Abstract
A sequence of 31 amino acids (S565-K595) in domain 6 of the light chain of high molecular weight kininogen (HK) has previously been shown to be responsible for the binding of plasma prekallikrein (PK) or kallikrein. To find effective peptides that might block binding between HK and PK on cell surfaces, a new series of synthetic peptides has now been prepared that incorporates portions of this binding domain sequence. For mapping the minimal sequence within HK, these new peptides were tested for their ability to compete with HK for binding PK in a cell-free system and on human umbilical vein endothelial cells (HUVEC). In the former, at pH 7.4, the kds for binding between kallikrein and either D567-K595, S565-P594, D567-S593, or D567-T591 were all similar to that for the binding of S565-K595 (0.2 to 0.4 μmol/L), but those for the binding of D568-K595, W569-K595, and D567-P589 were an order of magnitude greater (kd = 2 to 5 μmol/L). D567-S586, the shortest chain length of the N- and C-terminal truncation sequences tested, does not effectively compete with kininogen for kallikrein binding (kd = 100 μmol/L). These results imply that D567-T591, a 25-residue peptide (HK25c), contains sufficient structural information for binding kallikrein in solution. D567-T591 also is the minimum structural sequence to block binding of kallikrein to HUVEC-bound HK (IC50 = 50 nmol/L) and to inhibit PK activation to kallikrein on the cell surface (IC50 = 80 nmol/L). In addition, D567-T591 also inhibits the generation of kallikrein-activated urokinase, which activates plasminogen to plasmin (IC50 = 100 nmol/L). Thus, HK-derived peptides may be useful compounds for modulating excessive fibrinolysis and hypotension in sepsis and multiple trauma.
HIGH MOLECULAR weight kininogen (HK), a single-chain plasma glycoprotein, participates in contact activation reactions through specific interactions with prekallikrein and factor XI.1-3 In plasma, HK circulates in a stoichiometric complex with either prekallikrein (PK) or factor XI, but not both proteins.4,5 Our previous results showed that the binding domain sequences within HK for either of these latter two proteins are likely to be alternatively expressed on the surface of HK.6 HK binding to PK in plasma serves to promote surface-dependent activation of the zymogen and to position HK for efficient cleavage by the resulting enzyme, kallikrein.7 New evidence suggests that the intrinsic pathway of fibrinolysis may operate efficiently on cell surfaces and has led to the development of a model in which endothelial cells may facilitate the assembly of components of the contact and plasminogen activator systems in a manner that promotes the efficient activation of plasminogen. Such a model is supported by several observations.8,9 First, HK binds specifically to human umbilical vein endothelial cells (HUVEC).10,11 Second, prekallikrein binds to HUVEC in a kininogen-dependent manner.12 Third, FXIIa promotes the activation of plasminogen on HUVEC.12 Finally, kallikrein mediates the activation of prourokinase in a purified system3 as well as in plasma.13 Thus, the endothelial cell surface provides a locus for assembly of a proteolytic cascade leading to the kallikrein-mediated activation of prourokinase and culminating in cell surface plasminogen activation. The quantitative contribution of this pathway to the overall process of cell surface plasminogen activation is not currently understood. However, we postulated that peptides containing sequences common to the PK binding site of HK would serve as inhibitors of the binding of PK to endothelial cells and in turn inhibit the generation of cell surface plasminogen activator activity in the presence of cell-bound prourokinase (pro-UK).
HK has been found to bind to two sites on the heavy chain of PK, one in apple domain 1 and the other in apple domain 4.14-16 Recently, we showed the formation of a ternary complex between peptides from apple 1 and 4 of PK and a peptide from HK.17 The binding site sequence within HK for PK has been mapped to S565-K595 of domain 6 of the light chain of HK, and a synthetic peptide encompassing this sequence (HK31; Table 1) displays the same binding affinity for PK18 as does intact, native HK.19,20 HK31 assumes a folded structure in solution that is stabilized by long-range interactions between N- and C-terminal residues.21 HK31 contains essential features necessary for binding PK, such as an ordered N-terminus containing a short helix segment. N- or C-terminal truncations of this sequence cause detrimental perturbations in structure that result in peptides that possess diminished binding capacity.22 For example, deleting the first four N-terminal residues (HK27; W569-K595) decreases binding fourfold,6,19 probably because HK31 and HK27 assume different structures in solution.6 22
The Sequences of Truncated Peptides of HK31
| Peptide* . | Sequence† . |
|---|---|
| 565 570 575 580 585 590 595 | |
| HK31 | S D D D W I P D I Q T D P N G L S F N P I S D F P D T T S P K |
| N-terminal truncated peptides | |
| HK29 | D D W I P D I Q T D P N G L S F N P I S D F P D T T S P K |
| HK28 | D W I P D I Q T D P N G L S F N P I S D F P D T T S P K |
| HK27 | W I P D I Q T D P N G L S F N P I S D F P D T T S P K |
| C-terminal truncated peptides | |
| HK30c | S D D D W I P D I Q T D P N G L S F N P I S D F P D T T S P |
| HK27c | D D W I P D I Q T D P N G L S F N P I S D F P D T T S |
| HK25c | D D W I P D I Q T D P N G L S F N P I S D F P D T |
| HK23c | D D W I P D I Q T D P N G L S F N P I S D F P |
| HK20c | D D W I P D I Q T D P N G L S F N P I S |
| Peptide* . | Sequence† . |
|---|---|
| 565 570 575 580 585 590 595 | |
| HK31 | S D D D W I P D I Q T D P N G L S F N P I S D F P D T T S P K |
| N-terminal truncated peptides | |
| HK29 | D D W I P D I Q T D P N G L S F N P I S D F P D T T S P K |
| HK28 | D W I P D I Q T D P N G L S F N P I S D F P D T T S P K |
| HK27 | W I P D I Q T D P N G L S F N P I S D F P D T T S P K |
| C-terminal truncated peptides | |
| HK30c | S D D D W I P D I Q T D P N G L S F N P I S D F P D T T S P |
| HK27c | D D W I P D I Q T D P N G L S F N P I S D F P D T T S |
| HK25c | D D W I P D I Q T D P N G L S F N P I S D F P D T |
| HK23c | D D W I P D I Q T D P N G L S F N P I S D F P |
| HK20c | D D W I P D I Q T D P N G L S F N P I S |
HK31 = S565 − K595.
Each peptide was prepared as the C-terminal amide and acetylated at the N-terminus.
The one-letter code for amino acids is used.
To further define the minimum sequence within HK necessary to bind PK, additional truncated peptides based on HK31 were prepared and tested for their ability to compete with surface-bound kininogen for PK binding, using a competitive amidolytic assay.15 The shortest chain length peptide that retained high affinity for PK was D567-T591 (HK25c); loss of additional residues in HK25c dramatically decreased binding. HK25c blocked the binding of PK to HUVEC-bound HK and, by doing so, decreased the activation of PK on the cell surface to kallikrein.
MATERIALS AND METHODS
Materials.PK, kallikrein, HK, and Glu-plasminogen, purified from human plasma, were purchased from Enzyme Research Laboratories (South Bend, IN). Recombinant prourokinase was kindly provided by Drs Jack Henkin and Andrew Mazar (Abbott Laboratories, Abbott Park, IL). Chromogenic kallikrein substrate S2302, H-D-Pro-Phe-Arg-p-nitroaniline 2HCl, was purchased from Kabi Diagnostica AB (Mölndal, Sweden). The fluorescent plasmin substrate, H-D-Val-Leu-Lys-AMC, was obtained from Bachem (King of Prussia, PA), and 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) was purchased from Sigma (St Louis, MO).
Radioactive labeling of plasma kallikrein.Plasma kallikrein was radiolabeled with Na125I by incubating the protein with Iodo-Beads (Pierce Co, Rockford, IL) for 15 minutes at room temperature. Free iodine was separated from the iodinated protein by applying 0.12 mL of the iodination mixture to a column of Sephadex G-10 (10 mL) in 50 mmol/L phosphate buffer, pH 7.2.
Peptide synthesis.All peptides were synthesized by solid-phase methods at Commonwealth Biotechnologies, Inc (Richmond, VA). Peptides were purified by reverse-phase high-performance liquid chromatography, and their sequences were verified by amino acid analysis.6,14,21 22 The purities were calculated based on the theoretical peptide content per milligram of lyophilized solid. The purities of the peptides range from 90% to 98%. The sequences of the kininogen-based peptides prepared for this study are shown in Table 1. All peptides were soluble in aqueous buffers up to 10 mg/mL.
Endothelial cell culture.HUVEC were isolated as previously described23,24 and cultured in Medium 199 containing 10% fetal bovine serum (Hyclone, Logan, UT), 75 μg/mL crude endothelial cell growth factor (prepared using the method of Maciag et al25 ), 2 mmol/L glutamine, and penicillin-streptomycin. Cells were initially cultured in 10-cm2 dishes (Falcon Labware, Lincoln Park, NJ), plated in 96-well fibronectin-coated tissue culture plates (Nunc, Roskilde, Denmark), and used for assays upon achieving confluence. Cells from passages 2 through 5 were used in these studies.
Dissociation constants.Binding assays for the determination of the dissociation constants (kds) between the peptides and PK were performed in 96-well microtiter plates, as previously described.14 In this assay, the ability of test peptides to compete with native HK for binding of kallikrein is assessed by assay of residual kallikrein activity against 0.4 mmol/L S2302, as determined from the change in absorbance at 405 nm. To determine the pH dependence of peptide binding, incubation of the test peptides in the HK-coated microtiter well was performed in a buffer containing 50 mmol/L glycine, 50 mmol/L sodium acetate, and 50 mmol/L sodium phosphate. The addition of small amounts of either acid or base to this solution results in a new pH as determined by means of a pH probe.6,22 kd values were calculated by Ao /(Ao − A) = 1 + (Kd /ao ), where Ao is the absorbance of a control in the absence of peptide, A is the absorbance measured in the presence of a particular concentration of peptide (ao ), and Kd is the slope of the plotted line.14 The data were fitted by means of a least squares fit.
Measurement of kallikrein activity on endothelial cells.Before each experiment, HUVEC were washed three times (0.4 mL total volume) at 23°C with HEPES-bovine serum albumin (BSA) buffer (137 mmol/L NaCl, 4 mmol/L KCL, 1 mmol/L CaCl2 , 11 mmol/L glucose, 10 mmol/L HEPES, 0.5 mg/mL cell culture grade BSA, pH 7.4). Next, cells were incubated with 70 nmol/L HK for 1 hour at 4°C in HEPES-BSA buffer containing 50 μmol/L ZnCl2 . Unbound HK was removed by successive washes, and cells were then incubated for 1 additional hour in the same buffer containing 10 nmol/L kallikrein (with or without HK peptide or prekallikrein). Wells were then washed three times and incubated with 100 μL/well of a solution containing 0.4 mmol/L substrate S2302. The absorbance increase with time at 405 nm was measured for 50 minutes using a microtiter plate reader (Bio-Tek, Winooski, VT).
Measurement of PK activation to kallikrein in the presence of HK-bound HUVEC.HK was bound to HUVEC as described above. PK (20 nmol/L), HK25c, and kallikrein substrate S2302 were then added, cells were incubated at 23°C for 24 minutes, and the absorbance increase in each well at 405 nm was measured. HEPES-BSA buffer containing ZnCl2 (50 μmol/L) was used to wash the cells and was used as the binding and assay buffer.
Measurement of plasminogen activation on endothelial cells.To measure the ability of HUVEC exposed to HK and PK to promote the activation of cell-bound plasminogen, HUVEC were incubated for 1 hour in HEPES-BSA buffer containing ZnCl2 (50 μmol/L) and 70 nmol/L HK. Cells were then washed in the same buffer and incubated in the same buffer (100 μL/well) containing 2.5 nmol/L or 50 nmol/L, depending on the experiment, of PK, HK peptides, 4.5 nmol/L pro-UK, 60 nmol/L Glu-plasminogen, and 50 μmol/L H-D-Val-Leu-Lys-AMC. The emission fluorescence intensity at 460 nm (excitation wavelength, 360 nm) was then measured at room temperature using a fluorescence plate reader (Cytofluor 2300; Millipore, Bedford, MA).
RESULTS
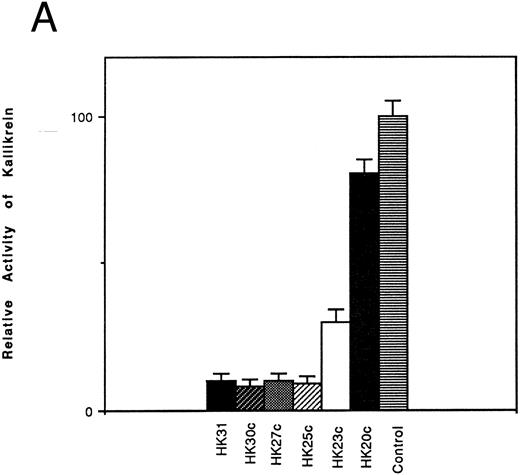
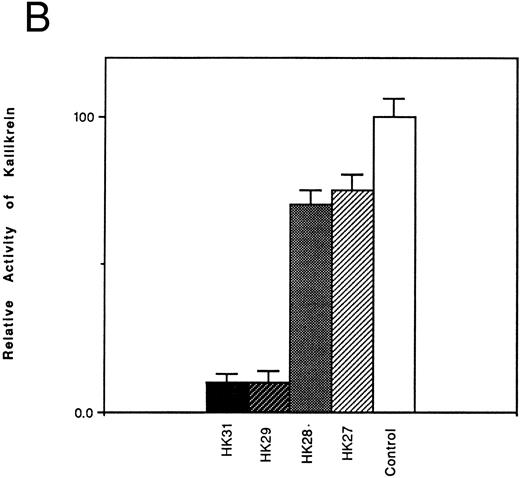
Inhibition of the binding of kallikrein to kininogen by HK-derived peptides.The ability of N- and C-terminal truncated peptides to inhibit the binding of kallikrein to HK was determined by measuring the binding of kallikrein (2.9 nmol/L) to HK immobilized in microplate wells, in the absence and presence of specific peptides (4.5 μmol/L). A decrease in kallikrein binding to immobilized HK was detected by demonstrating a decrease in the hydrolysis rate of the chromogenic kallikrein substrate S2302. HK30c, HK27c, and HK25c blocked PK binding with potency similar to HK31 (90%), whereas HK23c (68%) and HK20c (19%) were less potent (Fig 1A). For N-terminal truncated peptides, HK29 blocked the binding of PK to HK with potency similar to HK31 (90%), whereas HK28 and HK27 were less potent (30% and 25% inhibition, respectively; Fig 1B).
Effect of truncated HK peptides on the binding of kallikrein to HK. HK peptides, either (A) C-terminal truncated or (B) N-terminal truncated (4.5 μmol/L), were incubated with a single concentration of kallikrein (2.9 nmol/L) in HK-coated wells for 2 hours. After washing, the activity of bound kallikrein was determined using chromogenic substrate S2302 (0.4 mmol/L). The activity of the control experiment in which no HK peptides were added was considered to be 100%. The data shown are the mean ± SEM of three experiments.
Effect of truncated HK peptides on the binding of kallikrein to HK. HK peptides, either (A) C-terminal truncated or (B) N-terminal truncated (4.5 μmol/L), were incubated with a single concentration of kallikrein (2.9 nmol/L) in HK-coated wells for 2 hours. After washing, the activity of bound kallikrein was determined using chromogenic substrate S2302 (0.4 mmol/L). The activity of the control experiment in which no HK peptides were added was considered to be 100%. The data shown are the mean ± SEM of three experiments.
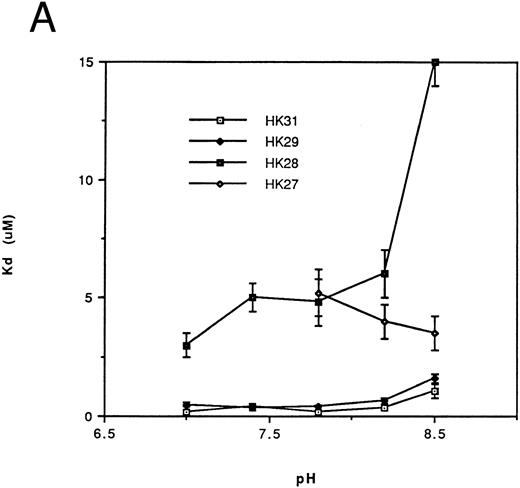
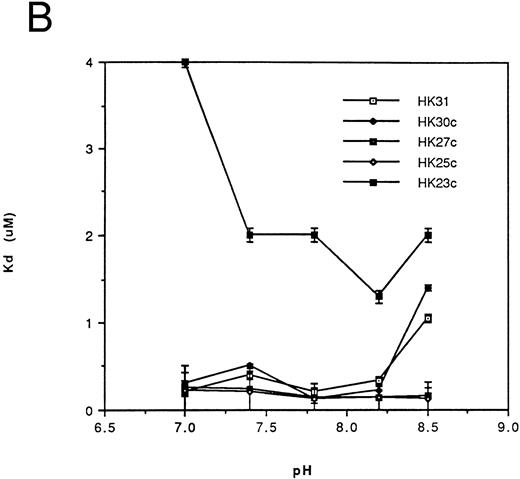
Affinity of binding of HK peptides to kallikrein.kds for the binding of HK peptides to kallikrein were determined by competitive amidolytic assay as a function of pH. The kds of HK31 and HK29 (Fig 2A) or of HK30c, HK27c, and HK25c (Fig 2B) were similar (0.2 to 0.45 μmol/L). HK28, HK27 (Fig 2A), and HK23c had higher kds (3 to 5 μmol/L), but only HK20c showed the most dramatic change in binding affinity (kd = 100 μmol/L; data not shown).
Dependence of the kd on pH for binding of the N-terminal truncated peptides (A) and the C-terminal truncated peptides (B) of HK 31 to kallikrein. After adjustment of buffer pH, the ability of peptides to inhibit the binding of kallikrein to immobilized HK was determined using an amidolytic assay. kd values were obtained as described in the Materials and Methods. Each kd is a mean of at least triplicate determinations.
Dependence of the kd on pH for binding of the N-terminal truncated peptides (A) and the C-terminal truncated peptides (B) of HK 31 to kallikrein. After adjustment of buffer pH, the ability of peptides to inhibit the binding of kallikrein to immobilized HK was determined using an amidolytic assay. kd values were obtained as described in the Materials and Methods. Each kd is a mean of at least triplicate determinations.
Thus, HK25c, a 25-residue peptide, retains the minimum sequence necessary for maintenance of HK binding to kallikrein. The removal of one additional N-terminal residue or two additional C-terminal residues of HK25c decreases its affinity at least 10-fold. An increase in the chain length of HK25c at either the N- or C-terminus, such as HK31, HK29, HK30c, or HK27c, does not increase the ability to bind to kallikrein.
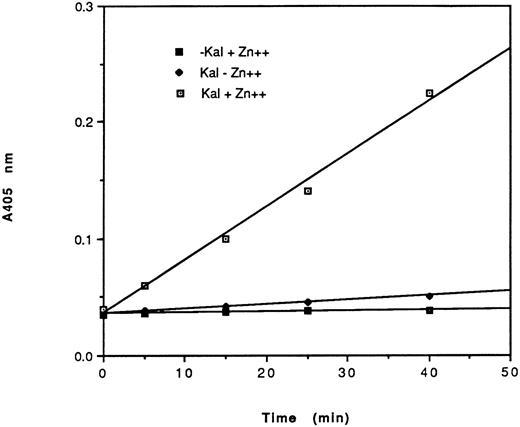
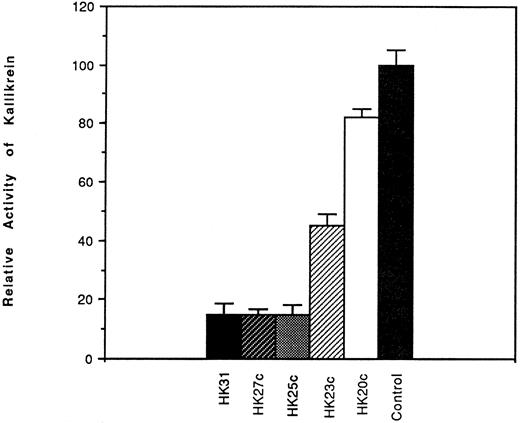
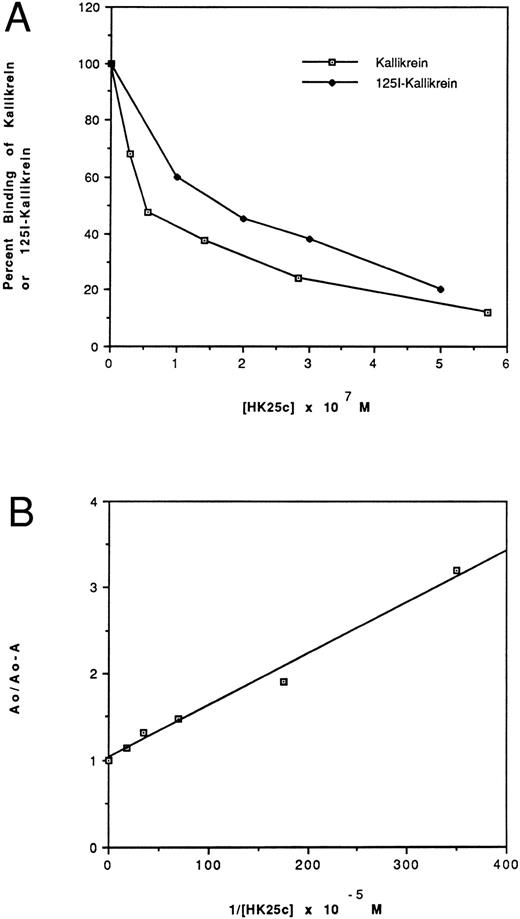
Effect of HK peptides on the binding of kallikrein and 125I-kallikrein to HUVEC-bound HK.In the presence of HK and Zn2+, kallikrein can bind to HUVEC effectively, as judged by amidolytic assay. However, in the absence of Zn2+, HK does not specifically bind to HUVEC; therefore, less kallikrein activity is detectable on the endothelial cell surface (Fig 3). To confirm that the synthetic HK peptides also effectively inhibit the binding of kallikrein to HUVEC-bound HK, various peptides were added along with kallikrein to HUVEC-bound HK (Fig 4). HK25c, HK27c, or HK31 (0.5 μmol/L) can inhibit 85% of kallikrein binding to HUVEC-bound HK, but HK23c and HK20c were much less effective. HK25c is therefore the minimum structural sequence required for inhibition. The IC50 of HK25c for this inhibition is 50 nmol/L and was 150 nmol/L for 125I-kallikrein (Fig 5A), indicating that the kallikrein measured by activity binds similarly to that measured by protein. The kd for binding of HK25c and kallikrein is 70 ± 10 nmol/L (mean ± SD; Fig 5B).
Amidolytic activity of HUVEC-bound kallikrein. HUVEC were incubated with 70 nmol/L HK in the presence (⊡, ▪) or absence (♦) of 50 μmol/L Zn2+. After washing, cells were further incubated in the presence (⊡, ♦) or absence (▪) of 10 nmol/L kallikrein. After additional washes, the amidolytic activity of cell-bound kallikrein was determined using S2302 (0.4 mmol/L). The data shown are from a single experiment run in triplicate of a total of three experiments. HEPES-BSA containing ZnCl2 (50 μmol/L) was used as the washing and assay buffers.
Amidolytic activity of HUVEC-bound kallikrein. HUVEC were incubated with 70 nmol/L HK in the presence (⊡, ▪) or absence (♦) of 50 μmol/L Zn2+. After washing, cells were further incubated in the presence (⊡, ♦) or absence (▪) of 10 nmol/L kallikrein. After additional washes, the amidolytic activity of cell-bound kallikrein was determined using S2302 (0.4 mmol/L). The data shown are from a single experiment run in triplicate of a total of three experiments. HEPES-BSA containing ZnCl2 (50 μmol/L) was used as the washing and assay buffers.
Effects of HK peptides on the binding of kallikrein to HUVEC. HUVEC were incubated with 70 nmol/L HK in the presence of 50 μmol/L Zn2+. After washing, 10 nmol/L kallikrein was added in the absence or presence of HK peptide (0.53 μmol/L). After further washing, bound kallikrein was measured using S2302. Binding of kallikrein to cells in the absence of peptides was considered to be 100%. The data shown are the mean ± SEM of three experiments. HEPES-BSA containing ZnCl2 (50 μmol/L) was used as the washing and assay buffers.
Effects of HK peptides on the binding of kallikrein to HUVEC. HUVEC were incubated with 70 nmol/L HK in the presence of 50 μmol/L Zn2+. After washing, 10 nmol/L kallikrein was added in the absence or presence of HK peptide (0.53 μmol/L). After further washing, bound kallikrein was measured using S2302. Binding of kallikrein to cells in the absence of peptides was considered to be 100%. The data shown are the mean ± SEM of three experiments. HEPES-BSA containing ZnCl2 (50 μmol/L) was used as the washing and assay buffers.
Effect of HK25c on the binding of kallikrein (⊡) and 125I-kallikrein (♦) to HUVEC-bound HK. (A) After preincubation with HK, HUVEC were incubated with increasing concentrations of HK25c in the presence of 10 nmol/L kallikrein or 125I-kallikrein. The ability of HK25c to inhibit kallikrein binding to cell-bound HK was determined by measuring the residual cell-associated kallikrein activity and 125I-kallikrein by radioactivity. The IC50 of HK25c determined in this manner was 50 ± 10 nmol/L (SD) for kallikrein and 150 nmol/L for 125I-kallikrein. (B) The kd of HK25c and kallikrein averaged from three binding assay plots of HK25c is 70 ± 10 nmol/L (SD).
Effect of HK25c on the binding of kallikrein (⊡) and 125I-kallikrein (♦) to HUVEC-bound HK. (A) After preincubation with HK, HUVEC were incubated with increasing concentrations of HK25c in the presence of 10 nmol/L kallikrein or 125I-kallikrein. The ability of HK25c to inhibit kallikrein binding to cell-bound HK was determined by measuring the residual cell-associated kallikrein activity and 125I-kallikrein by radioactivity. The IC50 of HK25c determined in this manner was 50 ± 10 nmol/L (SD) for kallikrein and 150 nmol/L for 125I-kallikrein. (B) The kd of HK25c and kallikrein averaged from three binding assay plots of HK25c is 70 ± 10 nmol/L (SD).
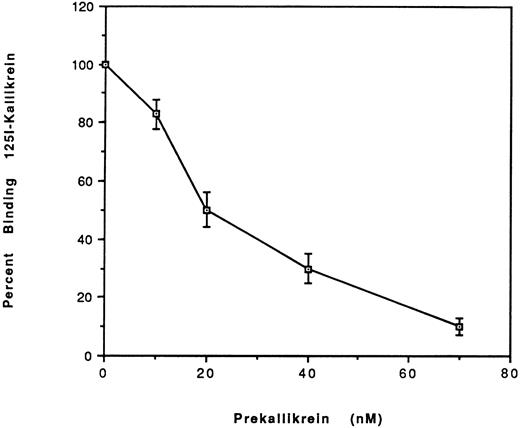
Comparison of the binding of prekallikrein with kallikrein to HUVEC.To address the question of whether PK binding to HUVEC-bound HK differed from kallikrein, we studied the binding of 125I-kallikrein (20 nmol/L) in the presence of increasing concentrations of unlabeled PK (Fig 6). The IC50 determined was 20 nmol/L, which indicates that the binding affinities for PK and kallikrein are similar.
Effect of plasma prekallikrein on the binding of 125I-kallikrein to HUVEC-bound HK. After preincubation with HK, HUVEC were incubated with increasing concentrations of plasma prekallikrein in the presence of 125I-kallikrein (20 nmol/L). The percentage of specific binding was calculated by obtaining the ratio of the specific binding in the presence of plasma PK to the specific binding in the absence of PK. Nonspecific binding is defined as the binding in the absence of HK and was subtracted from the total binding to yield the specific binding. The results are the mean ± SEM of three separate experiments.
Effect of plasma prekallikrein on the binding of 125I-kallikrein to HUVEC-bound HK. After preincubation with HK, HUVEC were incubated with increasing concentrations of plasma prekallikrein in the presence of 125I-kallikrein (20 nmol/L). The percentage of specific binding was calculated by obtaining the ratio of the specific binding in the presence of plasma PK to the specific binding in the absence of PK. Nonspecific binding is defined as the binding in the absence of HK and was subtracted from the total binding to yield the specific binding. The results are the mean ± SEM of three separate experiments.
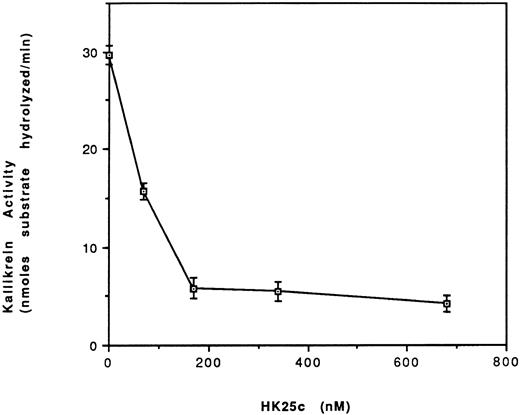
Effect of HK25c on the activation of PK to kallikrein in the presence of HK and HUVEC.We have shown that HK peptides can inhibit the binding of kallikrein to HUVEC-bound HK with an IC50 = 50 nmol/L. We therefore tested whether the most potent short peptide, HK25c, could prevent the activation of PK to kallikrein by preventing its binding to HUVEC-bound HK (Fig 7). Increasing the concentration of HK25c progressively inhibits the formation of kallikrein with an IC50 = 80 ± 8 nmol/L.
Effect of HK25c on the activation of PK to kallikrein in the presence of HK and HUVEC. HUVEC were incubated with HK (70 nmol/L) for 60 minutes at 4°C and the residual HK was removed. Prekallikrein (20 nmol/L), HK25c, and S2302 (0.37 mmol/L) were added and incubated at 23°C for 24 minutes. Kallikrein activity is expressed as nanomoles of substrate S2302 hydrolyzed per minute (mean ± SEM of 3 separate experiments).
Effect of HK25c on the activation of PK to kallikrein in the presence of HK and HUVEC. HUVEC were incubated with HK (70 nmol/L) for 60 minutes at 4°C and the residual HK was removed. Prekallikrein (20 nmol/L), HK25c, and S2302 (0.37 mmol/L) were added and incubated at 23°C for 24 minutes. Kallikrein activity is expressed as nanomoles of substrate S2302 hydrolyzed per minute (mean ± SEM of 3 separate experiments).
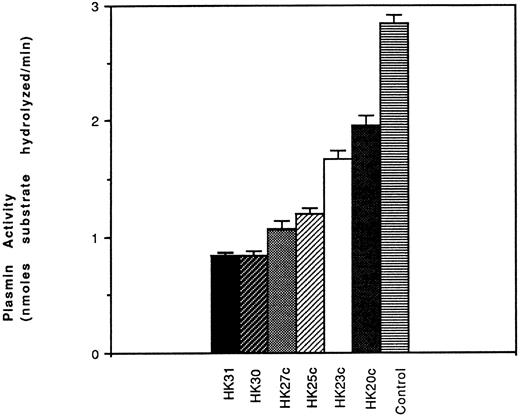
Effect of HK peptides on the generation of plasmin.To establish that urokinase-like plasminogen activator (uPA)-dependent plasmin generation was dependent on prekallikrein binding and activation on the surface of HUVEC, PK, plasminogen, and pro-UK were added together to HUVEC preincubated with HK (in the absence or presence of HK peptides). After 60 minutes of incubation, plasmin formation was measured. We found that several of the peptides, including HK31, HK30, HK27c, and HK25c, inhibited cell surface PA activity. HK25c was the shortest peptide with the ability to inhibit the PK binding to HUVEC-bound HK necessary for plasmin function. Among HK31, HK30, HK27c, and HK25c, HK25c was the shortest effective peptide capable of inhibiting the binding of PK to HK-bound HUVEC and, therefore, the generation of plasmin (Fig 8).
Effect of HK peptides on activation of plasminogen. HUVEC were preincubated with HK (70 nmol/L) for 60 minutes at 4°C. After removing the residual HK, PK (50 nmol/L), HK peptide, plasminogen (60 nmol/L), prourokinase (4.5 nmol/L), and H-D-Val-Leu-Lys-amino methyl coumarin (50 μmol/L) were added and incubated at 23°C for 60 minutes. The plasmin activity is represented as nanomoles of substrate hydrolyzed per minute (mean ± SEM of 3 separate experiments).
Effect of HK peptides on activation of plasminogen. HUVEC were preincubated with HK (70 nmol/L) for 60 minutes at 4°C. After removing the residual HK, PK (50 nmol/L), HK peptide, plasminogen (60 nmol/L), prourokinase (4.5 nmol/L), and H-D-Val-Leu-Lys-amino methyl coumarin (50 μmol/L) were added and incubated at 23°C for 60 minutes. The plasmin activity is represented as nanomoles of substrate hydrolyzed per minute (mean ± SEM of 3 separate experiments).
Requirements for plasminogen activation by u-PA on HUVEC to HUVEC-bound HK.Although Lenich et al12 indicated that factor XIIa is required for activation of plasminogen on HUVEC, recent studies by Motta et al9 indicate that this reaction may be independent of factor XII. To distinguish between these possibilities, we examined plasminogen activation in mixtures containing various combinations of HK, PK, and HUVEC on the activation of plasminogen in the presence of pro-UK (Table 2). Pro-UK by itself does not convert plasminogen to plasmin. When HUVEC, HK, and PK are present, a significant stimulation of plasminogen activation is observed. If prekallikrein is omitted, no plasminogen activation occurs, showing that prekallikrein is required for pro-UK activation. Taken together, these findings suggest that cell surface PA activity is promoted by a complex of PK and HK, which assembles on the HUVEC surface. We propose that PK binds to HUVEC-bound HK and, following its conversion to kallikrein, converts prourokinase to urokinase, which, in turn, hydrolyzes plasminogen to plasmin. Because no factor XII is present, the conversion of PK to kallikrein must be mediated by an enzyme present on the surface of HUVEC. This hypothesis is supported by the fact that DTNB blocks the conversion of prekallikrein to kallikrein as measured by S2302 activity (data not shown).
Effect of HUVEC, HK, and PK on the Activation of Plasminogen by Kallikrein-Activated Pro-UK
| . | HUVEC . | HK . | PK* . | Plasminogen . | Pro-UK . | Plasmin Substrate . | Net Cleavage of Plasmin Substrate (nmol in 46 min) . |
|---|---|---|---|---|---|---|---|
| a | + | + | + | + | + | + | 195 ± 5 |
| b | + | + | + | + | + | 0 | |
| c | + | + | + | + | + | 58 ± 5 | |
| d | + | + | + | + | + | 56 ± 4 | |
| e | + | + | + | + | 56 ± 4 | ||
| f | + | + | + | 0 |
| . | HUVEC . | HK . | PK* . | Plasminogen . | Pro-UK . | Plasmin Substrate . | Net Cleavage of Plasmin Substrate (nmol in 46 min) . |
|---|---|---|---|---|---|---|---|
| a | + | + | + | + | + | + | 195 ± 5 |
| b | + | + | + | + | + | 0 | |
| c | + | + | + | + | + | 58 ± 5 | |
| d | + | + | + | + | + | 56 ± 4 | |
| e | + | + | + | + | 56 ± 4 | ||
| f | + | + | + | 0 |
HK (70 nmol/L) was preincubated with HUVEC for 60 minutes at 4°C, and then excess HK was removed. The complete system for the generation of plasmin consists of HEPES-BSA buffer, pH 7.4, Zn2+ (50 μmol/L), HUVEC-bound HK, PK (50 nmol/L), plasminogen (60 nmol/L), and pro-UK (4.5 nmol/L) and the plasmin substrate (H-D-Val-Leu-Lys-amino methyl coumarin, 50 μmol/L). The resulting solution was incubated for 46 minutes at 23°C. The fluorescence emission intensity was recorded at 450 nm (λexcit = 360 nm) and used to calculate the amount of substrate hydrolyzed. HEPES-BSA buffer containing ZnCl2 (50 μmol/L) was used for all experiments.
PK contains 1% to 2% kallikrein.
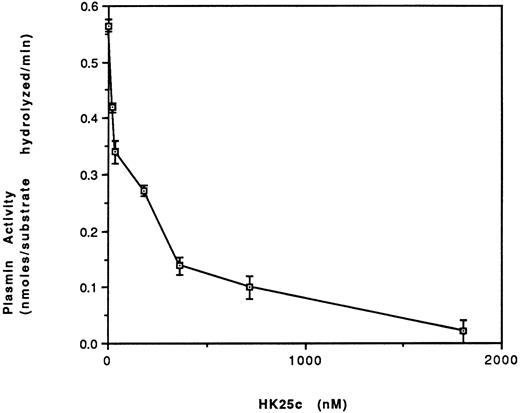
Effect of HK25c on plasmin formation.Increasing concentrations of HK25c progressively inhibit plasmin formation on HUVEC in the presence of HK, PK, prourokinase, and plasminogen with an IC50 = 100 ± 10 nmol/L SEM (Fig 9).
The effect of HK25c on the activation of plasminogen. HUVEC were incubated with HK (70 nmol/L) for 60 minutes at 4°C and then the residual HK was removed. PK (2.5 nmol/L), HK25c, plasminogen (60 nmol/L), prourokinase (4.5 nmol/L), and H-D-Val-Leu-Lys-amino methyl coumarin (50 nmol/L) were added in the presence of HEPES buffer, pH 7.0, and Zn2+ (50 μmol/L) and incubated for 170 minutes at 23°C. The plasmin activity is represented as nanomoles of substrate hydrolyzed per minute (mean ± SEM of 3 separate experiments).
The effect of HK25c on the activation of plasminogen. HUVEC were incubated with HK (70 nmol/L) for 60 minutes at 4°C and then the residual HK was removed. PK (2.5 nmol/L), HK25c, plasminogen (60 nmol/L), prourokinase (4.5 nmol/L), and H-D-Val-Leu-Lys-amino methyl coumarin (50 nmol/L) were added in the presence of HEPES buffer, pH 7.0, and Zn2+ (50 μmol/L) and incubated for 170 minutes at 23°C. The plasmin activity is represented as nanomoles of substrate hydrolyzed per minute (mean ± SEM of 3 separate experiments).
DISCUSSION
Mapping the minimal sequence necessary to allow specific interactions between HK and PK is critical for understanding the mechanisms that mediate complex formation between these two proteins. We performed some studies with kallikrein, measuring enzymatic activity, and others with prekallikrein. We therefore compared the binding to HUVEC-bound HK of unlabeled kallikrein with 125I-kallikrein. The binding of kallikrein showed a threefold decrease in affinity, which might be due to modification of tyrosine or histidine during radiolabeling, as previously noted.26 We also studied the inhibition of the binding to HUVEC-bound HK of 125I-kallikrein by prekallikrein and found the two proteins to be equivalent. Specific binding of prekallikrein or kallikrein is mediated via domain 6 of kininogen. Previous studies6,19-22 showed that HK31 contains sufficient structural features for binding of prekallikrein or kallikrein, and HK31 is frequently used to block the binding of kallikrein to HK in in vitro studies.27 These results provide the rationale for using peptides to solve the solution phase structures of the relevant binding domains of each of these proteins.
Vogel et al18 mapped the minimal HK binding sequence in PK to W569-K595, and You et al22 showed that C-terminal deletions of this core sequence cause perturbations in structure that result in peptides that do not bind to kallikrein. To obtain an effective peptide for inhibiting the binding of PK to HK, we reinvestigated the minimal sequence within D6 domain of HK necessary for this interaction.
Truncated peptides were prepared in which single amino acids were deleted from the N-terminus of HK31. We found that retention of D567 was critical for binding (an intermolecular event), consistent with previous studies demonstrating that D572 is essential for long-range, intramolecular electrostatic interactions6,21 22 that maintain the structure of HK31 in solution. Elimination of D566 did not adversely affect binding, consistent with the fact that D566 is not conserved between human, rat, and bovine kininogens. On the other hand, D567 and D572 are conserved between kininogens from these species.
C-terminal truncated peptides were also synthesized and tested for their ability to bind kallikrein. D567 was retained in these peptides for reasons discussed above. It has been shown that, in solution, an ion-pair found between K595 and D572 stabilizes the conformation of HK31.22 However, surprisingly, HK30, HK27c, and HK25c without K595 showed binding affinities similar to those of HK31. Thus, binding can still take place even in the absence of K595, suggesting that specific residues in prekallikrein may contribute molecular interactions, resulting in stabilization of the HK25c structure. We found that HK25c is the minimal sequence required for binding kallikrein; further deletions at the N- or C-terminal ends of HK25c cause a marked loss in binding ability. These results also suggest that K595 may contribute less to the binding of PK than to stabilizing the conformation of HK31.
It has recently been suggested that an intrinsic fibrinolytic pathway28 involving FXIIa, HK, prekallikrein, pro-UK, and plasminogen may form on the endothelial cell surface.12 Our studies confirm and extend preliminary reports9 suggesting that factor XII is not required for stimulation of cell surface plasminogen activator activity on HUVEC. Moreover, inhibition of this process by DTNB suggests that participation of a cysteine protease on the HUVEC surface is involved.9 According to this model, the binding of prekallikrein to endothelial cells is believed to be dependent on the presence of HK. Therefore, we hypothesized that we could use HK peptides to block binding of prekallikrein to HUVEC-bound HK and thereby effectively inhibit the activation of the fibrinolytic pathway. To test this hypothesis, HK31, HK27c, HK25c, HK23c, and HK20c were evaluated for their ability to inhibit the binding of kallikrein (Fig 1) to HUVEC-bound HK. We found that HK31, HK27c, and HK25c were most potent in this regard, whereas HK23c and HK20c were less effective. These results are consistent with our studies using a cell-free binding assay for measuring the binding of HK peptides to kallikrein (Fig 2). Also similar to the cell-free studies, HK25c was found to possess the minimal sequence capable of blocking the binding of kallikrein to HUVEC-bound HK (IC50 = 50 nmol/L; Fig 5) and inhibiting both the activation of PK to kallikrein (IC50 = 80 nmol/L; Fig 6), as well as the generation of plasmin (IC50 = 100 nmol/L; Fig 8). The kd for binding of HK25c to kallikrein, obtained in the presence of cells, is 70 nmol/L, which is also close to that (200 nmol/L) determined in the cell-free system.
For plasmin formation by prourokinase, prekallikrein is an absolute requirement (Table 2).13 29 This study shows that HUVEC, by providing a binding site for HK, localizes PK on its surface, allowing the activation of PK by a surface protease. Additional studies will be required to localize these binding sites and determine their physical proximity to cell-bound uPA and plasmin.
The inhibition of kallikrein or prekallikrein binding to HUVEC-bound HK by HK25c might have two physiologic consequences. First, such inhibition may decrease bradykinin liberation from HK. Bradykinin contributes to the development of hypotension in septicemia and multiple trauma. Second, inhibition of PK binding to cell-bound HK may also decrease the generation of plasmin on cell surfaces. Because both hypotension and hemorrhage are two of the major complications leading to the high morbidity and mortality of the systemic inflammatory response syndrome, peptides such as HK25c may serve as templates for the development of pharmaceutical agents that may be beneficial in such conditions and be of potential benefit in limiting hemorrhage resulting from excessive vascular fibrinolytic activity.
ACKNOWLEDGMENT
The authors thank Drs Jack Henkin and Andrew Mazar for their kind gift of recombinant prourokinase. The authors also thank the nursing staff of the Labor and Delivery Unit at Temple University Hospital for providing the umbilical cords used to isolate HUVEC and Rita Stewart and JoAnn Hamilton for their skills in preparing the manuscript.
Supported in part by National Institutes of Health Grants No. HL5489 (R.W.C.) and HL50827 (K.R.M.), and a grant from Commonwealth Biotechnologies, Inc (R.B.H.).
Address reprint requests to Robert W. Colman, MD, Sol Sherry Thrombosis Research Center, Temple University School of Medicine, 3400 N Broad St, Philadelphia, PA 19140.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal