculum. Moreover, the level of serum monoclonal Ig, which was undetectable in control SCID-hu mice, correlated with tumor cell mass. In contrast to other studies, growth of human MM cells was rarely found in murine BM in SCID-hu mice, suggesting an affinity of human MM cells for human BM. Moreover, the presence of isolated MM cells without tumor formation in lung, kidney, liver, heart, and blood vessels further suggests that tumor cells circulate but grow only in specific environments in our SCID-hu model. We also confirmed MM cell growth and related limb paralysis in SCID mice injected intravenouly with higher numbers (107) of MM cells, as noted in prior reports.20-22 However, in the present study, the subcutaneous injection of fewer (104) MM cells did not result in tumor growth, suggesting that both cell doses and mode of innoculation may affect MM cell growth in SCID mice. These observations suggest that species-specific interactions between BMSCs and MM cells may exist allowing for the homing of human MM cells to human BMSCs and further highlight the importance of our model to understand migration and localization of human MM cells within the human BM microenvironment.
We and others have shown that adherence of MM cells to BMSCs in vitro can not only localize tumor cells in the BM microenvironment, but also trigger IL-6 secretion.1,2,15 This is related, at least in part, to induction of IL-6 transcription in BMSCs conferred through NF-κB.2 In the current study, metastasis of MM cells was observed only at 12 weeks, after extensive localized tumor cell growth in the left implant, and was accompanied by elevation of human IL-6 in mouse serum. The current SCID-hu MM mouse model therefore may allow for isolation and characterization of those human MM cells responsive to IL-6, as well as study of the mechanisms regulating IL-6 production in the marrow environment in vivo. Prior MM models in SCID mice that lack human BMSCs cannot be used for these studies, because murine IL-6 does not act on human cells. In an initial attempt to define triggers of IL-6 secretion in our model, we assayed for human IL-1β and TNF-α within the sera of SCID-hu mice bearing human MM cells, because both can be produced by MM cells and/or BMSCs and can upregulate IL-6 gene transcription. In addition, IL-1β and TNF-α increase inflammation and stimulate IL-6 production.50 Our data show that the observed elevations of human IL-6 in sera of SCID-hu mice were not accompanied either by increases in human IL-1β and TNF-α or by inflammation around bone implants and other organs. Our in vitro studies suggest that these MM cells do not secrete IL-6 and that adherence of MM cells to BMSCs triggers IL-6 secretion primarily from BMSCs.1 Future studies using this model will delineate in vivo mechanisms regulating IL-6 production in the marrow microenvironment and specifically define whether human tumor cells adhering to human BMSCs activate NF-κB and IL-6 transcription in BMSCs in vivo, as is noted in vitro.2 Finally, IL-6 is also a survival factor for MM cells because it can prevent tumor cell apoptosis triggered by dexamethasone, serum starvation, and Fas.25-28 It is possible that the elevated levels of serum human IL-6 seen in the SCID-hu mouse model may prevent apoptosis of MM cells within the BM as well as at distant sites. This model may therefore be useful for defining the critical role of the human BM microenvironment not only in supporting localized growth and survival of MM cells in BM, but also in the progression and dissemination of disease.
In a recent study, Ahsmann et al23 have suggested that the simultaneous introduction of tumor and accessory cells from peripheral blood or BM facilitates MM cell engraftment in SCID mice. In our study, the lack of tumor cell growth in SCID mice, coupled with growth of tumor cells in SCID-hu mice, also highlights the importance of the human BM microenvironment to facilitate human MM cell growth. Taken together, these studies further suggest that factors may exist in SCID mice that inhibit MM cell growth but that can be overcome by human BM cells. In SCID mice lacking a human bone microenvironment, for example, it is possible that most MM cells in the innoculum are rapidly cleared by the mouse reticuloendothelial system.51 In this case, the human bone implant within SCID-hu mice may counteract tumor cell clearance by providing a supportive microenvironment to which human MM cells home by specific interactions mediated by adhesion molecules. Alternatively, it is possible that the human BM microenvironment within SCID-hu mice may be overcoming MM growth-suppressive factors present in SCID mice, because the MM cells used in this study grow rapidly in vitro without the need for specific growth factors.43 Ongoing studies will define the advantages of the SCID-hu model for tumor cell growth, using IL-6–dependent and IL-6–independent cell lines as well as freshly isolated patient tumor cell samples.
In our study, characteristic monoclonal tubular staining for κ or λ Ig light chains was observed in renal tubules, without involvement of glomerulae, at 12 weeks after MM cell injection into SCID-hu mice. Although we did not monitor renal function in these animals, myeloma nephropathy is characterized by the tubular deposition of monoclonal light chains. Prior animal models of renal disease in MM have injected Bence Jones proteins intraperitoneally into mice and shown that particular proteins produce distinctive types of protein deposition in renal tissue.52 In addition, IL-6 transgenic mice develop progressive renal pathology characterized initially by glomerulonephritis, followed by focal glomerulosclerosis, and finally by extensive tubular damage, reflecting changes observed in patients at terminal stages of MM.53 Ongoing studies will determine whether this SCID-hu human MM cell model, if observed at longer intervals after tumor cell injection, may mimic pathological changes and renal dysfunction characteristic of MM kidney.
Supported by National Institutes of Health Grant No. CA 50947.
Address reprint requests to Kenneth C Anderson, MD, Dana-Farber Cancer Institute, 44 Binney St, Boston, MA 02215.

![Fig. 1. Human MM cells grow in fetal human bone implants within SCID-hu mice. SCID-hu mice were treated with irradiation (400 cGy), injected directly into the marrow cavity of the left fetal human bone implant with 5 × 104 ARH-77 MM cells or with PBS, and killed 12 weeks later. H&E-stained tissue sections of the left human fetal bone marrow implant from SCID-hu mice injected with ARH-77 MM cells (original magnification × 100 [A]) or PBS (original magnification × 40 [B]) are shown. / Immunoperoxidase staining of the same human fetal bone marrow implant from SCID-hu mice injected with ARH-77 MM cells was also performed using antihuman κ (original magnification × 100 [C]) and antihuman λ (original magnification × 100 [D]) Ig light chain Abs.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/2/10.1182_blood.v90.2.754/4/m_bl_0023f1a.jpeg?Expires=1769423242&Signature=cbpzCGNcfTzkDal2BH0PHGEGDnV8VXLwJiwSF9wfv44UgtRlP4eXI~RizBV4gxgD~W0V9lnTc5R1f24P9LiAa4W3A~YlYYCFjz~07wIgSoxTlIpFtoE-ydW7CoSaVB47nGfqpHD3iBa2dLAXNKDLU2rYG0Z-gW1Xk9VNa6lblzKU5lPO88m~V6bn94oyhAc2CozAGkKF~WYS0yYQNdF~NcOiHRGq~ogK1NrosLybRYzRV2lvuFrqVrbukucANed2nDoVooKXCnH9JIXREkmgs-fuDt96pjI8QliSQMO3B-PHJmEFkB6P4cKmclJNP4wraafuJ7Z2ta3SUOZ-FI5M5Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. Human MM cells grow in fetal human bone implants within SCID-hu mice. SCID-hu mice were treated with irradiation (400 cGy), injected directly into the marrow cavity of the left fetal human bone implant with 5 × 104 ARH-77 MM cells or with PBS, and killed 12 weeks later. H&E-stained tissue sections of the left human fetal bone marrow implant from SCID-hu mice injected with ARH-77 MM cells (original magnification × 100 [A]) or PBS (original magnification × 40 [B]) are shown. / Immunoperoxidase staining of the same human fetal bone marrow implant from SCID-hu mice injected with ARH-77 MM cells was also performed using antihuman κ (original magnification × 100 [C]) and antihuman λ (original magnification × 100 [D]) Ig light chain Abs.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/2/10.1182_blood.v90.2.754/4/m_bl_0023f1b.jpeg?Expires=1769423242&Signature=SlFd4GoQY-j09w0Ant9OORYAhr2Mou5sXqLGbAoARsS1N2QkI1NV9iicv5KsX6sq9Br9hsH2IUEgGDkfALWunXGmFzRWq~CW8q2yCZ7oK2ag2v1fgNtAs0NtZ1~s7PIz-mTnQdfH~jb6mA-VU3VQB1y9t47CRg0r72JZ3b~6cAtK9RI8lS0DJhlZtVGpzJHItEVDrrNZxQc0pKL3bMTAfiEr9iXZ59WZmQSDgnxn0~RzO2k59d31gZu-GmcUDJDw4OPQrDFp4KNMHu6el-nUri-aMLt9G~Wt~P3I3UxnpYM7~X9fc1OJqOj1lwokuPT~o~xDg3kt1AglmD0zJMjRzw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. Human MM cells grow in fetal human bone implants within SCID-hu mice. SCID-hu mice were treated with irradiation (400 cGy), injected directly into the marrow cavity of the left fetal human bone implant with 5 × 104 ARH-77 MM cells or with PBS, and killed 12 weeks later. H&E-stained tissue sections of the left human fetal bone marrow implant from SCID-hu mice injected with ARH-77 MM cells (original magnification × 100 [A]) or PBS (original magnification × 40 [B]) are shown. / Immunoperoxidase staining of the same human fetal bone marrow implant from SCID-hu mice injected with ARH-77 MM cells was also performed using antihuman κ (original magnification × 100 [C]) and antihuman λ (original magnification × 100 [D]) Ig light chain Abs.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/2/10.1182_blood.v90.2.754/4/m_bl_0023f1c.jpeg?Expires=1769423242&Signature=eO7-sRRP-qouC8FtlL3ytWLr4BfFNzVJyZE9bTHPASXztItF54dVhzF6j3u3Kgzf6RX3mmigPyg7VX09iJmbO6JZBaTlpvTaiMA-TvG~9QvscImYts7sAbmg7qPPchbb7fMwvpCTIF5fqVnJmOz1ENRCtaE3dVcUwkqZg~1CxbrCatiRS3zOx-dO0LNl7AePq1Gkztn9RxXrN12pvTdUPha9zQxqYNLeiC9yyiPfrkVwyOjxouOXxldpTiNxRR10L~n8Hqy1ZQtqCleHyWChl9QCESVpLIOdZ0kKW1gExh4OiRK7~Wr~3C5aBprX5Iwu-Z~eVRlvt67iEGhMQNiZKg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. Human MM cells grow in fetal human bone implants within SCID-hu mice. SCID-hu mice were treated with irradiation (400 cGy), injected directly into the marrow cavity of the left fetal human bone implant with 5 × 104 ARH-77 MM cells or with PBS, and killed 12 weeks later. H&E-stained tissue sections of the left human fetal bone marrow implant from SCID-hu mice injected with ARH-77 MM cells (original magnification × 100 [A]) or PBS (original magnification × 40 [B]) are shown. / Immunoperoxidase staining of the same human fetal bone marrow implant from SCID-hu mice injected with ARH-77 MM cells was also performed using antihuman κ (original magnification × 100 [C]) and antihuman λ (original magnification × 100 [D]) Ig light chain Abs.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/2/10.1182_blood.v90.2.754/4/m_bl_0023f1d.jpeg?Expires=1769423242&Signature=xt8~oq-DaO5YYOt0sfLA0Qw2KyN-hgYrA75b4Qr1vQDsAEkSezU898vC61yzz0x9~EDx~JbVB~G~hMzYkXilKh9Y8zzW2hBJCiF1~QHM-UKqU9KqM4Pf4-CQLGe8OsYBpIvCa46EIisqCLKIetMJ7hPL-TOgclBVpXEGKlXcrpnoivJrSIqK6TgK9z~TU5FUiMOWxbdqMw0s8FXMsTgEdNb-L5UiF8R7gZvv2lxv5WMFQOKTNL68yHv9zfPEAglmz4KRNOKLI9Zk01wdYfRvOy~8WisAuJsYBdY-SbJ~BWI8HVlR552XQ20TTsOwSaJ5t0HczEu~MpsVmCoNhwjDyQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Metastasis of MM cells within SCID-hu mice. SCID-hu mice were treated with irradiation (400 cGy), injected directly into the marrow cavity of the left fetal human bone implant with 5 × 104 ARH-77 MM cells, and killed 12 weeks later. H&E (original magnification × 100 [A] and × 400 [B]) and immunoperoxidase staining with anti-κ Ig Ab (original magnification × 100 [C] and × 1,000 [D]) of the right-sided human fetal bone marrow implant is shown. / Mediastinum (original magnification × 40 [E]) and murine vertebral BM (original magnification × 400 [F ]) were also stained with H&E.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/2/10.1182_blood.v90.2.754/4/m_bl_0023f3a.jpeg?Expires=1769423242&Signature=Vba2ka8hJrhsO9dUUfse1vsc3WRTfHmDx3MQN48wWe~BqmBTd3tnd25TTeTnkhm5jgcMJaQhXJnDvU-e123Ez0kWSEJuecw~U0SXggbG4j8LS~3fDfunk2oFOOvF-6JqpAqRC6QmWfwtzNyHv~l81iDzZpyIvFXrHkR9jn3H~-hRRrRffY29paDO1ZAhQvioeazO-KALONwwOhREYOe7K-z86ZcqnAq7qUMIHQKs7FRHAF8EWdJqEHkb-gU67tw4iN2fgsL4Lu3yMX9XNnhmXIgItuqTlF4uPBda6PTtWI3vK7hyZWEHEfDdmd8yoVjbH0SPxss6~MJqESxvLhbqoQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Metastasis of MM cells within SCID-hu mice. SCID-hu mice were treated with irradiation (400 cGy), injected directly into the marrow cavity of the left fetal human bone implant with 5 × 104 ARH-77 MM cells, and killed 12 weeks later. H&E (original magnification × 100 [A] and × 400 [B]) and immunoperoxidase staining with anti-κ Ig Ab (original magnification × 100 [C] and × 1,000 [D]) of the right-sided human fetal bone marrow implant is shown. / Mediastinum (original magnification × 40 [E]) and murine vertebral BM (original magnification × 400 [F ]) were also stained with H&E.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/2/10.1182_blood.v90.2.754/4/m_bl_0023f3b.jpeg?Expires=1769423242&Signature=XMvy0drYjapA7g-8TzUfg2qhF14dwoaAmTE6OGsWbWkOTLZgqImxybRpLzxyzi3c27Msrs0Kn~uPHYkfeG~gvXGZVs3p3vGnEIjkmCVQLyFDYD6yBvPCjmXSz3upqlo59oyVo~tRPq2R0nVPK2CCuyxX35MO1g75OZVvvIs9bl1eo9RgEF0OSK~UY5Z5AgWqBLQ2QgAT3m6UtoV7tLlyzHTKxguW61Pm0VSASoDRkdC1X-rnlkVhn8t7I3phszbR6yO6yjzfl67gOjGpkH5mKbHQpFBGBqkAmU1ODaxhIqK41W3qbCKnK4Rem9-AtrKiF0LwHMfTlV6Mns1rEHsQPQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Metastasis of MM cells within SCID-hu mice. SCID-hu mice were treated with irradiation (400 cGy), injected directly into the marrow cavity of the left fetal human bone implant with 5 × 104 ARH-77 MM cells, and killed 12 weeks later. H&E (original magnification × 100 [A] and × 400 [B]) and immunoperoxidase staining with anti-κ Ig Ab (original magnification × 100 [C] and × 1,000 [D]) of the right-sided human fetal bone marrow implant is shown. / Mediastinum (original magnification × 40 [E]) and murine vertebral BM (original magnification × 400 [F ]) were also stained with H&E.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/2/10.1182_blood.v90.2.754/4/m_bl_0023f3c.jpeg?Expires=1769423242&Signature=W-Jrk5c~tchJnLf2Xk5U3yz~LGGmXj0DQ2eASQ1zy22gj5boVOOxAY-Io2~lJWm5bqVh7ZL4IAtA~H3KPapH~G5PSlcy-grrWc3w~NOrCK55R~NstGfxMwtrxBVzCxEZx68UavbEXptgMuV6Klzjm3VOi-AsUbV26Oja67XhoCdxgl~piGMroS1Ba6K6b8Lyl23k-o~U2et1TYzEmpVqUsvn1fnF8eUp0~I~0EpAs33ImlCl3rWw2vSfnFTmBJED0VIhvWIaLBlL81MwGZnx9q-7e2FRG-75BaZthJ6GdVWlDISfsd6ctb4Oxh8Q9uim-lPe1a5PjtoSKYYPrJNXKA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Metastasis of MM cells within SCID-hu mice. SCID-hu mice were treated with irradiation (400 cGy), injected directly into the marrow cavity of the left fetal human bone implant with 5 × 104 ARH-77 MM cells, and killed 12 weeks later. H&E (original magnification × 100 [A] and × 400 [B]) and immunoperoxidase staining with anti-κ Ig Ab (original magnification × 100 [C] and × 1,000 [D]) of the right-sided human fetal bone marrow implant is shown. / Mediastinum (original magnification × 40 [E]) and murine vertebral BM (original magnification × 400 [F ]) were also stained with H&E.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/2/10.1182_blood.v90.2.754/4/m_bl_0023f3d.jpeg?Expires=1769423242&Signature=iBWVBZjlCKMnOAG95rV7XMd7fDdj71JwYY24CtVTmS1UgZO9snpZFQNWNM1UHiUPqRlo0yI7e90MDN9xeJ2k8v1buVjz3cvBCY49YH3ASmpHu4N01Etagep6NfxnMoW28OKz4iHYdXjGyPzrZxxpvWCGpiYd66z31varGfDQxRoRrmf~VFxlCeY7SlOgYa~ocqmLYhId38HIf1ShWOWmwhnlKxg4GJjGxTXmUmttaMPkku0xtrXqJz0dbRtiXGkzezbw0lK6Egv-ycASqx2qgo0ctYX1odbNTF1M7Bh-4DgLrvYdKz5hXDbH3AzFdA9m4d3L79nwqI~DS7i8QaXP~g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Metastasis of MM cells within SCID-hu mice. SCID-hu mice were treated with irradiation (400 cGy), injected directly into the marrow cavity of the left fetal human bone implant with 5 × 104 ARH-77 MM cells, and killed 12 weeks later. H&E (original magnification × 100 [A] and × 400 [B]) and immunoperoxidase staining with anti-κ Ig Ab (original magnification × 100 [C] and × 1,000 [D]) of the right-sided human fetal bone marrow implant is shown. / Mediastinum (original magnification × 40 [E]) and murine vertebral BM (original magnification × 400 [F ]) were also stained with H&E.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/2/10.1182_blood.v90.2.754/4/m_bl_0023f3e.jpeg?Expires=1769423242&Signature=2hyuXDAEPT7O4CrJSsU0ctcqeDiYxoUQjadk3lsnZ9SSLaAoSxnby4NPe9pDpXeBVNLi5R9ZkM0j1jhRUeOtj0b9ulUJoBNbavgBK1PaEAtqAi39LUfRjzwEAFwNL7y4WwjLcapyGcItKUONPy2c0jd0BScDWXoEx1z-sUSv9R1dqrV6Er--~Qp6MhhaCQHXrynvRZCuJ4qnvUYkqqu8Qv1rbZwxCoRPdnyWnKDoAX6mpsi9LCPiCxkkoX6LXYTtYwGOhKWhoTeO9nTdC2Sq7p97niULyx1~H-dC7lxbjyGj2rMHF9FTb7ham3kj8iBBzwK8qNCQyWl62-hEGU8XeQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Metastasis of MM cells within SCID-hu mice. SCID-hu mice were treated with irradiation (400 cGy), injected directly into the marrow cavity of the left fetal human bone implant with 5 × 104 ARH-77 MM cells, and killed 12 weeks later. H&E (original magnification × 100 [A] and × 400 [B]) and immunoperoxidase staining with anti-κ Ig Ab (original magnification × 100 [C] and × 1,000 [D]) of the right-sided human fetal bone marrow implant is shown. / Mediastinum (original magnification × 40 [E]) and murine vertebral BM (original magnification × 400 [F ]) were also stained with H&E.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/2/10.1182_blood.v90.2.754/4/m_bl_0023f3f.jpeg?Expires=1769423242&Signature=ovz6YJHPq05DUOH-KGbzCzVW8UAb29UtTqLeq0D-SApg~JzD0JG5T3yyqQ8tzlGQJ3cNANr0RO0KeE-eKp4rUXRf~QPgFlEhUtQdAUpWvt2mAzauMCHSPu8-FR0C2BTazmNjiMHj1Hu8OEgIudsNXrJu-yKuwfG-XCCms-sQ9v4AMU6v0AXDFHN6kDFu~~1ZpG0UcbbL8fFeFAQIyxRWoUa5N~9F5Gq5ZlutV4IHLxTzdwLvyl0n469dtN0DHsA0jpgf380p--xajMEP3OK12eA9TR8kzKpsU0DhgbwHCk1ZOJd6JYPfe83w3~vCLzE2bEjZzDz-233mTjnKwbvg0Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Growth of human MM cells within SCID-hu mice triggers human IL-6 secretion. RPMI-8226 MM cells (1 × 104) were injected directly into the marrow cavity of the left human fetal bone implant in SCID-hu mice, and secretion of human IL-6 in mouse sera at 4, 8, and 12 weeks was determined by ELISA (A). ARH-77, OCI-My5, U-266, and RPMI-8226 MM cells ([▧] 1 × 104 MM cells; and [] 5 × 104 MM cells) were also injected directly into the marrow cavity of the left human fetal bone implant in SCID-hu mice, and the levels of human IL-6 in sera samples taken from these mice 12 weeks later were measured by ELISA (B).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/2/10.1182_blood.v90.2.754/4/m_bl_0023f5a.jpeg?Expires=1769423243&Signature=DPzwuKUyHk5-waBlc2tWD~smhz0z7a0MvqaZ-8RBmXz0OW2QlkewaFnejcLsCHBWTtmOM0R8aJlIonFY~V63b6OPeuHv7-n6lJ45LMSVrmyqDUuNboyT~YbW4kuK47JkTrUWmO7nDGojKEOStKqqMSrSLDR8QuiT2LV76MedWE8ZeXQpkPmmIKm1op5oPRTwvDH4Rm4-XxcWRH3Q3LumZakSvENlMUU2Y5h9wn9eBMji99z2hnmcs71QG~EcDKnx1f1hZ6d0EL0L1AFJh93u0uqn1sbt6LylEFEe1L5H3RZJ7Bs~g48WrgE5MMtoxdDA1OI41ynR56iXumLRfNs01Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Growth of human MM cells within SCID-hu mice triggers human IL-6 secretion. RPMI-8226 MM cells (1 × 104) were injected directly into the marrow cavity of the left human fetal bone implant in SCID-hu mice, and secretion of human IL-6 in mouse sera at 4, 8, and 12 weeks was determined by ELISA (A). ARH-77, OCI-My5, U-266, and RPMI-8226 MM cells ([▧] 1 × 104 MM cells; and [] 5 × 104 MM cells) were also injected directly into the marrow cavity of the left human fetal bone implant in SCID-hu mice, and the levels of human IL-6 in sera samples taken from these mice 12 weeks later were measured by ELISA (B).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/2/10.1182_blood.v90.2.754/4/m_bl_0023f5b.jpeg?Expires=1769423243&Signature=pcaSquNmxJD8za2By43~p1o4lHkQY-FtFuEtu-FdRSp71SARkY5Irj1NNaFj5vPH8ohTFuFlS~HLytvCaYHfqgVwre3la8bOT56I~K83kvYsGN5ZcCS~nsbxJSsvp~AC-t4atYL0tdkFvS31OFxLzAbYX0NaQlXlk2OK~Fzu6gfXxGxtaDHyE-BXTcA3ShTkNerBWO2RAvk5JPCXX9-4WjPX-aToVCqSxq9G~ZGI-gth7sUG9XwTWZ5ubhdfhSNw~1vuMjQgOiZy2g-zmyH-Cm7vObP6O7Af6fFPcbbK912xd8XODLXdx7G1NAP3Xda8D3U70jfH8ze2AdCRXHUuMg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Growth of human MM cells within SCID-hu mice triggers monoclonal Ig light chain renal tubular staining. U-266 MM cells (5 × 104) were injected directly into the marrow cavity of the left human fetal bone implant in SCID-hu mice. Mice were killed 12 weeks later and renal tissue was stained with rabbit antihuman λ (original magnification × 100 [A]) and κ (original magnification × 100 [B]) immunoperoxidase Abs.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/2/10.1182_blood.v90.2.754/4/m_bl_0023f6a.jpeg?Expires=1769423243&Signature=J6BmoMFv0OaQWTQ5LqpZty8mNAqLN2wsMG8SKhPTgu89UV2TuokJ9nrAroUwgqz92~voiYdNseVlDLMOwzp1EMkJSoz1LM~P~Z5x21MvUz7gOt3E~PvWfiifROnaCIY2Q3bA1iGKnaHV1BUXQH~4fDSsitY84MGoGpMk8VdZrQ~22X89G6KIKPKdKqBOSJo7Z9nQ4Oi5dJXoedYlh3RuL11iBb0MbXoi2Ho8~yDsvy4ILu6BqClmASIAcgTsX~OvA81bEBvMzl3fRFwOCnaf8lBUqwmKTmqOahUPw5XDXuP6AgStCMfgM0jhhtufL4Nuvi3BvsFLcSaTzXJlikTKLw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Growth of human MM cells within SCID-hu mice triggers monoclonal Ig light chain renal tubular staining. U-266 MM cells (5 × 104) were injected directly into the marrow cavity of the left human fetal bone implant in SCID-hu mice. Mice were killed 12 weeks later and renal tissue was stained with rabbit antihuman λ (original magnification × 100 [A]) and κ (original magnification × 100 [B]) immunoperoxidase Abs.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/2/10.1182_blood.v90.2.754/4/m_bl_0023f6b.jpeg?Expires=1769423243&Signature=31PrHrGSL~MZnDIc0vUpTT5pjkG5kq0XsnAyMoWfp~5NwRj6uzxdYSclLXjtsLkcFyrRpIgCWDy6LPjmol8LtOnBKxdsg03Ag0FUtiH9EBzMAj6DxoSC0EbQXnlZLPrYJl1CQjWEelZWM84M5pWyp5UaN5ssVYBFrLCUwjKy3qN4TzZiNMv-gDCV2PADJDyl7q8hl3xiWVZd7yv7-aiGCovuQKaG~tH44lKJORK0mUFkWbvD74Sj1a7dIMekwp1s6wtI6KQeAoYfxWigvADEsvC1RIYsl~98WR8qBKKRSU3apdQjGVsw-LfE5w8zMREcULSTXpog1VFYsaGVLED0oA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal