Abstract
Inorganic arsenic trioxide (As2O3 ) was recently shown to induce apoptosis in NB4 promyelocytic leukemic cells. We have investigated the effects of the organic arsenical, melarsoprol (a drug used for treatment of trypanosomiasis), upon induction of apoptosis in cell lines representative of chronic B-cell lymphoproliferative disorders. An Epstein-Barr virus (EBV)-transformed B-prolymphocytic cell line (JVM-2), an EBV-transformed B-cell chronic lymphocytic leukemia (B-CLL) cell line (I83CLL), and one non–EBV-transformed B-CLL cell line (WSU-CLL) were used as targets. Dose-response experiments with melarsoprol (10−7 to 10−9 mol/L) were performed over 96 hours. Unexpectedly, we found that melarsoprol caused a dose- and time-dependent inhibition of survival and growth in all three cell lines. In contrast, As2O3 at similar concentrations had no effect on either viability or growth. After 24 hours, all three cell lines treated with melarsoprol (10−7 mol/L) exhibited morphologic characteristics of apoptosis. We also observed prominent concentration-dependent downregulation of bcl-2 mRNA after 24 hours of exposure to melarsoprol in WSU-CLL, I83CLL, and JVM-2 cells. Decrease of bcl-2 protein expression was also observed in all three cell lines, whereas As2O3 had no effect on this parameter. We conclude that melarsoprol may inhibit the growth of lymphoid leukemic cell by promoting programmed cell death. Results of these studies suggest that melarsoprol shows promising therapeutic activity in these diseases, and a study to evaluate clinical effects of this drug has been initiated.
ARSENIC-CONTAINING compounds have been used as cancer treatment for hundreds of years.1-3 Inorganic potassium arsenite (Fowler's solution) was used to control elevated leukocyte counts in chronic myelogenous leukemia in the early 1900s.2 3 With the advent of x-ray therapy and conventional cytotoxic drugs, the use of arsenicals for cancer treatment in Western countries has been abandoned. However, in traditional Asian folk medicine, arsenicals are still used for this purpose.
Arsenic trioxide (As2O3 ) was recently reported to induce complete remission in a high proportion of patients with refractory acute promyelocytic leukemia (APL).4 More recently, in vitro studies in NB4 cells showed that As2O3 induced apoptosis5 that was associated with a downregulation of bcl-2. The only arsenical currently formulated for human use in Western countries is melarsoprol, which is an organic arsenic compound synthesized by complexing melarsen oxide with the metal-chelating drug, dimercaprol.6 This agent has been used to treat African trypanosomiasis since the 1950s.7
Chronic lymphocytic leukemia (CLL), the most frequent type of adult leukemia in Western countries, is a chronic lymphoproliferative disorder characterized by the progressive accumulation of clonally derived, CD5+, CD19+ B lymphocytes.8 It has been suggested that prolonged life span, due to dysregulation of apoptosis, is a major factor contributing to the delayed senescence of these transformed lymphocytes.9
We have investigated both melarsoprol and arsenic trioxide for its effects on various lymphoid cells.
In these studies, we unexpectedly found that melarsoprol, but not As2O3 , exhibited potent cytotoxic activity that was associated with downregulation of bcl-2 protein expression and induced apoptosis.
MATERIALS AND METHODS
Reagents.Melarsoprol (kindly supplied as Arsobal [36 mg/mL] by Rhone Poulenc Rorer, Collegeville, PA) was further diluted in propylene glycol at a stock concentration of 10−4 mol/L and stored at room temperature. Serial dilutions (10−6 to 10−9 mol/L) were also made with propylene glycol. As2O3 (Sigma, St Louis, MO) was dissolved in 1.65 mol/L sodium hydroxide (NaOH) at a stock solution of 10−3 mol/L. Serial dilutions (10−6 to 10−9 mol/L) were made in RPMI 1640.
Cell lines.The well-characterized permanent human B-cell leukemia cell line JVM-210,11 was established from the peripheral blood of a patient with B-prolymphocytic leukemia (PLL) by Epstein-Barr virus (EBV) immortalization. The human chronic B-cell leukemia cell line, I83CLL, was derived from the peripheral blood of a patient with B-cell chronic lymphocytic leukemia (B-CLL) by EBV immortalization.12 The human EBV-negative cell line, WSU-CLL (generously provided by Dr Ayad Al-Katib, Wayne State University School of Medicine, Detroit, MI), was established from a patient with advanced B-CLL.13 All three cell lines and the human promyelocytic cell line HL-60 (used as a positive control for bcl-2 expression) were cultured in RPMI 1640, supplemented with 1% penicillin/streptomycin, 1 mmol/L L-glutamine (GIBCO, Grand Island, NY), 10% heat-inactivated fetal bovine serum (FBS; GIBCO) at 37°C, 5% CO2 in air.
Patient samples.Peripheral blood was drawn from patients with documented B-CLL after obtaining informed consent. B-CLL cells were separated by Ficoll-Hypaque centrifugation, washed three times in phosphate-buffered saline (PBS), and resuspended in RPMI 1640 supplemented with 10% FBS, 1% penicillin/streptomycin, and 1 mmol/L L-glutamine. A total of 3 × 105 cells/mL was used for viability experiments, which were incubated with or without melarsoprol (0.5 × 10−6 to 10−9 mol/L) or arsenic trioxide (10−6 to 10−9 mol/L) for 24 to 96 hours at 37°C, 5% CO2 in air.
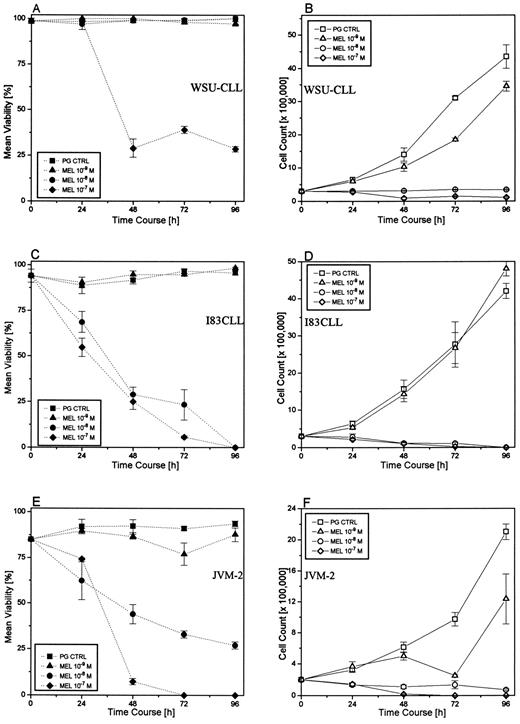
Effect of melarsoprol on the viability (A, C, and E) and cell growth (B, D, and F ) of B-CLL cell lines at various concentrations of melarsoprol for 96 hours. Control cells were cultured in the presence of polyethylene glycol alone (PG CTRL).
Effect of melarsoprol on the viability (A, C, and E) and cell growth (B, D, and F ) of B-CLL cell lines at various concentrations of melarsoprol for 96 hours. Control cells were cultured in the presence of polyethylene glycol alone (PG CTRL).
Effect of As2O3 on the viability (A, C, and E) and cell growth (B, D, and F ) of B-CLL cell lines at various concentrations of As2O3 for 96 hours. Control cells were cultured in the presence of the highest NaOH concentration used for dilutions of As2O3 (NaOH CTRL).
Effect of As2O3 on the viability (A, C, and E) and cell growth (B, D, and F ) of B-CLL cell lines at various concentrations of As2O3 for 96 hours. Control cells were cultured in the presence of the highest NaOH concentration used for dilutions of As2O3 (NaOH CTRL).
Morphologic changes showing features of apoptosis in B-CLL cell lines after treatment with melarsoprol for 24 hours (original magnification × 600). (A) Untreated WSU-CLL cells; (B) 10−8 mol/L melarsoprol-treated WSU-CLL cells; (C) untreated I83CLL cells; (D) 10−8 mol/L melarsoprol-treated I83CLL cells; (E) untreated JVM-2 cells; (F ) 10−8 mol/L melarsoprol-treated JVM-2 cells. The cells were stained using Giemsa-Wright stain.
Morphologic changes showing features of apoptosis in B-CLL cell lines after treatment with melarsoprol for 24 hours (original magnification × 600). (A) Untreated WSU-CLL cells; (B) 10−8 mol/L melarsoprol-treated WSU-CLL cells; (C) untreated I83CLL cells; (D) 10−8 mol/L melarsoprol-treated I83CLL cells; (E) untreated JVM-2 cells; (F ) 10−8 mol/L melarsoprol-treated JVM-2 cells. The cells were stained using Giemsa-Wright stain.
Viability and cell growth.Cell viability and growth were assessed by trypan blue (GIBCO) exclusion using a hemocytometer. Samples were collected and each sample was evaluated in triplicate.
Cell morphology.Using cellular and nuclear morphology, specific changes in DNA organization and cytoplasmic integrity, including membrane blebbing, cellular shrinkage, chromatin condensation, and fragmentation, were examined. For differential staining, cytocentrifuge preparations were stained using Wright-Giemsa stain (Diff Quick; Baxter, Miami, FL)
RNA isolation.Cells were lysed with 0.2 mL of RNAzolTMB (Tel-Test, Friendswood, TX) per 106 cells. Chloroform (0.2 mL/2 mL of lysate) was added to the samples; after vortexing, the suspension was incubated on ice for 5 minutes, followed by centrifugation at 12,000g at 4°C for 15 minutes. The aqueous phase was then transferred into a fresh tube and an equal volume of isopropanol was added. Samples were kept on ice for 60 minutes and centrifuged at 12,000g at 4°C for 15 minutes. The pellets were washed with 75% ethanol, dried, and resuspended in H2O. RNA concentration was determined by spectrophotometer at 260 nm.
Nonradioactive Northern blot analysis.Total cellular RNA (10 μg/lane) was size-fractionated by electrophoresis in a 1.2% agarose gel containing 0.7 mol/L formaldehyde, transferred to nylon membranes (Hybond N; Amersham, Arlington Heights, IL) by capillary suction overnight, and cross-linked by UV light (Stratalinker; Stratagene, La Jolla, CA). The cDNA probe for bcl-2, a 410-bp EcoRI fragment in pCR II vector (TA cloning vector; Invitrogen, San Diego, CA) digested with BamHI, was labeled with digoxygenin-UTP (DIG-UTP; Boehringer Mannheim, Indianapolis, IN) by in vitro transcription with respect to T7 RNA-polymerase. The cDNA probe for glyceraldehyde phosphate dehydrogenase (GAPDH), a 1,270-bp insert in pBS KS+ (ATCC), was linearized with EcoRI and labeled with DIG-UTP by in vitro transcription with respect to T7 RNA-polymerase. Hybridization was performed as previously described.14 Detection was accomplished by chemiluminescent reaction according to the Genius System (Boehringer Mannheim), as previously described.14
In situ deoxynucleotidyl transferase (TdT) assay.The in situ TdT assay was performed as previously described.14 15 Briefly, aliquots of cells were collected from liquid culture, centrifuged, and fixed in 0.3% buffered formaldehyde (pH 7.5). After washing with PBS, cells were resuspended in 70% ethanol (−20°C) and stored at −20°C. After rehydration with PBS, the cells were resuspended in 50 μL cacodylate buffer containing 0.2 mol/L potassium cacodylate, 2.5 mmol/L Tris-HCl (pH 6.6), 2.5 mmol/L cobalt chloride (CoCl2 ), 0.25 mg/mL bovine serum albumin (BSA), 7.5 U TdT, and 0.5 nmol/L biotinylated deoxyuracil-triphosphphate (b-dUTP; all reagents from Boehringer Mannheim). After an incubation period in this solution for 30 minutes at 37°C, cells were washed with PBS and resuspended in 100 mL of a solution containing 4× SSC, 25 μg/mL avidin-fluorescein-isothiocyanate (avidin-FITC; Boehringer Mannheim), 0.1% Triton X-100, and 5% wt/vol nonfat dry milk and incubated for 30 minutes in the dark at room temperature. Cells were washed with PBS containing 0.1% Triton X-100 and resuspended in 0.5 mL PBS containing 5 μg/mL propidium iodide (Sigma) and 0.1% RNAse A (Sigma). Green fluorescence detecting FITC levels and red fluorescence measuring propidium iodide content of individual cells were quantified on a FACStar (Becton Dickinson, Franklin Lakes, NJ). Data from 2 × 104 cells were collected and used for analysis.
Western blot analysis.Cells were lysed in a lysis buffer containing 20 mmol/L Tris-HCl, 1 mmol/L EGTA, 50 mmol/L NaVO4 , 50 mmol/L NaF, 0.01 U/mL aprotinin, 10 μg/mL leupeptin, 10 μg/mL pepstatin, 1 mmol/L elastinal, and 1 μmol/L phenylmethylsulfonyl fluoride (PMSF; all Sigma). The lysates were then sonicated using a ultrasonic homogenizer (4710 series; Cole Parmer Instruments, Chicago, IL) and centrifuged at 7,500g in a microfuge (Sorvall Instruments, Wilmington, DE), and the protein content of the lysates was determined (BioRad Protein Assay Kit I; BioRad, Melville, NY) at 595 nm with a BSA standard. Sample buffer containing 10% glycerol, 0.4% sodium dodecyl sulfate (SDS), 0.3% bromphenol blue, and 0.2% pyronin Y in 1× stacking buffer (Tris base 0.5 mol/L, 0.8% SDS) and 20% 2-mercaptoethanol was added to the cell lysates, which subsequently were heat-denaturated at 95°C for 3 minutes. Protein (10 μg/lane) was loaded on an SDS-polyacrylamide gel containing 12.5% polyacrylamide and size-fractionated by electrophoresis. Proteins were electroblotted onto Immobilon-P PVDF transfer membrane (Millipore, Bedford, MA) and immunostained with a mouse antihuman monoclonal bcl-2 antibody (1:5,000; Dako, Carpinteria, CA). Bound antibody was detected using the ECL chemiluminescence detection system (Amersham). Protein bands were quantified by computer densitometry.
RESULTS
Melarsoprol decreases viability and arrests cell growth in CLL cell lines.To investigate the potential effect of melarsoprol and As2O3 , respectively, on cell growth and survival, I83CLL, JVM-2, and WSU-CLL cells were used as targets. Treatment of the cells with melarsoprol (10−7 to 10−9 mol/L) over a time period of 96 hours led to a marked dose- and time-dependent inhibition of cell survival (Fig 1A through C) as determined using trypan blue exclusion. Exposure of the cell lines to 10−7 mol/L (WSU-CLL, I83CLL, and JVM-2) and 10 −8 mol/L (I83CLL and JVM-2) melarsoprol resulted in a significant decrease in cell viability, whereas lower concentrations (10−9 mol/L in I83CLL and JVM-2; 10−8 mol/L and 10−9 mol/L in WSU-CLL) were ineffective (Fig 1A through C).
Detection of apoptosis-associated DNA strand-breaks by in situ TdT assay in WSU-CLL cells. The cells were either untreated (CTRL) or exposed to melarsoprol for 24 and 48 hours, respectively. The dots represent the distribution of individual cells with respect to their b-dUTP incorporation and DNA content. The degree of b-dUTP complexes correlated with DNA strand-breaks per cell.
Detection of apoptosis-associated DNA strand-breaks by in situ TdT assay in WSU-CLL cells. The cells were either untreated (CTRL) or exposed to melarsoprol for 24 and 48 hours, respectively. The dots represent the distribution of individual cells with respect to their b-dUTP incorporation and DNA content. The degree of b-dUTP complexes correlated with DNA strand-breaks per cell.
Northern blot analysis of bcl-2 mRNA expression of three B-cell lines cultured in the presence of melarsoprol (A) or As2O3 (B) for 24 hours. Bcl-2–specific transcripts are shown at 8.5 kb.
Northern blot analysis of bcl-2 mRNA expression of three B-cell lines cultured in the presence of melarsoprol (A) or As2O3 (B) for 24 hours. Bcl-2–specific transcripts are shown at 8.5 kb.
Regulation of bcl-2 protein expression in I83CLL and WSU-CLL cells after treatment with melarsoprol. Cells (1 × 106/mL) were cultured alone (CTRL) or in the presence of melarsoprol for 24 hours.
Regulation of bcl-2 protein expression in I83CLL and WSU-CLL cells after treatment with melarsoprol. Cells (1 × 106/mL) were cultured alone (CTRL) or in the presence of melarsoprol for 24 hours.
In Vitro Effects of Melarsoprol on Cells From Patients With B-CLL
| Patient No. . | Day . | Mean Viability (%) . | ||||
|---|---|---|---|---|---|---|
| . | . | Control . | MEL 5 × 10−7 mol/L . | MEL 10−7 mol/L . | MEL 10−8 mol/L . | MEL 10−9 mol/L . |
| 1 | 1 | 83 ± 5 | 48 ± 6 | 74 ± 9 | 77 ± 2 | 66 ± 1 |
| 2 | 94 ± 1 | 52 ± 3 | 73 ± 4 | 74 ± 4 | 75 ± 6 | |
| 3 | 82 ± 5 | 43 ± 7 | 55 ± 3 | 73 ± 1 | 70 ± 4 | |
| 4 | 84 ± 4 | 49 ± 5 | 58 ± 7 | 66 ± 3 | 82 ± 7 | |
| 2 | 1 | 90 ± 2 | 59 ± 1 | 68 ± 9 | 81 ± 8 | 86 ± 2 |
| 2 | 90 ± 3 | 43 ± 8 | 66 ± 6 | 63 ± 7 | 78 ± 6 | |
| 3 | 91 ± 6 | 49 ± 11 | 64 ± 1 | 72 ± 3 | 77 ± 6 | |
| 4 | 77 ± 5 | 57 ± 17 | 48 ± 2 | 62 ± 13 | 64 ± 3 | |
| 3 | 1 | 87 ± 10 | 66 ± 7 | 82 ± 2 | 78 ± 7 | 79 ± 2 |
| 2 | 90 ± 2 | 57 ± 11 | 68 ± 8 | 74 ± 7 | 74 ± 7 | |
| 3 | 86 ± 8 | 59 ± 5 | 55 ± 6 | 53 ± 5 | 68 ± 2 | |
| 4 | 84 ± 4 | 41 ± 9 | 45 ± 6 | 52 ± 7 | 44 ± 11 | |
| 4 | 1 | 99 ± 2 | 65 ± 4 | 58 ± 7 | 68 ± 3 | 84 ± 4 |
| 2 | 87 ± 7 | 51 ± 1 | 52 ± 6 | 57 ± 4 | 69 ± 5 | |
| 3 | 71 ± 3 | 34 ± 3 | 41 ± 1 | 50 ± 11 | 48 ± 5 | |
| 4 | 64 ± 2 | 40 ± 4 | 30 ± 11 | 46 ± 3 | 46 ± 12 | |
| 5 | 1 | 86 ± 6 | 54 ± 2 | 57 ± 5 | 80 ± 11 | 82 ± 2 |
| 2 | 90 ± 3 | 47 ± 2 | 42 ± 9 | 54 ± 11 | 60 ± 8 | |
| 3 | 88 ± 4 | 55 ± 10 | 42 ± 6 | 51 ± 7 | 63 ± 8 | |
| 4 | 82 ± 7 | 46 ± 11 | 33 ± 9 | 51 ± 8 | 43 ± 11 | |
| Patient No. . | Day . | Mean Viability (%) . | ||||
|---|---|---|---|---|---|---|
| . | . | Control . | MEL 5 × 10−7 mol/L . | MEL 10−7 mol/L . | MEL 10−8 mol/L . | MEL 10−9 mol/L . |
| 1 | 1 | 83 ± 5 | 48 ± 6 | 74 ± 9 | 77 ± 2 | 66 ± 1 |
| 2 | 94 ± 1 | 52 ± 3 | 73 ± 4 | 74 ± 4 | 75 ± 6 | |
| 3 | 82 ± 5 | 43 ± 7 | 55 ± 3 | 73 ± 1 | 70 ± 4 | |
| 4 | 84 ± 4 | 49 ± 5 | 58 ± 7 | 66 ± 3 | 82 ± 7 | |
| 2 | 1 | 90 ± 2 | 59 ± 1 | 68 ± 9 | 81 ± 8 | 86 ± 2 |
| 2 | 90 ± 3 | 43 ± 8 | 66 ± 6 | 63 ± 7 | 78 ± 6 | |
| 3 | 91 ± 6 | 49 ± 11 | 64 ± 1 | 72 ± 3 | 77 ± 6 | |
| 4 | 77 ± 5 | 57 ± 17 | 48 ± 2 | 62 ± 13 | 64 ± 3 | |
| 3 | 1 | 87 ± 10 | 66 ± 7 | 82 ± 2 | 78 ± 7 | 79 ± 2 |
| 2 | 90 ± 2 | 57 ± 11 | 68 ± 8 | 74 ± 7 | 74 ± 7 | |
| 3 | 86 ± 8 | 59 ± 5 | 55 ± 6 | 53 ± 5 | 68 ± 2 | |
| 4 | 84 ± 4 | 41 ± 9 | 45 ± 6 | 52 ± 7 | 44 ± 11 | |
| 4 | 1 | 99 ± 2 | 65 ± 4 | 58 ± 7 | 68 ± 3 | 84 ± 4 |
| 2 | 87 ± 7 | 51 ± 1 | 52 ± 6 | 57 ± 4 | 69 ± 5 | |
| 3 | 71 ± 3 | 34 ± 3 | 41 ± 1 | 50 ± 11 | 48 ± 5 | |
| 4 | 64 ± 2 | 40 ± 4 | 30 ± 11 | 46 ± 3 | 46 ± 12 | |
| 5 | 1 | 86 ± 6 | 54 ± 2 | 57 ± 5 | 80 ± 11 | 82 ± 2 |
| 2 | 90 ± 3 | 47 ± 2 | 42 ± 9 | 54 ± 11 | 60 ± 8 | |
| 3 | 88 ± 4 | 55 ± 10 | 42 ± 6 | 51 ± 7 | 63 ± 8 | |
| 4 | 82 ± 7 | 46 ± 11 | 33 ± 9 | 51 ± 8 | 43 ± 11 | |
Numbers represent the mean and standard deviation evaluated in triplicate.
Abbreviation: MEL, melarsoprol.
In Vitro Effects of As2O3 on Cells From Patients With B-CLL
| Patient No. . | Day . | Mean Viability (%) . | ||||
|---|---|---|---|---|---|---|
| . | . | Control . | As2O3 10−6 mol/L . | As2O310−7 mol/L . | As2O310−8 mol/L . | As2O310−9 mol/L . |
| P1 | 1 | 87 ± 1 | ND | 91 ± 3 | 90 ± 4 | 89 ± 4 |
| 2 | 82 ± 5 | ND | 82 ± 1 | 83 ± 3 | 92 ± 4 | |
| 3 | 81 ± 4 | ND | 67 ± 2 | 74 ± 7 | 85 ± 8 | |
| 4 | 86 ± 3 | ND | 78 ± 4 | 84 ± 1 | 90 ± 4 | |
| P2 | 1 | ND | ND | ND | ND | ND |
| 2 | ND | ND | ND | ND | ND | |
| 3 | ND | ND | ND | ND | ND | |
| 4 | ND | ND | ND | ND | ND | |
| P3 | 1 | ND | ND | ND | ND | ND |
| 2 | ND | ND | ND | ND | ND | |
| 3 | ND | ND | ND | ND | ND | |
| 4 | ND | ND | ND | ND | ND | |
| P4 | 1 | 86 ± 1 | 87 ± 7 | 87 ± 8 | 88 ± 4 | 96 ± 6 |
| 2 | 89 ± 1 | 84 ± 5 | 87 ± 4 | 91 ± 8 | 91 ± 8 | |
| 3 | 83 ± 2 | 73 ± 5 | 76 ± 10 | 76 ± 8 | 84 ± 3 | |
| 4 | 80 ± 7 | 48 ± 9 | 72 ± 6 | 72 ± 11 | 71 ± 4 | |
| P5 | 1 | 81 ± 9 | 86 ± 6 | 85 ± 2 | 92 ± 7 | 88 ± 3 |
| 2 | 91 ± 0 | 92 ± 2 | 82 ± 16 | 86 ± 5 | 93 ± 2 | |
| 3 | 94 ± 3 | 93 ± 6 | 86 ± 8 | 94 ± 5 | 96 ± 7 | |
| 4 | 89 ± 7 | 92 ± 4 | 92 ± 3 | 91 ± 5 | 94 ± 2 | |
| Patient No. . | Day . | Mean Viability (%) . | ||||
|---|---|---|---|---|---|---|
| . | . | Control . | As2O3 10−6 mol/L . | As2O310−7 mol/L . | As2O310−8 mol/L . | As2O310−9 mol/L . |
| P1 | 1 | 87 ± 1 | ND | 91 ± 3 | 90 ± 4 | 89 ± 4 |
| 2 | 82 ± 5 | ND | 82 ± 1 | 83 ± 3 | 92 ± 4 | |
| 3 | 81 ± 4 | ND | 67 ± 2 | 74 ± 7 | 85 ± 8 | |
| 4 | 86 ± 3 | ND | 78 ± 4 | 84 ± 1 | 90 ± 4 | |
| P2 | 1 | ND | ND | ND | ND | ND |
| 2 | ND | ND | ND | ND | ND | |
| 3 | ND | ND | ND | ND | ND | |
| 4 | ND | ND | ND | ND | ND | |
| P3 | 1 | ND | ND | ND | ND | ND |
| 2 | ND | ND | ND | ND | ND | |
| 3 | ND | ND | ND | ND | ND | |
| 4 | ND | ND | ND | ND | ND | |
| P4 | 1 | 86 ± 1 | 87 ± 7 | 87 ± 8 | 88 ± 4 | 96 ± 6 |
| 2 | 89 ± 1 | 84 ± 5 | 87 ± 4 | 91 ± 8 | 91 ± 8 | |
| 3 | 83 ± 2 | 73 ± 5 | 76 ± 10 | 76 ± 8 | 84 ± 3 | |
| 4 | 80 ± 7 | 48 ± 9 | 72 ± 6 | 72 ± 11 | 71 ± 4 | |
| P5 | 1 | 81 ± 9 | 86 ± 6 | 85 ± 2 | 92 ± 7 | 88 ± 3 |
| 2 | 91 ± 0 | 92 ± 2 | 82 ± 16 | 86 ± 5 | 93 ± 2 | |
| 3 | 94 ± 3 | 93 ± 6 | 86 ± 8 | 94 ± 5 | 96 ± 7 | |
| 4 | 89 ± 7 | 92 ± 4 | 92 ± 3 | 91 ± 5 | 94 ± 2 | |
Numbers represent the mean and standard deviation evaluated in triplicate.
Abbreviation: ND, not done.
Exposure of the cells to melarsoprol also led to a marked dose- and time-dependent inhibition of cell growth. Treatment of the cells with the organic arsenical resulted in a 50% growth inhibitory concentration (IC50 ) of 10−7 and 10−8 mol/L in all three cell lines after 24 hours. Lower concentrations (10−9 mol/L) did not result in a growth arrest (Fig 1D through F ).
In contrast, exposure of all three cell lines to inorganic As2O3 at the same concentrations (10−7 to 10−9 mol/L) had no effect on the survival (Fig 2A through C) or growth of these cell lines (Fig 2D through F ).
Morphologic changes characteristic of apoptosis.Morphologic analysis showed characteristic apoptotic changes, such as chromatin condensation and fragmentation, membrane blebbing, and formation of apoptotic bodies, in all three cell line targets in a concentration-dependent manner after treatment with melarsoprol (10−7 to 10−9 mol/L; Fig 3)
Induction of apoptosis-related DNA strand-breaks by melarsoprol.WSU-CLL cells were treated with melarsoprol and examined for apoptosis-associated DNA strand breaks using the TdT assay. The intensity of labeling of apoptotic cells with biotinylated dUTP correlated with the number of DNA strand breaks per cell. The data showed that a significant number of cells exposed to the arsenical compound underwent apoptosis. Treatment of WSU-CLL cells with melarsoprol (10−8 mol/L) for 24 hours promoted apoptosis in 23.5% of the treated cells versus control cells, which exhibited 0.9% apoptosis. After 48 hours of incubation with melarsoprol, 85% of the cells were apoptotic, compared with untreated cells with 2.1% apoptosis (Fig 4).
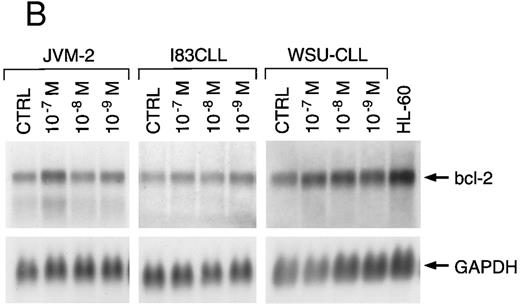
Regulation of bcl-2 expression by melarsoprol.We next examined the expression of bcl-2 mRNA and protein in I83CLL, JVM-2, and WSU-CLL cells after exposure to either the organic or the inorganic arsenical. Northern blot analysis showed a total loss of bcl-2 mRNA expression in I83CLL and WSU-CLL cells after 24 hours of incubation with 10−7 and 10−8 mol/L (Fig 5A). Melarsoprol concentrations as low as 10−9 mol/L still induced a significant decrease of bcl-2 transcripts. Similar effects were observed in JVM-2 cells (data not shown).
A dose-dependent response was also observed at the protein level. After 24 hours, bcl-2 protein expression was markedly reduced after incubation with 10−7 and 10−8 mol/L melarsoprol as compared with the expression of protein from untreated cells. A concentration of 10−9 mol/L had no effect at this time point (Fig 6). By contrast, no changes in bcl-2 expression were observed at either the mRNA (Fig 5B) or protein level (data not shown) after exposure to As2O3.
Melarsoprol decreases viability and cell growth in fresh CLL lymphocytes.Treatment of lymphocytes from five B-CLL patients of various clinical stages with melarsoprol (0.5−6 to 10−9 mol/L) resulted in a dose- and time-dependent decrease of viability (Table 1) and cell growth (not shown). In contrast, comparable concentrations of As2O3 (10−6 to 10−9 mol/L) generally had no effect on the growth or viability of freshly isolated cells from patients with B-CLL; however, a decline in viability to 48% (Table 2) and cell number (not shown) was noted with 10−6 mol/L As2O3 after 96 hours of exposure in one patient‘s sample (patient no. 4).
DISCUSSION
Recently, As2O3 , an inorganic arsenic compound, was found to induce apoptosis in NB4 APL cells,5,16 possibly by decreasing expression of bcl-2 mRNA and protein.5 Recently, clinical studies in China have shown that As2O3 treatment is an effective drug in patients with APL.17 Given As2O3’s lack of clinical availability in the West, we compared the effects of As2O3 with melarsoprol, an organic arsenical that has been well characterized as a treatment of late hemolymphatic and central nervous system African trypanosomiasis.7 Preliminary in vitro results by our group have suggested that this agent has broad efficacy against leukemic cell lines of both lymphoid and myeloid lineage.18 In all cell lines that responded to the drug, induction of apoptosis appeared to be a common feature. In trypanosomiasis, melarsoprol reversibly inhibits the cellular enzymes glutathione reductase and NADPH oxidase.19,20 Cellular resistance to the drug appears be mediated by the ars operon, which encodes repressor proteins that reduce arsenate to arsenite.21
In this study, we showed that melarsoprol unexpectedly reduced the growth and survival of cells that are characteristic of B-cell chronic lymphoid malignancies by triggering programmed cell death (apoptosis). These effects were associated with a dramatic decrease/loss of bcl-2 proto-oncogene expression, as observed at both mRNA and protein levels. By contrast, As2O3 has no major effect on viability or cell growth of these lymphoid cell lines or on the expression of apoptosis-related genes such as bcl-2 at in vivo pharmacologic concentrations ranging from 10−7 to 10−9 mol/L. Moreover, As2O3 did not significantly affect either the growth or viability of malignant lymphoid cells freshly isolated from patients with B-CLL. Because As2O3 had no effect on growth, survival, or apoptosis induction in other leukemic cell lines (U937 and HL-60),5 Chen et al5 suggested that this specific arsenical might be restricted to be a selective inducer of apoptosis in APL.5 However, our data using As2O3 in other myeloid leukemic cell lines do not confirm this speculation regarding such high selectivity.18
Apoptosis occurs during senescence of normal cells, and dysregulation of apoptosis may play a crucial role in the development of CLL.21-25 Altered expression of other gene products may also critically affect this process, including deletions of the retinoblastoma (RB) gene,26 p53 mutations,27 and overexpression of cyclin D2.28 Although bcl-2 gene rearrangement occurs infrequently in CLL, elevated levels of bcl-2 have been documented in most B-CLL cells.29 Thus, it is of particular interest that melarsoprol could induce apoptosis in cell lines derived from patients with this disease and in freshly isolated lymphocytes from B-CLL patients. As suggested by recent studies of As2O3 in NB4 cells, one mechanism by which melarsoprol may act is by regulating genes such as bcl-2 that are involved in this process. Whether bcl-2 selectively targeted by melarsoprol seems unlikely, and further investigations are underway to elucidate these additional mechanisms.
We conclude that melarsoprol appears be a potent inducer of programmed cell death in leukemic cell lines representative of chronic lymphoid cancers. Based on the data and related studies,18 we have initiated a clinical investigation broadly directed against both acute and chronic myeloid and lymphoid leukemias with this agent.30
Supported by grants from the Deutsche Forschungsgemeinschaft (Ko 1583/1-1) and the American Cancer Society (DHP-82).
Address correspondence to Andrea König, PhD, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, PO Box 320, New York, NY 10021.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal