Abstract
The expression and activity of receptor tyrosine kinases (RTK) at the cell surface can be modulated by several different pathways including the proteolytic release of the extracellular domain as a soluble receptor. We investigated the regulation of tie receptor expression, an orphan RTK restricted to cells of hematopoietic and endothelial lineages, on primary human endothelial cells and a stably transfected Chinese hamster ovary (CHO) cell line. Tie was expressed in cells as a doublet of 135 and 125 kD; the 135-kD band represented mature cell surface receptor containing sialic acid and N-linked oligosaccharide residues, whereas the 125-kD band represented an intracellular pool of immature receptor. Phorbol 12-myristate 13-acetate (PMA) had dramatic effects on tie expression at the cell surface. Within 15 minutes of PMA treatment, the 135-kD band disappeared from the cell surface and was accompanied by the appearance of a 100-kD band in cell supernatants. The 100-kD band continued to accumulate in the media throughout the duration of PMA treatment during which mature tie receptor was undetectable on the cell surface by fluorescence-activated cell sorting (FACS) or in cell lysates by immunoblot analysis. Using specific antibodies, this 100-kD species was shown to be a soluble form of the tie receptor containing the extracellular domain. PMA-dependent release of soluble tie was mediated through the activation of protein kinase C (PKC); soluble tie was not released in the presence of PKC inhibitors, an inactive PMA analog, or following the downregulation of PKC through chronic PMA treatment. These results indicate that tie receptor expression on endothelial cells is regulated by the release of a soluble extracellular fragment following activation of PKC. Parallel pathways regulating c-kit, tumor necrosis factor (TNF), and colony-stimulating factor (CSF) receptor expression suggest that the release of extracellular receptor fragments represents an alternative mechanism through which cells modulate responses to growth factors and cytokines.
THE INTEGRITY OF THE vascular endothelium is critical to the establishment and maintenance of vascular patency and normal physiology. In the healthy adult, endothelial cell turnover occurs rarely with some estimates of their half-life as high as 20 years. Consequently, the induction of endothelial cell proliferation and activation often signals the initiation of pathological processes such as wound healing, metastasis, and chronic inflammation.1-3 Understandably, the endothelial cell receptor-ligand interactions that control activation and proliferation have generated intense interest.
Receptor tyrosine kinases (RTKs) comprise a superfamily of cell surface receptors sharing structural similarities that are involved in regulating cell proliferation and activation.4 The multiple domain structure of RTKs allows activation signals received at the cell surface to be transmitted intracellularly, as well as amplified. As with most biological response modifiers, activation pathways are coupled with parallel deactivation pathways; in RTKs the intracellular domain also mediates inhibition of receptor activity. Although one pathway for receptor inhibition is through phosphotyrosine-specific phosphatase activity, recent evidence also suggests that protein kinase C (PKC) activation may play a role.5 For example, PKC-dependent phosphorylation of the epidermal growth factor receptor (EGF-R) leads to decreases in EGF binding and tyrosine kinase activity.6
Endothelial cells express several members of the RTK family.7 Stimulation of the Flk-1 receptor by vascular endothelial growth factor (VEGF) leads to endothelial cell proliferation, migration, and tubule formation in vitro. In vivo, Flk-1 activation stimulates increased vascular permeability and the induction of angiogenesis.8 Similar effects have been documented following fibroblast growth factor receptor (FGF-R) activation by basic fibroblast growth factor (bFGF).9 These results suggest a role for RTK activation in pathologic processes involving the endothelium. In the absence of angiogenesis, solid tumor growth is severely limited as a result of nutritional and oxygen deprivation.10 One way tumors circumvent this restriction is to express and secrete endothelial cell mitogens including bFGF and VEGF, in effect creating their own blood supply. The pivotal role played by endothelial RTKs in this process was demonstrated by Millauer et al11 showing that a dominant-negative mutant of Flk-1 inhibited the growth of several tumor types in vivo and by other investigators12 13 showing that neutralizing antibodies (Abs) to VEGF or FGF prevented glioblastoma growth in nude mice. Thus, RTK activation is critical in tumor progression, often entailing paracrine interactions between endothelial and tumor cells.
The tie RTK is expressed on endothelial cells and a subset of hematopoietic progenitor cells.14 Along with tek, it forms a subfamily of RTKs that possess fibronectin type III repeats, Ig loops, and EGF-like repeats. Tie is expressed on CD34+ hematopoietic stem cells and decreases during myeloid or erythroid differentiation.15 However, during megakaryocytic differentiation, a significant percentage of CD34- cells express tie. Similarly, treatment of megakaryoblastic leukemia cells with phorbol 12-myristate 13-acetate (PMA) upregulates tie expression at both the mRNA and protein levels.16 In embryos, tie expression is high in the extraembryonic and embryonic mesoderm at day 8.0, tissues that subsequently give rise to the vasculature.17 Moreover, tie knockout mice suffer from edema, hemorrhage, and a lack of vascular integrity resulting in death between E13.5 and 14.5.18,19 In adults, tie expression increases during wound healing and in proliferating ovarian capillaries after hormone-induced superovulation.20 These studies suggest that tie plays an important role in hematopoiesis and angiogenesis. Although the ligand for the tie RTK has yet to be identified, tie's tightly regulated expression suggests that it is involved in endothelial cell activation and proliferation. We examined the regulation of tie expression on endothelial cells and stably transfected Chinese hamster ovary (CHO) cells and found that a soluble, clipped form of the tie receptor is released in response to PKC activation.
MATERIALS AND METHODS
Reagents.N-Glycosidase, neuraminidase, O-glycosidase, tunicamycin, L-1-Chloro-3-[4-tosylamido]-7-amino-2-heptanone-HCl (TLCK), phosphoramidon, and staurosporin were purchased from Boehringer Mannheim (Indianapolis, IN). PMA, 3-[(3-cholamidopropyl)dimethyammonio]-1-propanesulfonate (CHAPS) and o-phenanthroline were purchased from Sigma (St Louis, MO) and 4α-PMA, GF109203X, and N-(2-Guanidinoethyl)-5-isoquinoline sulfonamide-2HCl (HA) 1004 were purchased from BioMol (Lexington, KY). Bovine serum albumin (BSA) (endotoxin- and lipid-free), benzamidine, and NCO-700 were purchased from Calbiochem (La Jolla, CA). 35S-Translabel was purchased from ICN (Irvine, CA). A recombinant fragment of the tie receptor representing the soluble extracellular domain (amino acids 305-772) was purified from the conditioned media of stably transfected CHO cells.21
Antibodies.5D2 and 21G6 are immunoprecipitating and immunoblotting monoclonal antibodies (MoAbs), respectively, which were made against the recombinant extracellular domain of tie. 42G10, a third MoAb used in fluorescence-activated cell sorting (FACS) analysis, and rabbit polyclonal antitiex were also made against recombinant extracellular tie. Balb/c mice or rabbits were inoculated subcutaneously with 45 μg recombinant tie and standard protocols were used for the production and screening of resulting hybridomas and Abs.22 SC is a rabbit polyclonal Ab made against a C-terminus peptide of tie (amino acids 1121-1138) from Santa Cruz Biochemicals (Santa Cruz, CA).
Cells.Human umbilical vein endothelial cells (HUVEC) and dermal microvascular endothelial cells (MDEC) were purchased from Cell Systems (Kirkland, WA) and maintained in growth media provided by the vendor. HUVEC and MDEC cultures were used between passages 2 and 8. CHO cells deficient in dihydrofolate reductase activity (CHO-) were transfected with a pDSRα2-based expression vector containing full-length tie cDNA by the CaPO4 method.21 23 Transfected cells were cloned and analyzed for tie receptor expression by Western blotting using MoAb 21G6. CHO cell lines stably transfected with full-length tie (CHO clones 2 and 37) or vector alone (CHO-) were grown in Dulbecco's modified Eagle's medium (DMEM) containing 10% dialyzed fetal bovine serum (FBS).
PMA treatment.Cells grown to confluency in 60 or 100 mm dishes were rinsed once in basal media (DMEM + 0.5% FBS + 1% BSA), followed by incubation, for the indicated time, in basal media containing 10 ng/mL PMA or 4α-PMA. For chronic PMA treatment, cells were incubated in 1 μmol/L PMA for 24 hours before a second stimulation, where indicated, with 10 ng/mL PMA for 1 hour. Cells were preincubated with staurosporin (100 nmol/L), GF109203X (200 nmol/L), or protease inhibitors at the indicated concentrations, for 15 minutes before the addition of 10 ng/mL PMA for 1 hour. Cells were incubated with HA 1004 (25 μmol/L), an inhibitor of protein kinase A, for 1 hour. Following these incubations, conditioned media was collected and the cell layer rinsed with cold phosphate-buffered saline (PBS) and lysed before immunoprecipitation and immunoblotting, or enzyme-linked immunosorbent assay (ELISA) analysis as described below.
Immunoprecipitations and immunoblots.Cells were lysed for 5 minutes on ice in buffer containing 50 mmol/L Tris, 100 mmol/L NaCl, 1% Triton X-100, 1 mmol/L NaVO3, and protease inhibitors (1 μg/mL leupeptin, 1 μg/mL aprotinin, 1 μg/mL pepstatin, and 10 μg/mL soybean trypsin inhibitor). Protease inhibitors and Triton X-100 to a final concentration of 1% were also added to conditioned media samples. Immunoprecipitations were performed on equivalent numbers of cells for each sample in an experiment. Lysates and conditioned media samples were centrifuged at 14,000 rpm for 10 minutes at 4°C to pellet nuclei and cell debris, and the supernatants precleared with protein G Sepharose for 30 minutes at 4°C. For immunoprecipitations, cell lysates or conditioned media were incubated with 3 μg of Ab for 1 hour at room temperature (RT). Immunocomplexes were incubated with protein G Sepharose overnight at 4°C. The immunoprecipitates were then washed three times with 1% Tx-100 lysis buffer, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Novex, San Diego, CA) and electroblotted onto polyvinylidene fluoride (PVDF ) membranes (Millipore Corp, Bedford, MA). Blots were blocked for 1 hour, incubated for an additional hour with primary Ab, and followed by a 30-minute incubation with secondary Ab conjugated to horseradish peroxidase (HRP). Bands were detected using the ECL system (Amersham, Arlington Heights, IL). For competition experiments, MoAb 5D2 was preincubated with 30 μg recombinant soluble tie for 1 hour before immunoprecipitation.
Metabolic labeling.HUVEC or CHO clone 37 cells were washed once, then incubated for 30 minutes in methionine-, cysteine-free DMEM containing 0.5% FBS and 1% BSA. 35S-Translabel (100 to 200 μCi/mL) was added for a 30-minute pulse, after which cells were washed again and incubated in complete growth medium for the indicated chase period. Cell lysates and conditioned media were collected and processed for immunoprecipitation as described above, separated by SDS-PAGE, and analyzed by autoradiography.
Glycosylation analysis.HUVECs or CHO clone 37 cells were metabolically labeled with 35S-Translabel for 2 hours. Cell lysates were prepared and immunoprecipitated with anti-tie MoAb 5D2 as described above. Immunocomplexes were resuspended in 30 μL of buffer containing 0.5% SDS, 10 mmol/L Tris pH 7, 1% β-mercaptoethanol, 1 mmol/L EDTA, 1 μg/mL leupeptin, 1 μg/mL aprotinin, 1 μg/mL pepstatin, and 10 μg/mL soybean trypsin inhibitor and boiled for 5 minutes. For N-glycosidase digestion, 16.7 U/mL N-glycosidase and 18.8 mmol/L CHAPS was added to 15 μL of boiled immunoprecipitate. For neuraminidase digestion, 0.83 U/mL neuraminidase and 18.8 mmol/L CHAPS was added to 15 μL of boiled immunoprecipitate. For combined neuraminidase and O-glycosidase digestion, 10 U/mL neuraminidase, 0.07 U/mL O-glycosidase and 18.8 mmol/L CHAPS was added to 15 μL of boiled immunoprecipitate. Digestions were performed for 20 hours at 37°C, after which 10 μL of 4x sample buffer was added, samples boiled again, and analyzed by SDS-PAGE followed by autoradiography. Mock samples were treated with buffer alone under identical conditions. Tunicamycin was used to inhibit N-linked glycosylation of the tie receptor. CHO clone 37 cells were preincubated with 5 μg/mL tunicamycin for 1.5 hours and then labeled with 35S-Translabel for 2 hours in the presence of tunicamycin. Cells were lysed and immunoprecipitated with MoAb 5D2 as described above.
Tie receptor expression on endothelial cells and transfected CHO cells. Cell lysates were prepared as described in Materials and Methods and immunoprecipitated with anti-tie MoAb 5D2. Immunoprecipitates were analyzed by SDS-PAGE and immunoblotted with another anti-tie MoAb, 21G6. Molecular weight markers as indicated. CHO-, CHO cells transfected with vector alone; CHO, cl.2, CHO cells transfected with full-length tie receptor, clone 2; CHO, cl.37, CHO cells transfected with full-length tie receptor, clone 37; HUVEC, human umbilical vein endothelial cells; MDEC; human microvascular dermal endothelial cells. Double arrows, tie receptor doublet.
Tie receptor expression on endothelial cells and transfected CHO cells. Cell lysates were prepared as described in Materials and Methods and immunoprecipitated with anti-tie MoAb 5D2. Immunoprecipitates were analyzed by SDS-PAGE and immunoblotted with another anti-tie MoAb, 21G6. Molecular weight markers as indicated. CHO-, CHO cells transfected with vector alone; CHO, cl.2, CHO cells transfected with full-length tie receptor, clone 2; CHO, cl.37, CHO cells transfected with full-length tie receptor, clone 37; HUVEC, human umbilical vein endothelial cells; MDEC; human microvascular dermal endothelial cells. Double arrows, tie receptor doublet.
Cell surface immunoprecipitation.Cells were removed from culture dishes with PBS containing 5 mmol/L EGTA and 5 mmol/L EDTA at room temperature for 5 minutes. The intact cells were washed twice with cold PBS containing 1% BSA and incubated with 50 μg/mL MoAb 5D2 on ice for 90 minutes. After extensive washing in PBS containing 1% BSA, cells were lysed in 1% Triton X-100 lysis buffer. Lysates were precleared and immunocomplexes captured with protein G Sepharose as described above.
FACS analysis.HUVECs treated with PMA for the indicated times were washed with ice cold PBS and harvested using versine-EDTA. Cells were washed twice with PBS/0.5% FBS before being plated, as a single cell suspension, into 96-well U-bottom plates at 5 × 105 cells/well. After centrifugation, cell pellets were resuspended and incubated with MoAb 42G10 conjugated to phycoerythrin for 45 minutes on ice. Cells were washed twice more before analysis on a FACScan flow cytometer (Becton Dickinson, Milpitas, CA).
ELISA assays.Immulon 4 plates (Dynatech, Chantilly, VA) were coated for 4 hours at RT with 2 μg/mL polyclonal anti-tiex and blocked overnight with 200 μg/mL gelatin (Difco, Detroit, MI) in PBS. Aliquots of supernatants from HUVECs treated with 10 ng/mL PMA in the presence or absence of protease inhibitors were added to duplicate wells and incubated for 2 hours at RT. Wells were then incubated with MoAb 21G6 for 1 hour at 37°C, followed by a 2-hour incubation with antimouse-HRP (Amersham, Arlington Heights, IL). Plates were developed with the 3,3′,5,5′-tetramethylbenzidine (TMB) substrate kit (KPL, Gaithersburg, MD), color development stopped with 1 mol/L H3PO4, and the optical density (OD) read at 450 nm.
RESULTS
Recent studies on endothelial cell-specific RTKs have shown that their expression can vary significantly depending on cell origin, activation state, and whether cells are transformed.7 14 Therefore, we first evaluated tie receptor expression in normal, primary endothelial cells, as well as in CHO cells stably transfected with full-length tie cDNA. A doublet of approximately 125 and 135 kD was specifically immunoprecipitated from CHO cells transfected with full-length tie cDNA (Fig 1). Corresponding bands were absent from CHO cells transfected with vector alone. Both HUVECs and MDECs expressed a doublet of identical size to that seen in CHO clone 2 and clone 37 cells, although at lower expression levels. These data suggested that tie expression in primary endothelial cells and stably transfected CHO cells was similar; a doublet of 125 to 135 kD bands in roughly equal proportions.
To determine the relationship between the bands in the tie doublet, deglycosylation experiments were performed on stably transfected CHO cells (clone 37) and HUVECs. Treatment with N-glycosidase resulted in the immunoprecipitation of a single band having a lower Mr than either of the bands in the doublet (Fig 2, N-Gly lanes), suggesting that tie contained N-linked oligosaccharides. This was confirmed by treating cells with 5 μg/mL tunicamycin, resulting in the immunoprecipitation of a single band with an identical Mr (data not shown). Additionally, treatment with neuraminidase, alone or in combination with O-glycosidase (Fig 2, Neur and Neur +O lanes), resulted in the immunoprecipitation of bands comigrating with the lower band of the doublet. These results suggested that the lower band of the doublet represented an immature form of the tie receptor lacking sialic acid residues, and containing N-linked, but not O-linked, sugars.
Tie receptor glycosylation. Cells were metabolically labeled with 35S-Translabel for 2 hours before preparation of cell lysates and immunoprecipitation with anti-tie MoAb 5D2 as described in Materials and Methods. Immunoprecipitates were treated with either buffer (MOCK), N-glycosidase (N-Gly), neuraminidase (Neur.), or a combination of O-glycosidase and neuraminidase (Neur. + O) for 20 hours at 37°C before SDS-PAGE and autoradiography. Molecular weight markers as indicated.
Tie receptor glycosylation. Cells were metabolically labeled with 35S-Translabel for 2 hours before preparation of cell lysates and immunoprecipitation with anti-tie MoAb 5D2 as described in Materials and Methods. Immunoprecipitates were treated with either buffer (MOCK), N-glycosidase (N-Gly), neuraminidase (Neur.), or a combination of O-glycosidase and neuraminidase (Neur. + O) for 20 hours at 37°C before SDS-PAGE and autoradiography. Molecular weight markers as indicated.
To confirm the precursor-product relationship between the lower and upper bands of the tie doublet, pulse chase experiments using 35S-cysteine/methionine metabolically labeled cells were performed. Figure 3 shows that after a 30-minute pulse label, only the lower band of the tie doublet is immunoprecipitated (0′ lanes). However, over the next several hours, the label is gradually chased into the upper band of the doublet; in CHO cells, after 3 hours the lower doublet band is no longer labeled, whereas, in HUVECs after 2 hours, there is approximately equal distribution of label between the upper and lower bands. In both cells, after a 5-hour chase, only a minimum amount of label remains associated with tie. These experiments also showed that tie's half-life is relatively short, on the order of a few hours. Moreover, when tie antibodies were incubated with HUVECs before lysis (cell surface immunoprecipitation), only the upper band of the doublet was seen, indicating that the upper, but not the lower, tie receptor band is expressed at the cell surface (data not shown). These data showed that the lower band of the doublet represents an intracellular, immature form of tie, which subsequently matures by acquiring sialic acid residues before being transported to and expressed on the cell surface.
Pulse-chase analysis of tie receptor expression by HUVECs and CHO cells. CHO, clone 37 cells (A) or HUVECs (B) were preincubated in methionine-, cysteine-free media for 30 minutes to deplete intracellular amino acid pools. 35S-Translabel was added for a 30-minute pulse followed by incubation in complete growth media for the indicated chase period. Cell lysates were prepared as described in Materials and Methods and immunoprecipitated with anti-tie MoAb 5D2. Immunoprecipitates were analyzed by SDS-PAGE and autoradiography. The chase period is indicated across the top of each lane. Molecular weight markers as indicated.
Pulse-chase analysis of tie receptor expression by HUVECs and CHO cells. CHO, clone 37 cells (A) or HUVECs (B) were preincubated in methionine-, cysteine-free media for 30 minutes to deplete intracellular amino acid pools. 35S-Translabel was added for a 30-minute pulse followed by incubation in complete growth media for the indicated chase period. Cell lysates were prepared as described in Materials and Methods and immunoprecipitated with anti-tie MoAb 5D2. Immunoprecipitates were analyzed by SDS-PAGE and autoradiography. The chase period is indicated across the top of each lane. Molecular weight markers as indicated.
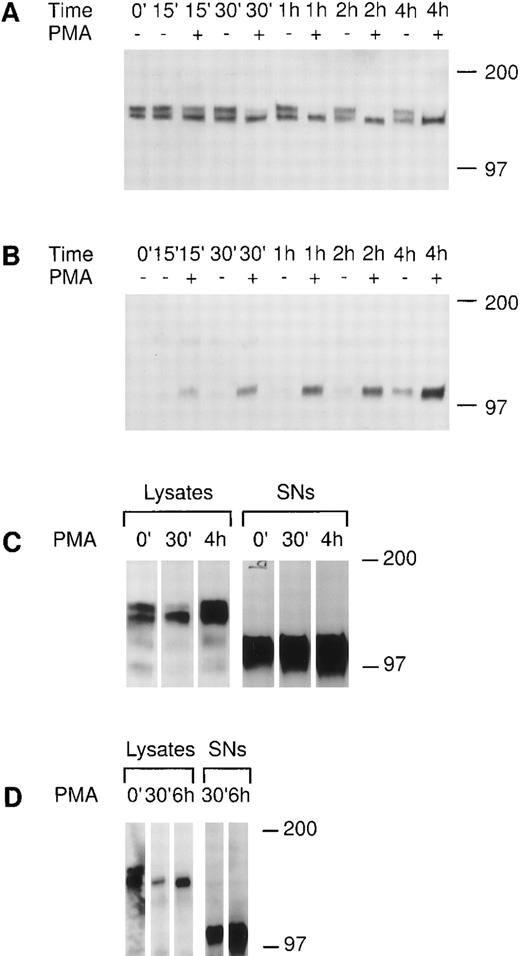
We investigated the regulation of tie expression by the biological response modifier PMA. PMA is known to activate PKC, induce tubule formation, increase urokinase plasminogen activator synthesis, and inhibit proliferation in response to mitogens in endothelial cells.24-26 Tie receptor expression was assessed in HUVECs treated with 10 ng/mL PMA (16 nmol/L). Figure 4A shows the rapid loss of mature tie from the cell surface within 30 minutes of PMA treatment. The disappearance of cell surface tie was sustained over several hours in the presence of PMA. No significant decrease in mature tie expression was noted in the absence of PMA, and levels of intracellular, immature tie appeared to be relatively stable. Concurrent with the loss of cell surface tie expression in PMA-treated endothelial cells, an immunoreactive tie band at ≈100 kD appeared in the conditioned media (Fig 4B). The 100-kD band first appeared after 15 minutes of PMA treatment, paralleling the kinetics of tie receptor disappearance, and accumulated during the 4-hour time course. There is a low level of spontaneous soluble tie release in HUVECs, as well, as shown by the appearance of a 100-kD band after 2 hours in basal media. CHO clone 37 cells were also treated with PMA and tie expression evaluated (Fig 4C). PMA stimulated the loss of mature tie from the cell surface within 30 minutes and the concurrent accumulation of a soluble tie fragment in the conditioned media. However, in contrast to HUVECs, the CHO clones constitutively released a significant amount of soluble tie into the conditioned media (Fig 4C, lane 0′). Moreover, the CHO clone 37 cells recovered quickly; within 4 hours cell-associated tie expression had reached and even surpassed the levels of tie seen in untreated cells. PMA effects were also assayed in MDECs (Fig 4D); PMA stimulated a decrease in cell surface tie expression within 30 minutes and a concomitant accumulation of a 100-kD soluble tie fragment in the supernatant. These results suggested that PMA-mediated activation of PKC induced the loss of cell surface-associated tie receptor and the rapid release of a 100-kD soluble tie fragment into the media.
PMA stimulates the release of tie from the cell surface and the accumulation of a soluble tie fragment in the media. HUVECs were incubated in basal media with or without 10 ng/mL PMA for the indicated length of time, after which the conditioned media was collected, and the cell layer rinsed and lysed as described in Materials and Methods. Cell lysates (A) or conditioned media (B) were immunoprecipitated with anti-tiex. Immunoprecipitates were analyzed on SDS-PAGE followed by immunoblotting with anti-tie MoAb 21G6. Accumulation of a protein band at ≈100 kD can be observed in the conditioned media of PMA-treated cells starting at 15 minutes. (C) CHO, clone 37 cells were treated with PMA for the indicated times, after which, cell lysates or conditioned media supernatants (SNs) were immunoprecipitated with anti-tie MoAb 5D2. Immunoprecipitates were analyzed by SDS-PAGE and immunoblotting with anti-tie MoAb 21G6. CHO clones constitutively released a significant level of soluble tie into the media, although treatment with PMA increased soluble tie levels significantly. (D) MDEC cells were treated with PMA for the indicated times before preparation of cell lysates or collection of conditioned media supernatants (SNs) for analysis by immunoprecipitation and immunoblotting as described. Molecular weight markers as indicated.
PMA stimulates the release of tie from the cell surface and the accumulation of a soluble tie fragment in the media. HUVECs were incubated in basal media with or without 10 ng/mL PMA for the indicated length of time, after which the conditioned media was collected, and the cell layer rinsed and lysed as described in Materials and Methods. Cell lysates (A) or conditioned media (B) were immunoprecipitated with anti-tiex. Immunoprecipitates were analyzed on SDS-PAGE followed by immunoblotting with anti-tie MoAb 21G6. Accumulation of a protein band at ≈100 kD can be observed in the conditioned media of PMA-treated cells starting at 15 minutes. (C) CHO, clone 37 cells were treated with PMA for the indicated times, after which, cell lysates or conditioned media supernatants (SNs) were immunoprecipitated with anti-tie MoAb 5D2. Immunoprecipitates were analyzed by SDS-PAGE and immunoblotting with anti-tie MoAb 21G6. CHO clones constitutively released a significant level of soluble tie into the media, although treatment with PMA increased soluble tie levels significantly. (D) MDEC cells were treated with PMA for the indicated times before preparation of cell lysates or collection of conditioned media supernatants (SNs) for analysis by immunoprecipitation and immunoblotting as described. Molecular weight markers as indicated.
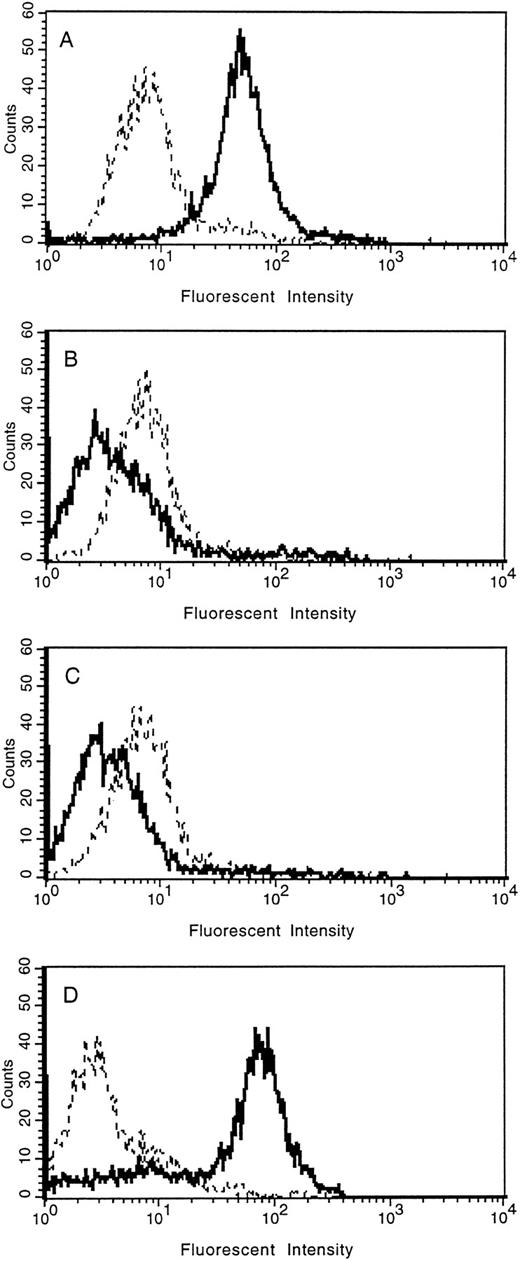
The loss of tie expression from the surface of endothelial cells was confirmed by FACS analysis (Fig 5). HUVECs were treated with PMA for 30 minutes, 2 hours, overnight, or left untreated. Cell surface expression of tie was reduced to background levels within 30 minutes of PMA treatment compared with control cells (compare Fig 5A with 5B). Parallel to immunoblot results, tie expression on HUVECs remained at background levels even after 2 hours of PMA treatment (Fig 5C). In contrast, after overnight stimulation with PMA (Fig 5D), tie cell surface expression recovered to levels slightly higher than those seen in control cells. FACS analysis also indicated that the population of HUVECs expressing tie, both in the control and PMA-treated samples, was homogeneous.
FACS analysis of tie expression on HUVECs treated with PMA. HUVECs in 100-mm dishes were left untreated (A), or treated with PMA for 30 minutes (B), 2 hours (C), or overnight (D). Cells were harvested with EDTA at 4°C and incubated with either control IgG (- - -) or anti-tie MoAb 42G10 () in PBS containing 1% BSA. FACS analysis was performed as described in Materials and Methods.
FACS analysis of tie expression on HUVECs treated with PMA. HUVECs in 100-mm dishes were left untreated (A), or treated with PMA for 30 minutes (B), 2 hours (C), or overnight (D). Cells were harvested with EDTA at 4°C and incubated with either control IgG (- - -) or anti-tie MoAb 42G10 () in PBS containing 1% BSA. FACS analysis was performed as described in Materials and Methods.
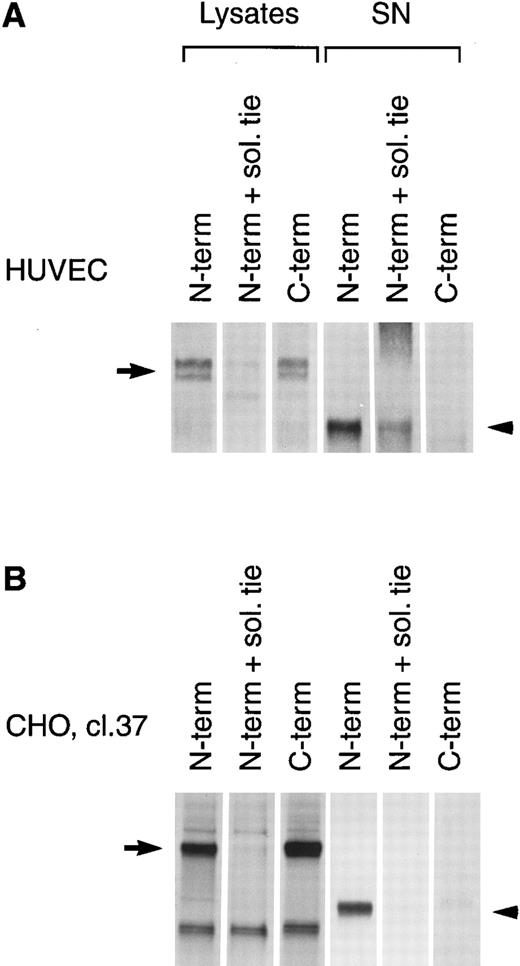
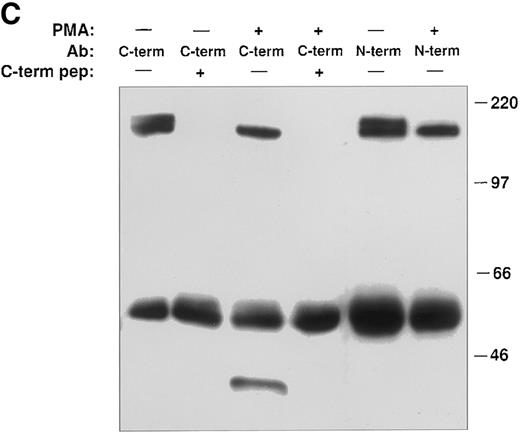
PMA induces cleavage of the tie receptor into an N-terminal 100 kD soluble fragment and a C-terminal 40 kD cell-associated fragment. Cell lysates prepared from untreated HUVECs (A) or CHO, clone 37 cells (B) were immunoprecipitated with antibodies recognizing either the N-terminus (MoAb 5D2) or the C-terminus (SC Ab) of the tie receptor and analyzed by SDS-PAGE on a 6% (A) or 8% (B) gel. Conditioned media supernatants were prepared from HUVECs (A) or CHO, clone 37 cells (B) that had been incubated with 10 ng/mL PMA for 1 hour, followed by immunoprecipitation with Abs recognizing either the N- or C-terminal of tie and analysis on SDS-PAGE. For samples labeled N-term + sol. tie, the N-terminal Ab was preincubated with 30 μg of recombinant soluble tie before immunoprecipitations were performed. Arrows, full-length cell-associated tie; arrowheads, soluble tie. (C) HUVECs were treated in the presence or absence of 10 ng/mL PMA, as indicated, for 1 hour followed by the preparation of cell lysates. Immunoprecipitations were performed with polyclonal Abs specific for the C-terminus (SC Ab) or N-terminus (anti-tiex) of tie followed by analysis on an 8% gel and immunoblotting with the SC Ab. To show specificity, in lanes labeled C-term pep +, the SC Ab was preincubated with the C-terminus tie peptide (amino acids 1121-1138) before immunoprecipitation.
PMA induces cleavage of the tie receptor into an N-terminal 100 kD soluble fragment and a C-terminal 40 kD cell-associated fragment. Cell lysates prepared from untreated HUVECs (A) or CHO, clone 37 cells (B) were immunoprecipitated with antibodies recognizing either the N-terminus (MoAb 5D2) or the C-terminus (SC Ab) of the tie receptor and analyzed by SDS-PAGE on a 6% (A) or 8% (B) gel. Conditioned media supernatants were prepared from HUVECs (A) or CHO, clone 37 cells (B) that had been incubated with 10 ng/mL PMA for 1 hour, followed by immunoprecipitation with Abs recognizing either the N- or C-terminal of tie and analysis on SDS-PAGE. For samples labeled N-term + sol. tie, the N-terminal Ab was preincubated with 30 μg of recombinant soluble tie before immunoprecipitations were performed. Arrows, full-length cell-associated tie; arrowheads, soluble tie. (C) HUVECs were treated in the presence or absence of 10 ng/mL PMA, as indicated, for 1 hour followed by the preparation of cell lysates. Immunoprecipitations were performed with polyclonal Abs specific for the C-terminus (SC Ab) or N-terminus (anti-tiex) of tie followed by analysis on an 8% gel and immunoblotting with the SC Ab. To show specificity, in lanes labeled C-term pep +, the SC Ab was preincubated with the C-terminus tie peptide (amino acids 1121-1138) before immunoprecipitation.
The identity of the 100-kD band as the soluble extracellular domain of the tie receptor was confirmed by using antibodies specific for either the N- or C-terminus of tie. Both antibodies recognized the tie receptor in lysates of untreated HUVECs and CHO clone 37 cells (Fig 6, N-terminus and C-terminus lanes). Preincubating the N-terminus Ab with excess recombinant soluble tie before immunoprecipitation (Fig 6A and 6B, N-term + sol. tie lanes) abolished immunoreactivity, demonstrating specificity. Supernatants and cell lysates from PMA-treated cells were collected and immunoprecipitated. The 100-kD band is recognized by N-terminus, but not C-terminus Abs, as would be expected if the soluble 100-kD fragment represented the extracellular domain of tie. Additionally, recombinant soluble tie inhibited recognition of the 100-kD band by the N-terminus Ab, confirming its identity. Conversely, in cell lysates, C-terminus, but not N-terminus Abs, immunoprecipitated a 40-kD band, probably representing the cell-associated transmembrane and cytoplasmic domains of tie (Fig 6C). The specificity of this interaction was demonstrated by preincubating the Ab with a C-terminus peptide, which abolished reactivity. Thus, PMA stimulated the release of a 100-kD soluble extracellular fragment of tie into the media, while a 40-kD receptor fragment remained cell-associated.
Chronic PMA treatment of cells leads to the downregulation of PKC and the inability of additional PMA to activate PKC.6 27 We used chronic PMA treatment with or without short-term PMA treatment as a way to link PMA-dependent modulation of tie expression with the PKC pathway. HUVECs were incubated with 1 μmol/L PMA for 24 hours, 16 nmol/L PMA for 1 hour, or both before immunoprecipitation of tie from cell lysates or supernatants (Fig 7). Cells treated chronically with PMA did not respond to further PMA stimulation; there was no appreciable release of soluble tie into the media after an additional 1 hour PMA exposure. Indeed, it appeared that tie expression under these conditions increased, which may reflect faster kinetics of tie receptor processing in PMA-treated HUVECs, as suggested by comparing tie expression in the presence or absence of PMA in pulse-chase experiments (data not shown). Additionally, cells treated with PMA for 24 hours did not show the decreased cell surface tie expression and increased release of soluble tie seen in cells treated with PMA for 1 hour. This data demonstrated that downregulation of PKC inhibited the PMA-dependent release of soluble tie from HUVECs and provided a connection between PKC activation and modulation of tie receptor expression.
Inhibition of soluble tie release by downregulation of PKC. HUVECs were incubated with 16 nmol/L (10 ng/mL) PMA for 1 hour, 1 μmol/L PMA for 24 hours, or both, as indicated, before harvesting of conditioned media supernatants and preparation of cell lysates. Lysates and supernatants were immunoprecipitated with anti-tie MoAbs, and analyzed by SDS-PAGE followed by immunoblotting as described in Materials and Methods.
Inhibition of soluble tie release by downregulation of PKC. HUVECs were incubated with 16 nmol/L (10 ng/mL) PMA for 1 hour, 1 μmol/L PMA for 24 hours, or both, as indicated, before harvesting of conditioned media supernatants and preparation of cell lysates. Lysates and supernatants were immunoprecipitated with anti-tie MoAbs, and analyzed by SDS-PAGE followed by immunoblotting as described in Materials and Methods.
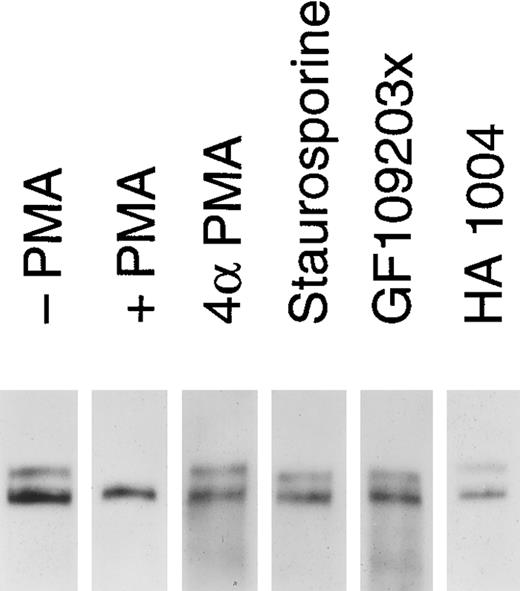
Release of soluble tie from HUVECs is mediated through PKC activation. HUVECs were incubated with basal media alone (-PMA), 10 ng/mL PMA (+ PMA), 10 ng/mL 4αPMA (4αPMA) or 25 μmol/L HA 1004 (HA 1004) for 1 hour, or preincubated for 15 minutes with 100 nmol/L staurosporin (Staurosporin) or 200 nmol/L GF 109203X (GF109203x) before the addition of PMA for 1 hour. Preparation of cell lysates was performed as described in Materials and Methods. Samples were analyzed by immunoprecipitation using anti-tie MoAbs, SDS-PAGE, and immunoblotting as previously described.
Release of soluble tie from HUVECs is mediated through PKC activation. HUVECs were incubated with basal media alone (-PMA), 10 ng/mL PMA (+ PMA), 10 ng/mL 4αPMA (4αPMA) or 25 μmol/L HA 1004 (HA 1004) for 1 hour, or preincubated for 15 minutes with 100 nmol/L staurosporin (Staurosporin) or 200 nmol/L GF 109203X (GF109203x) before the addition of PMA for 1 hour. Preparation of cell lysates was performed as described in Materials and Methods. Samples were analyzed by immunoprecipitation using anti-tie MoAbs, SDS-PAGE, and immunoblotting as previously described.
We investigated whether the loss of tie expression at the cell surface was specific for the PKC pathway. HUVECs were incubated with an inactive PMA analog, PKC inhibitors or an inhibitor of protein kinase A (PKA), and analyzed for tie receptor expression (Fig 8). HUVECs incubated with 4αPMA, an inactive PMA analog, for 1 hour did not demonstrate the loss of cell surface tie observed in the presence of PMA. Similarly, preincubating cells with staurosporin or GF109203X inhibited the PMA-dependent decrease in tie expression. On the other hand, HA 1004 did not stimulate the release of soluble tie. These results suggested that the generation of soluble tie from cell-associated receptor specifically requires the activation of PKC.
To establish the nature of the protease involved, a panel of protease inhibitors was tested for their effect on PMA-mediated release of soluble tie from HUVECs. The results, shown in Table 1, demonstrated that soluble tie release was not significantly inhibited by reagents specific for serine-, aspartate-, cysteine-, trypsin-, or metallo-proteases. However, soluble tie release was completely inhibited by 100 μmol/L EGTA. In contrast, EDTA had no effect. Additionally, inhibition by EGTA could be reversed by adding either 100 or 500 μmol/L exogenous Ca2+. This data indicates that the protease that cleaves tie on HUVECs in response to PMA is Ca2+-dependent, but leaves its identity unresolved.
Inhibition of Tie Release by Protease Inhibitors and Chelation of Divalent Cations
| Inhibitor . | Concentration . | Soluble Tie Release (% control)* . |
|---|---|---|
| Leupeptin | 1 μg/mL | 100 |
| Benzamidine | 1 mmol/L | 92 |
| TLCK | 40 μg/mL | 96 |
| PMSF | 500 μmol/L | 107 |
| Aprotinin | 1 μg/mL | 119 |
| Pepstatin | 1 μg/mL | 87 |
| NCO-700 | 100 μmol/L | 66 |
| Phosphoramidon | 100 μg/mL | 125 |
| o-Phenanthroline | 20 μmol/L | 92 |
| EDTA | 100 μmol/L | 103 |
| EGTA | 100 μmol/L | 4 |
| EGTA + Ca2+ | 100 μmol/L + 100 μmol/L | 88 |
| EGTA + Ca2+ | 100 μmol/L + 500 μmol/L | 100 |
| Inhibitor . | Concentration . | Soluble Tie Release (% control)* . |
|---|---|---|
| Leupeptin | 1 μg/mL | 100 |
| Benzamidine | 1 mmol/L | 92 |
| TLCK | 40 μg/mL | 96 |
| PMSF | 500 μmol/L | 107 |
| Aprotinin | 1 μg/mL | 119 |
| Pepstatin | 1 μg/mL | 87 |
| NCO-700 | 100 μmol/L | 66 |
| Phosphoramidon | 100 μg/mL | 125 |
| o-Phenanthroline | 20 μmol/L | 92 |
| EDTA | 100 μmol/L | 103 |
| EGTA | 100 μmol/L | 4 |
| EGTA + Ca2+ | 100 μmol/L + 100 μmol/L | 88 |
| EGTA + Ca2+ | 100 μmol/L + 500 μmol/L | 100 |
HUVECs were pretreated with protease inhibitors, at the indicated concentrations, for 15 minutes before incubation with 10 ng/mL PMA for 1 hour. Supernatants were collected and assayed for soluble tie release by ELISA as described in Materials and Methods. Experiments with EDTA/EGTA were performed in PBS (−Ca2+/Mg2+) + 1% FBS instead of basal media. Results are expressed as % control, where 100% control represents soluble tie released in response to 10 ng/mL PMA in the absence of protease inhibitors or divalent cation chelators.
DISCUSSION
This study investigated the regulation of tie receptor expression, an orphan RTK restricted to cells of hematopoietic and endothelial cell lineages. Using HUVECs and CHO cells transfected with full-length tie, it was shown that tie expression at the cell surface is limited to the mature receptor, the upper band of the doublet immunoprecipitated by tie specific Abs from cell lysates. Furthermore, PMA induced a rapid loss of tie from the cell surface concomitant with the accumulation of a 100-kD soluble tie fragment in the media representing the extracellular domain. A 40-kD tie fragment remained cell-associated probably containing transmembrane and cytoplasmic domains. Reagents that inhibited PKC activity prevented the release of soluble tie in response to PMA, as did chronic PMA treatment. These experiments showed that the PMA-mediated release of soluble tie into the media is specific for the PKC pathway. Together, these results suggested that tie receptor expression on endothelial cells is modulated by the activation of PKC, which leads to the release of soluble tie from the cell surface, abrogating tie signaling.
The most thoroughly studied endothelial cell RTKs are the bFGF and VEGF receptors.7 Activation of FGFR-1 stimulates several endothelial cell responses including proliferation, migration, and tubule formation.9 Similarly, activation of Flk-1, the VEGF receptor, induces a parallel group of endothelial cell responses including increased vascular permeability.28 These functional responses are commonly linked to angiogenesis and pathological conditions that rely on the development of new blood vessels such as tumor growth, metastatic dissemination, diabetic retinopathy, and rheumatoid arthritis.2 The importance of RTKs in these processes is demonstrated by the inhibition of tumor growth in vivo by a dominant-negative mutation of Flk-1.11 Delivered by a retroviral expression vector, signal transduction-defective Flk-1 receptors inhibited tumor growth by inhibiting tumor-associated angiogenesis. Similarly, neutralizing antibodies to bFGF and VEGF administered in vivo inhibit tumor growth, as well.12 13 Because the established vascular endothelium of adults is essentially quiescent, therapeutic design strategies are currently taking advantage of the link between RTK activation and angiogenesis.
Recent work has suggested a connection between tie expression on endothelial cells and neovascularization, including tumor-associated angiogenesis.8,29,30 In adults, tie expression is limited to the highly vascularized placenta and lung, and to tissues undergoing angiogenesis-dependent remodeling, such as in wound healing and hormone-induced superovulation.20 Tie expression increases dramatically in the endothelium of new blood vessels surrounding cutaneous and brain metastases of melanoma compared with weak tie expression in normal skin capillaries.30 Interestingly, the capillary endothelial cells associated with metastatic tumors were negative for FGFR-1, suggesting that FGF does not play a critical role in the development of these blood vessels. Similar findings were reported in a comparison of the vasculature surrounding gliomas versus normal brain tissue, specifically, tie expression in the endothelium is positively correlated to tumor invasiveness.29 These studies suggest that tie plays an essential role in tumor-associated angiogenesis, as well as in neovascularization during embryogenesis.
RTK activity can be downregulated through several different pathways4 including the abrogation of cell surface receptor expression by release of the extracellular domain in soluble form. The release of a receptor fragment from the cell membrane may have a dual function in modulating receptor activity.31,32 The primary effect is to remove the receptor ligand-binding domain from the cell surface, thus inhibiting ligand-dependent signal transduction. Equally important, soluble receptor fragments often retain their capacity to bind ligand, serving as a sink for extracellular ligand, but without accompanying receptor activation. Several RTKs release a soluble receptor fragment from the cell surface including CSF-1R, c-kit, and axl.33-35 For CSF-1R, ligand binding induces receptor endocytosis in transfected NIH 3T3 cells, but not the release of soluble receptor, while PMA stimulates soluble receptor release, but not internalization. Similarly, PMA induces the release of soluble c-kit in bone marrow-derived mast cells, and soluble axl in the AF6295 murine tumor cell line.
Evidence is accumulating that the mechanisms by which soluble RTKs are generated involve multiple pathways. Although soluble EGF and VEGF receptors can be generated through alternate splicing,36,37 several RTKs undergo limited proteolysis to generate soluble receptors.33-35,38 Based on the PMA-mediated appearance of soluble receptors in conditioned media, proteolysis is most likely activated through a PKC-dependent mechanism. We were able to directly demonstrate this by showing that PKC inhibitors, or chronic PMA treatment, which downregulates PKC activity, prevented soluble tie release. In contrast, PMA-mediated formation of soluble c-kit in bone marrow-derived mast cells is PKC-independent; the PKC inhibitor H-7 did not block the generation of soluble c-kit, but did block PKC activation.38 These divergent results suggest that PMA-dependent generation of soluble RTKs may occur through both PKC-dependent and -independent pathways.
Even less is known about the identity of the proteases responsible for cell surface RTK cleavage. Similar to our results showing that EGTA inhibited soluble tie generation, EDTA inhibited soluble c-kit generation in bone marrow-derived mast cells,34 suggesting the activation of a divalent cation-dependent protease. None of the other protease inhibitors tested showed a significant effect on soluble tie formation indicating that a novel Ca2+-activated protease may be involved. Similarly, PMA-mediated induction of soluble interleukin (IL)-6 receptors was unaffected by a wide range of protease inhibitors suggesting the activity of a novel protease.39 Another unresolved question is whether a consensus amino acid sequence targets RTKs for PMA-mediated proteolysis. Unlike the PMA-dependent proteolysis of TGFα, where a C-terminal valine is thought to be critical,40 tie has neither a C-terminal valine or similar juxtamembrane sequence.
There has been much speculation on the role of soluble receptors in cellular functional responses.31,32 Among several scenarios are (1) cleavage of cell surface receptors abrogates signal transduction in the presence of ligand, (2) the release of receptor fragments serves to bind and remove free ligand from circulation, thereby dampening cellular responses, and (3) soluble receptor and full-length receptor may form crippled heterodimers that are unable to signal. Thus, proteolytic cleavage of RTKs from the cell surface may result in a rapid and multipronged termination of activation. Unfortunately, experimental evidence is limited. Although embryonic fibroblasts overexpressing soluble EGF-R are refractory to TGFα-dependent transformation,41 and chemical cross-linking studies suggest that soluble EGF-R is capable of forming an inactive heterodimer with intact EGF-R,42 it is not clear if this mechanism is functional in vivo. Similarly, PMA abrogates the adhesion of c-kit+ mast cells to c-kit ligand+ fibroblasts, yet biochemical analysis shows that a significant amount of c-kit on PMA-treated mast cells remains intact and cell-associated.38 Therefore, the connection between proteolytic generation of soluble RTKs and cell functional responses is still unclear.
More information is available on the relationship between soluble cytokine receptors and pathology.31,43 High serum levels of soluble IL-2 receptors are positively correlated with a more invasive B-cell chronic lymphocytic leukemia phenotype and the loss of T-cell mitogenic responsiveness.44 Similarly, elevated levels of soluble IL-2 receptors have been found in patients with rheumatoid arthritis and multiple sclerosis.45,46 Mechanistically, the binding and consequent inhibition of IL-2 function by soluble IL-2 receptors in vivo may contribute to the immunosuppression observed in these diseases. Soluble TNF receptors are also detected in the serum of cancer patients, but not in healthy controls.47,48 Because soluble TNF receptors inhibit the TNF-mediated killing of L929 cells, these results suggest that tumor cells may stimulate the generation of soluble TNF receptors to protect themselves from TNF-dependent cytotoxicity. Interest has also been spurred in using soluble receptor fragments therapeutically. Administration of soluble IL-2 or IL-6 receptors could modulate the growth of lymphocytic tumors, while soluble IL-4 receptors might prove useful in modulating allergic reactions.31 Soluble IL-1 receptor has already been shown to inhibit alloreactivity in animal models.49 Whether the therapeutic potential of soluble cytokine receptors can be extended to soluble RTKs has yet to be investigated, but the parallels are clear. It is not too far a step to anticipate that soluble RTK receptors, such as tie, could be used therapeutically to modulate pathological processes including angiogenesis.
ACKNOWLEDGMENT
We would like to thank D. Dumont, K. Alitalo, C. Saris, B. Varnum, and D. Lyons for their helpful suggestions and interest and T. Burgess, K. Farrell, and S. Suchard for many lively conversations throughout the course of this study and critical review of the manuscript.
Address reprint requests to Rachel Yabkowitz, PhD, Mammalian Cell Molecular Biology, M/S 14-2-C, Amgen Inc, 1840 DeHavilland Dr, Thousand Oaks, CA 91320-1789.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal