Abstract
In contrast to other neoplasms, antigen-specific autologous cytolytic T cells have not been detected in patients with human pre-B–cell leukemias. The absence of efficient B7 family (B7-1/CD80; B7-2/CD86) -mediated costimulation has been shown to be a major defect in tumor cells' capacity to function as antigen-presenting cells. We show here the generation of autologous anti–pre-B–cell leukemia-specific cytolytic T-cell lines from the marrows of 10 of 15 patients with pre-B–cell malignancies. T-cell costimulation via CD28 is an absolute requirement for the generation of these autologous cytolytic T cells (CTL). Although costimulation could be delivered by either bystander B7 transfectants or professional antigen-presenting cells (indirect costimulation), optimal priming and CTL expansion required that the costimulatory signal was expressed by the tumor cell (direct costimulation). These anti–pre-B–cell leukemia-specific CTL lysed both unstimulated and CD40-stimulated tumor cells from each patient studied but did not lyse either K562 or CD40-stimulated allogeneic B cells. Cytolysis was mediated by the induction of tumor cell apoptosis by CD8+ T cells via the perforin-granzyme pathway. Although we were able to generate anti–leukemia-specific CTL from the bone marrow, we were unable to generate such CTL from the peripheral blood of these patients. These studies show that antigen-specific CTL can be generated from the bone marrow of patients with pre-B–cell leukemias and these findings should facilitate the design of adoptive T-cell–mediated immunotherapy trials for the treatment of patients with B-cell precursor malignancies.
OPTIMAL ANTIGEN-SPECIFIC T-cell expansion requires both a T-cell receptor signal conferring antigenic specificity and the delivery of a costimulatory signal that induces interleukin-2 (IL-2) production resulting in T-cell clonal expansion.1-4 Although many tumors can present peptide antigens in the context of the major histocompatibility complex to the T-cell receptor, few primary murine or human neoplastic cells express requisite costimulatory molecules necessary to induce clonal T-cell expansion and the generation of anti–tumor-specific cytolytic T lymphocytes (CTL).5-8 Nevertheless, autologous CTL have been detected and can be expanded from some patients with a variety of human malignancies.9-21
In contrast to other neoplasms, antigen-specific autologous CTL have not been detected in patients with human pre-B–cell leukemias. We have previously shown that primary human pre-B leukemia cells are either inefficient or ineffective allogeneic antigen-presenting cells (allo-APC). Such pre-B acute lymphoblastic leukemia (ALL) cells can be induced to express B7-family members (B7-1/CD80; B7-2/CD86) by signaling through the CD40 molecule on their cell surface. Moreover, we have shown that B7 expression is necessary to induce alloantigen-specific T-cell proliferation and that lack of B7 family member expression results in alloantigen-specific T-cell unresponsiveness.8 These observations support the hypothesis that the absence of B7 family-mediated costimulation contributes significantly to the paucity of antigen-specific T-cell–mediated immunity observed in patients with pre-B–cell malignancies.
In the present report, we attempted to generate anti–pre-B–cell leukemia-specific autologous T-cell lines from the bone marrows of patients with pre-B–cell ALL or pre-B–cell blastic crisis chronic myelogenous leukemia (BC-CML). We show that T-cell costimulation via CD28 is an absolute requirement for the generation of anti–pre-B leukemia autologous CTL. Although costimulation could be delivered by either bystander B7 transfectants or professional antigen-presenting cells (indirect costimulation), optimal priming and CTL expansion require that the costimulatory signal is expressed by the tumor cell (direct costimulation). Our results show that autologous anti–pre-B leukemia-specific CTL can be generated and that direct costimulation by B7 family members is required for their optimal expansion. These observations provide the basis for studies that may ultimately result in the design of adoptive T-cell–mediated immunotherapy clinical protocols for the treatment of patients with B-cell precursor malignancies.
MATERIALS AND METHODS
Pre-B–cell leukemia samples.Pre-B–cell ALL and pre-B–cell BC-CML cells were obtained from the bone marrow of patients with more than 90% marrow involvement. Appropriate informed consent and Institutional Review Board approval was obtained for all sample collections. Samples were enriched by density centrifugation over Ficoll-Hypaque and then washed twice in RPMI-1640 supplemented with 10% (vol/vol) fetal bovine serum (FBS) and 2 mmol/L L-glutamine (further referred to as RPMI-10 media).
B7-transfectants.The Chinese hamster ovarian cell line (CHO) was transfected by electroporation with either human B7-1 (t-B7-1), B7-2 (t-B7-2), or the resistance construct alone (t-Neo). The preparation and selection of transfectants has been previously reported.22 23 Transfectants were maintained in 45% Dulbecco's modified Eagle's medium (DMEM), 45% F12, 10% FBS, glutamine, 15 μg/mL gentamicin, and 200 μg/mL of G418 (hereafter referred as Tr-media). For the establishment of the t-B7 or t-Neo layers, cells were irradiated at 128 Gy, washed twice, plated at 5 × 105 cells/well in 6-well plates or 4 × 104 cells/well in 96-well plates in Tr-media without G418, and incubated overnight at 37°C in a 5% CO2 humidified atmosphere.
Allogeneic T cells, dendritic cells (DCs), and interferon-γ (IFN-γ)–activated monocytes.Peripheral blood T cells, DCs, and monocytes were obtained from healthy unrelated volunteers. Mononuclear cells (MNCs) were separated by density centrifugation over Ficoll-Hypaque. Non-T cells (B cells, natural killer [NK] cells, monocyte/macrophages, and DCs) were depleted by two rounds of magnetic bead depletion of cells expressing CD19 (monoclonal antibody [MoAb] B4), CD11b (MoAb Mo1), CD14 (MoAb Mo2), MHC II (MoAb 9-49), or CD56 (MoAb 3B8 or N901). The purified T cells were 95% to 99% CD3+ T cells. For the preparation of DCs, MNCs were plated for 1 hour at 37°C for the elimination of plastic-adherent cells, followed by magnetic bead depletion of cells expressing CD19, CD3 (MoAb OKT3), or CD56. The resulting fraction was cultured for 7 days in RPMI-10 media supplemented with recombinant human granulocyte-macrophage colony-stimulating factor (rhuGM-CSF; 50 ng/mL) and recombinant human IL-4 (rhuIL-4; 5 ng/mL). For the last 3 days of the culture, tumor necrosis factor-α (TNF-α; 50 U/mL) was added. The cells were then harvested and magnetic bead depletion was performed to eliminate cells expressing CD14, CD3, or CD56. Phenotype was confirmed by the expression of major histocompatibility complex (MHC) class I and II, CD83 (MoAb HB15), CD1a, B7-1, and B7-2. For the preparation of monocytes, the MNC adherent fraction was harvested, cryopreserved, and thawed 2 days before the experiment. After the removal of dead cells by density centrifugation, the monocyte fraction was depleted of cells expressing CD83, CD3, CD19, and CD56 by two-round magnetic bead depletion. The monocytes were then cultured for 48 hours in RPMI-10 supplemented with 100 U/mL of IFN-γ. Phenotype was confirmed by the expression of MHC I and II, CD14, B7-1, and B7-2.
MoAbs and fusion proteins.MoAbs were used as purified Ig. The anti–B7-1 (clone 4B2.C4), anti–B7-2 (clone 2B7), and anti-CD28 (clone 3D10) MoAbs and the CTLA4-Ig and control-Ig (C-Ig) fusion proteins were obtained were from Repligen Corp (Cambridge, MA). Anti-CD28 MoAb (clone 9.3) was kindly provided by Dr C. June (Bethesda, MD). Anti-FAS MoAb (clone ZB4) was kindly provided by Immunotech (Marseille, France). Anti-MHC I (W6/32), anti-MHC II (9-49), and anti-CD8 (T8) MoAbs were produced and purified in our laboratory. Fluorochrome-conjugation of anti-MHC I, anti–B7-1, C-Ig, and CTLA4-Ig was performed in our laboratory. Fluorochrome-conjugated MoAbs anti-MHC II, CD83, CD1a, and CD14 were kindly provided from Coulter (Miami, FL). Phycoerythrin (PE)-conjugated anti–B7-2 MoAb (clone IT2.2) was obtained from Pharmingen (San Diego, CA). The soluble CD40L (sCD40L) is a fusion protein of murine CD40L and CD8α chain24 and was kindly provided by Dr P. Lane (Basel, Switzerland).
Phenotypic analysis.Expression of cell surface molecules was determined by direct labeling using standard methodology. Fc receptors were blocked by incubation with mouse Ig before the addition of the specific MoAbs. The MoAbs used were fluorescein isothiocyanate (FITC)-conjugates anti-MHC I, anti–B7-1, anti-CD14, and CTLA4-Ig and PE-conjugates anti-MHC II, anti–B7-2, anti-CD1a, and anti-CD83. Irrelevant isotype-matched antibodies (IgG subclasses) or control fusion protein were used as negative controls. Appropriate controls were used to determine optimal voltage settings and electronic subtraction for the spectral fluorescence overlap correction. Samples were analyzed in a Coulter Elite flow cytometer and data were acquired in listmode files. At least 5,000 positive events were measured for each sample.
Stimulation of B-cell precursor ALL cells by CD40 cross-linking.Pre-B leukemia cells were stimulated by CD40 cross-linking, as previously described.8Briefly, NIH3T3 cells were stably transfected with the CD40L coding region (t-CD40L) and cultured in Tr-media. For the establishment of the t-CD40L or t-mock layers, cells were irradiated at 96 Gy, plated at 5 × 105 cells/well in 6-well plates in media without G418, and incubated overnight at 37°C in a 5% CO2 humidified atmosphere. Before culture of the pre-B leukemia cells, the plates were washed twice with phosphate-buffered saline, and tumor cells were cultured at 1 to 2 × 106 cells/mL in Iscove's modified Dulbecco's medium (IMDM) supplemented with 2% FBS, 0.5 mg/mL deionized bovine serum albumin, glutamine, 50 μg/mL iron-saturated transferrin, 5 μg/mL insulin, and 2 ng/mL of IL-4 (a gift of Immunex, Seattle, WA) hereafter referred to as B-cell media. On day 6, CD40-stimulated pre-B leukemia cells (CD40-ALL) were harvested, washed on IMDM, and used for phenotypic analysis and functional studies.
Primary and secondary mixed lymphocyte reaction (MLR).Irradiated (32 Gy) pre-B leukemia cells were used as stimulators, cocultured at 5 × 104 cells/well in a final volume of 200 mL with allogeneic T cells at 1 × 105 cells/well in 96-well round-bottom plates, and incubated for 5 days at 37°C in a 5% CO2/95% air humidified atmosphere. The stimulator/responder cell ratio and time of incubation were determined as the optimal culture conditions. The culture medium used was RPMI supplemented with 4% heat-inactivated human AB serum (NABI, Miami, FL), glutamine, and 25 mmol/L of HEPES buffer (further referred to as HS-4 media). All microcultures were performed in triplicate. Cells were pulsed with 1 μCi of [3H]-Thymidine (Du Pont, Boston, MA) for the last 18 hours of the culture period. Cells were then harvested onto glass fiber filters and the [3H]-Thymidine incorporation was measured by liquid scintillation spectrophotometry (1205 Betaplate; Pharmacia). Stimulation indexes (SI) were calculated for each individual experiment as follows: SI = cpm(T cells + ALL cells)/cpm(T cells). Costimulatory or blocking MoAbs or fusion proteins were added to the MLR microcultures as indicated, including anti-CD28 (2.5 μg/mL) or CTLA4-Ig (10 μg/mL). Appropriate isotype-matched irrelevant antibodies and a control-Ig fusion protein were used as controls at the same concentration.
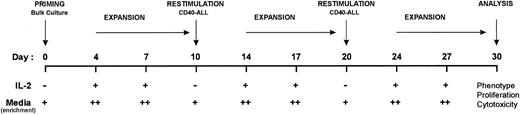
Generation of anti–leukemia-specific T-cell lines.The generation and expansion of autologous anti–leukemia-specific T-cell lines was performed using the methodology depicted in Fig 2. High-density (5 × 106/mL) bulk cultures of bone marrow (>90% marrow infiltration) were initiated in the presence of (1) a feeder layer of CHO B7-1 (t-B7-1) and/or CHO B7-2 (t-B7-2), (2) anti-CD28 MoAb (2.5 μg/mL), or (3) 25% (vol/vol) soluble CD40L (sCD40L) in the presence or absence of rhuIL-2 (10 U/mL). Cultures were performed in RPMI supplemented with 2% human AB serum (further referred as RPMI-HS2) and incubated at 37°C. After 4 days, cells were harvested, and dead cells were eliminated by density centrifugation over Ficoll-Hypaque. Live cells were then cultured at 1 to 2 × 106/mL in IMDM supplemented with 4% human AB serum (further referred as IMDM-HS4) and rhuIL-2 (10 U/mL) and incubated at 37°C. On day 7, fresh IMDM-HS4 media and IL-2 were added. On day 10, cells were harvested, dead cells were eliminated, and the bulk culture was restimulated with syngeneic CD40-stimulated leukemia cells. The same sequence was repeated twice, as described in Fig 2.
Protocol for the generation and expansion of autologous anti–leukemia-specific T-cell lines from the bone marrow of patients with pre-B–cell leukemias. T-cell lines were primed from bulk bone marrow in the presence of sCD40L, CD28 MoAb, or bystander costimulators and expanded by restimulation with syngeneic CD40-stimulated leukemia cells and IL-2 (10 U/mL) when indicated. Media enrichment was performed as follows: + for RPMI-HS2; ++ for IMDM-HS4.
Protocol for the generation and expansion of autologous anti–leukemia-specific T-cell lines from the bone marrow of patients with pre-B–cell leukemias. T-cell lines were primed from bulk bone marrow in the presence of sCD40L, CD28 MoAb, or bystander costimulators and expanded by restimulation with syngeneic CD40-stimulated leukemia cells and IL-2 (10 U/mL) when indicated. Media enrichment was performed as follows: + for RPMI-HS2; ++ for IMDM-HS4.
Cytotoxic assay.Cell-mediated toxicity was determined using a standard 51Cr-release assay.25 Unstimulated and CD40-stimulated pre-B leukemia cells were used as targets, as well as CD40-stimulated allogeneic B cells, autologous malignant, and K562 cells. Target cells were incubated with 0.1 mCi of 51Cr per 106 cells for 4 hours at 37°C, followed by 5 washes in HS-4 media. Labeled target cells were then plated in 96-well U-bottom plates at 5,000 cells/well. T-cell lines generated from the patient's bone marrow were used as effector cells and plated at different effector/target ratios (5:1 to 40:1). All of the experiments were performed in triplicate. The plates were centrifuged for 3 minutes at 300 rpm and incubated for 5 hours at 37°C. After the incubation period, the supernatants were harvested using the Skatron filters (Skatron Instruments, Sterling, VA) and radioactivity was measured in an automatic gamma counter (LKB Wallac, Turku, Finland). Specific lysis was determined for each individual experiment as: Specific Lysis (%) = ([Experimental 51Cr Release − Spontaneous 51Cr Release]/[Maximum 51Cr Release/Spontaneous 51Cr Release]) × 100. Maximum release was determined by the addition of 2% sodium dodecyl sulfate to the target cells. For the blocking experiments, anti–LFA-3, anti–ICAM-1, anti-MHC I, and anti-Fas MoAbs were used at 10 μg/mL. CD8 depletion was performed by magnetic bead depletion, and the depleted population was greater than 95% CD4+ T cells. Brefeldin A was used at 5 μg/mL.
DNA fragmentation.To determine the mechanism of cytolysis, target cells were cultured with T-cell lines at effector-to-target ratio of 40:1 for 6 hours at 37°C in HS-4 media. Unstimulated and CD40-stimulated pre-B leukemia cells were used as target. After incubation, cells were harvested and lysed using a NP-40 buffer containing 10 mmol/L EDTA, 50 mmol/L Tris-HCl, 0.5% (wt/vol) N-laurylsarcosine, and 0.5 mg/mL proteinase K. The DNA crude preparation was loaded on a 3% agarose gel, electrophoresed, and photographed.
Statistical analysis.The statistical significance between the treatment groups was determined using the Wilcoxon test for matched-pairs.
RESULTS
Direct T-cell costimulation is superior to bystander costimulation in repairing defective pre-B–cell ALL antigen-presenting cell function.In an effort to generate anti–ALL-specific T-cell–mediated immunity, we first sought to develop a simple yet efficient method to correct the inability of pre-B ALL cells to function as alloAPC. Therefore, we compared multiple methods of CD28-mediated costimulation using either bystander costimulators (B7 family transfectants or professional APC) or direct costimulators (CD40-stimulated tumor cells).
The intensity of B7-1 and B7-2 expression by bystander costimulators or direct costimulators was compared by direct immunofluorescence. As shown in Fig 1A, B7 family transfectants consistently expressed high levels of either B7-1 or B7-2. Although mature DCs and IFN-γ–activated monocytes expressed both B7-1 and B7-2, DCs consistently expressed higher levels (>1 log MIF ) than monocytes. Whereas CD40-stimulated pre-B ALL cells consistently expressed high levels of both B7-1 and B7-2 (comparable to DCs), none of the unstimulated pre-B cell ALL tested expressed B7-1 and, of 56 pre-B ALLs examined to date, variable levels of B7-2 could be detected on 34 of them (a representative B7-1−, B7-2− case shown in Fig 1A).
(A) Expression of B7-1 and B7-2 molecules by CHO-transfectants, professional APCs, and pre-B ALL cells (unstimulated and CD40-stimulated). The histograms shown for DCs and macrophages are from a single donor and are representative of five donors. Open areas represent fluorescence distribution of the B7-1 or B7-2 molecules and solid areas represent that of isotype-matched control antibodies. The cell number is shown on the Y-axis.
(B) Proliferative responses of purified allogeneic CD3+ T cells to irradiated pre-B ALL cells in the presence or absence of direct or bystander costimulation. Proliferation was assessed by measuring [3H] thymidine incorporation for the last 18 hours of the 6-day assay. The SI were calculated for each individual experiment as follows: SI = cpm(T cells + ALL cells)/cpm(T cells) . Bars represent mean ± SEM of the SI from the MLRs using 4 different patients and 5 different allogeneic T-cell donors. Allogeneic DCs and monocytes were syngeneic with the T cells. Control-Ig (C-Ig) and CTLA4-Ig fusion proteins were used at 10 μg/mL. Anti-CD28 MoAb (CD28) was used at 2.5 μg/mL. (C) Secondary proliferative responses of T cells primed by pre-B ALL cells in the presence of bystander costimulation. Proliferation was assessed by measuring [3H] thymidine incorporation for the last 18 hours of the 6-day assay. Bars represent mean ± SEM of the SI.
(A) Expression of B7-1 and B7-2 molecules by CHO-transfectants, professional APCs, and pre-B ALL cells (unstimulated and CD40-stimulated). The histograms shown for DCs and macrophages are from a single donor and are representative of five donors. Open areas represent fluorescence distribution of the B7-1 or B7-2 molecules and solid areas represent that of isotype-matched control antibodies. The cell number is shown on the Y-axis.
(B) Proliferative responses of purified allogeneic CD3+ T cells to irradiated pre-B ALL cells in the presence or absence of direct or bystander costimulation. Proliferation was assessed by measuring [3H] thymidine incorporation for the last 18 hours of the 6-day assay. The SI were calculated for each individual experiment as follows: SI = cpm(T cells + ALL cells)/cpm(T cells) . Bars represent mean ± SEM of the SI from the MLRs using 4 different patients and 5 different allogeneic T-cell donors. Allogeneic DCs and monocytes were syngeneic with the T cells. Control-Ig (C-Ig) and CTLA4-Ig fusion proteins were used at 10 μg/mL. Anti-CD28 MoAb (CD28) was used at 2.5 μg/mL. (C) Secondary proliferative responses of T cells primed by pre-B ALL cells in the presence of bystander costimulation. Proliferation was assessed by measuring [3H] thymidine incorporation for the last 18 hours of the 6-day assay. Bars represent mean ± SEM of the SI.
To compare direct and bystander costimulators for their capacity to costimulate alloantigen-induced T-cell proliferation (allogeneic MLR), allogeneic T cells from 5 distinct unrelated donors were isolated (>95% CD3+) and used as responders (Fig 1B). Each allogeneic T-cell responder was cocultured with each of four B7-1−, B7-2− pre-B ALL cells to present alloantigen to the T-cell receptor (TCR signal). Costimulation was provided by (1) direct costimulation (anti-CD28 MoAb or CD40 stimulation of ALL cells; Fig 1B, upper panel), (2) bystander transfectants (B7 family transfectants, t-B7-1 and/or t-B7-2; Fig 1B, middle panel), or (3) bystander allogeneic professional APCs (mature DCs or activated monocytes; Fig 1B, lower panel). As expected, pre-B ALL cells alone could not induce allogeneic T-cell proliferation (Fig 1B, upper panel). As we have previously observed, the addition of anti-CD28 MoAb or previous stimulation of the tumor cells by CD40 cross-linking provided sufficient direct costimulation to induce highly significant allogeneic T-cell proliferation. Somewhat surprisingly, transfectants expressing B7-1, B7-2, or the combination provided significantly inferior costimulation compared with either anti-CD28 MoAb (P < .001) or CD40-ALL cells (P < .0001; Fig 1B, middle panel). Although the magnitude of the response was low, such bystander costimulation resulted in a consistently higher (P < .01) level of T-cell alloreactivity compared with control transfectants or unstimulated pre-B ALL cells alone (Fig 1B, middle panel). Bystander costimulation was induced by B7 family members, because it was abrogated by the B7-family–specific blocker CTLA4-Ig (Fig 1B, middle panel).
We next sought to determine whether professional APCs also could provide bystander costimulation and whether this would be superior to bystander costimulation provided by transfectants. DCs and monocytes autologous for the T-cell donor were expanded, activated, purified (>95% pure), and added to the coculture of responder T cells and the B7-1−, B7-2− stimulator pre-B ALL cells. Similar to B7 family transfectants, both mature DCs and IFN-γ activated monocytes provided bystander costimulation that was totally abrogated by CTLA4-Ig (Fig 1B, lower panel). The observed low stimulation index was comparable to that observed with B7 family transfectants. DCs were consistently more effective (SI range, 4.8 to 11.3; P < .05) than monocytes (SI range, 3.7 to 7.5) as bystander costimulators.
To determine whether the costimulation provided by transfectants resulted in alloantigen-specific immunity, allogeneic T cells initially primed by pre-B ALL cells in the presence of bystander costimulation were tested in a secondary MLR against (1) primary target (pre-B ALL), (2) CD40-stimulated ALL from the identical patient (CD40-ALL), or (3) CD40-stimulated B cells from an unrelated donor (CD40-B). As shown in Fig 1C, allogeneic T cells initially primed with pre-B ALL cells in the presence of transfectants or CD40-ALL proliferated on rechallenge to either unstimulated or CD40-stimulated ALL cells but not to CD40-B cells from unrelated donors, thus demonstrating the antigen specificity of these responses.
Direct T-cell costimulation is superior to bystander costimulation in the generation and expansion of autologous anti–pre-B ALL–specific T-cell immunity.Considering the variable effectiveness of direct versus bystander costimulation in the generation of alloreactive anti-ALL specific T cells, we sought to determine whether autologous anti–leukemia-specific T-cell immunity could be generated using these methodologies (Fig 2). Pre-B ALL bone marrows (>90% leukemia CD10+ CD19+ sμ− cells) were primed in bulk culture (1 × 108 bone marrow MNCs per condition) at high density either in the presence of anti-CD28 MoAb or soluble CD40L (direct costimulation) or irradiated B7-transfected CHO cells (bystander costimulators). Soluble CD40L was used for priming because it provided comparable levels of costimulation to that observed with previously activated CD40-ALL cells (data not shown).
(A) T-cell lines generated from the bone marrow of leukemia patients recognize and proliferate when challenged by unstimulated and CD40-stimulated leukemia cells: Comparison of direct or bystander costimulation. Proliferation was assessed by measuring [3H] thymidine incorporation for the last 18 hours of the 3-day assay. The results shown are from one experiment and are representative of three independent experiments (3 different patients).
(B) T-cell lines generated from the bone marrow of leukemia patients are capable of lysing both unstimulated and CD40-stimulated leukemia cells: Comparison of direct or bystander costimulation. Cytotoxicity was assessed as described in the Materials and Methods. The results shown are from one experiment and are representative of three independent experiments (3 different patients).
(A) T-cell lines generated from the bone marrow of leukemia patients recognize and proliferate when challenged by unstimulated and CD40-stimulated leukemia cells: Comparison of direct or bystander costimulation. Proliferation was assessed by measuring [3H] thymidine incorporation for the last 18 hours of the 3-day assay. The results shown are from one experiment and are representative of three independent experiments (3 different patients).
(B) T-cell lines generated from the bone marrow of leukemia patients are capable of lysing both unstimulated and CD40-stimulated leukemia cells: Comparison of direct or bystander costimulation. Cytotoxicity was assessed as described in the Materials and Methods. The results shown are from one experiment and are representative of three independent experiments (3 different patients).
The methodologic schema used for the generation of autologous anti–ALL-specific T-cell immunity is summarized in Fig 2 and is divided into stages: (1) priming, (2) repetitive expansion and restimulation, and (3) analysis of T-cell lines generated. As shown, antigen-specific T cells were primed in bulk culture, expanded, and restimulated with CD40-ALL cells, followed by another round of expansion and restimulation. As detailed in the Materials and Methods, cultures were initially primed with either direct or bystander costimulators and restimulated on days 10 and 20 with irradiated CD40-ALL cells. Proliferating autologous T cells were harvested on day 30 and phenotype and function was determined. Figure 3A and B and Table 1 summarize a representative patient (ALL-17) and identical results were observed for two others (ALL-21 and ALL-28). No viable cells were detected from cocultures of bone marrow cells with CHO-Neo transfectants (Table 1). After 30 days, virtually all cells in these cultures expressed CD3 and TCRαβ and lacked NK markers (data not shown). By day 30, the autologous T-cell lines generated in the presence of bystander costimulation (t-B7-1, t-B7-2, or t-B7-1 + t-B7-2 transfectant cells) specifically proliferated on rechallenge with unstimulated and CD40-stimulated syngeneic pre-B ALL but not to third-party allogeneic CD40-B cells (Fig 3A, upper panel, bystander costimulation). Likewise, autologous T-cell lines generated under these conditions induced specific cytolysis of either unstimulated or CD40-stimulated syngeneic pre-B ALL cells but not third-party (CD40-B) or NK-sensitive targets (K562; Fig 3B, upper panel, bystander costimulation).
Expansion of Autologous T Cells From Bulk Cultures of Pre-B–Cell ALL Bone Marrow in the Presence of Direct or Bystander Costimulation
| Conditions . | T-Cell Number . | T-Cell Expansion . | |||
|---|---|---|---|---|---|
| . | CD4+ . | CD8+ . | (fold increase) . | ||
| . | . | . | Total . | CD4+ . | CD8+ . |
| t-NEO | 0 | 0 | 0 | 0 | 0 |
| t-B7-1 | 1.3 × 106 | 3.2 × 106 | 3.1 | 1.7 | 4.6 |
| t-B7-2 | 1.1 × 106 | 2.2 × 106 | 2.2 | 1.4 | 3.1 |
| t-B7-1 + t-B7-2 | 1.3 × 106 | 2.6 × 106 | 2.6 | 1.7 | 3.7 |
| CD28 MoAb | 13.2 × 106 | 34.3 × 106 | 32.7 | 16.5 | 49.0 |
| CD28 MoAb + IL-2 | 17.1 × 106 | 36.9 × 106 | 37.3 | 21.4 | 52.7 |
| sCD40L | 18.1 × 106 | 48.9 × 106 | 44.7 | 22.6 | 69.8 |
| sCD40L + IL-2 | 23.0 × 106 | 46.8 × 106 | 48.0 | 28.8 | 66.9 |
| Conditions . | T-Cell Number . | T-Cell Expansion . | |||
|---|---|---|---|---|---|
| . | CD4+ . | CD8+ . | (fold increase) . | ||
| . | . | . | Total . | CD4+ . | CD8+ . |
| t-NEO | 0 | 0 | 0 | 0 | 0 |
| t-B7-1 | 1.3 × 106 | 3.2 × 106 | 3.1 | 1.7 | 4.6 |
| t-B7-2 | 1.1 × 106 | 2.2 × 106 | 2.2 | 1.4 | 3.1 |
| t-B7-1 + t-B7-2 | 1.3 × 106 | 2.6 × 106 | 2.6 | 1.7 | 3.7 |
| CD28 MoAb | 13.2 × 106 | 34.3 × 106 | 32.7 | 16.5 | 49.0 |
| CD28 MoAb + IL-2 | 17.1 × 106 | 36.9 × 106 | 37.3 | 21.4 | 52.7 |
| sCD40L | 18.1 × 106 | 48.9 × 106 | 44.7 | 22.6 | 69.8 |
| sCD40L + IL-2 | 23.0 × 106 | 46.8 × 106 | 48.0 | 28.8 | 66.9 |
At day 0, 1 × 108 MNCs were plated per condition in the bulk culture. For the patient represented here (ALL-17), T cells represented 1.5% of the bone marrow MNCs and the initial number of CD4+ and CD8+ T cells per condition was, respectively, 0.8 × 106 and 0.7 × 106 cells.
Alternatively, the use of either anti-CD28 MoAb or sCD40L during priming resulted in a significant increase in specific T-cell proliferation on rechallenge with either unstimulated and CD40-stimulated syngeneic pre-B ALL cells (Fig 3A, lower panel, direct costimulation) and increased specific cytolysis (Fig 3B, lower panel, direct costimulation). The addition of IL-2 during priming with anti-CD28 MoAb or sCD40L resulted in 25% to 30% reduction in specific T-cell proliferation on rechallenge with tumor cells as compared with priming with either anti-CD28 MoAb or sCD40L alone (Fig 3A, lower panel). This reduction is likely due to the dilution effect of antileukemia CTL by nonspecific T cells that resulted from the addition of IL-2 during the priming phase. Moreover, the addition of IL-2 during priming resulted in the generation of T cells capable of proliferating to and killing allogeneic CD40-B cells, thus suggesting that IL-2 signaling also induced the expansion of nonspecific T cells (Fig 3B, lower panel; P < .05).
Phenotypic analysis of the multiple T-cell lines generated under the various priming conditions was undertaken. At priming, the CD4+ and CD8+ T cells represented less than 2% of the total MNC fraction (data not shown) and for the ALL-17 represented, respectively, 0.8% and 0.7% of the bone marrow MNCs. By day 30, the majority of cells expressed CD8, with a ratio CD8:CD4 of 2:1, regardless of the conditions used during priming (Table 1). However, the absolute numbers of T cells (both CD4+ and CD8+) were dependent on the conditions used for the priming phase. Strikingly, the expansion of the T-cell lines, calculated from the initial number of T cells present in the primary bone marrow culture, were significantly lower (10- to 22-fold; P < .001) for both CD4+ and CD8+ when the B7-transfectants were used as bystander costimulators as compared with direct costimulation by either CD28 MoAb or sCD40L (Table 1). Taken together, these results show that, although bystander B7-costimulation during priming can contribute to the generation of anti–leukemia-specific cytotoxic T-cell lines, it leads to T-cell expansion significantly inferior to that induced by direct costimulation.
Priming pre-B–cell tumors with soluble CD40 ligand permits the generation and expansion of autologous anti–leukemia-specific cytotoxic T cells in 10 of 15 patients.Because sCD40L stimulation resulted in optimal generation and expansion of autologous anti–leukemia-specific T cells in the 3 pre-B ALL patients tested, we sought to determine whether this methodology could be generalized. Patients with pre-B ALL and pre-B BC-CML with more than 90% of marrow involvement were selected, and the bone marrow MNC fraction was cultured at high density in the presence of sCD40L. Phenotypic analysis of the bulk bone marrow showed low numbers of T cells (Table 2), which represented from 1.6% to 4.5% of the MNCs (0.4% to 2.7% CD4+ cells; 0.7% to 1.9% CD8+ cells). After 30 days in culture, cell expansion was observed in 10 of the 15 patients (Table 2). The cells generated were CD3+ T cells and in all cases tested expressed TCRαβ (data not shown). Phenotypic analysis showed that the majority of these cells were CD8+ T cells, representing 58% to 80% of the total cells. The T-cell expansion was determined comparing the total number of T cells generated in the system with the initial number of T cells in the bulk marrow and ranged from 6.5- to 53.8-fold increase. As shown in Table 2, the T-cell expansion was variable for both CD4+ and CD8+ T cells, ranging from 7- to 29.9-fold for the CD4+ subset and 5.7- to 84-fold to the CD8+ subset. Interestingly, in 9 of 10 cases (except for CML-1), the expansion of CD8+ cells was 2.2- to 4.3-fold higher than observed for CD4+ cells.
Generation and Expansion of Autologous Antileukemia T-Cell Lines From the Bone Marrow of Patients With Pre-B–Cell Leukemias (Pre-B ALL and Pre-B CML)
| Patient . | Day 0 (T-cell number) . | Day 30 (T-cell number) . | T-Cell Expansion . | |||
|---|---|---|---|---|---|---|
| . | CD4+ . | CD8+ . | CD4+ . | CD8+ . | (fold increase) . | |
| . | . | . | . | . | CD4+ . | CD8+ . |
| ALL-17 | 0.8 × 106 | 0.7 × 106 | 18 × 106 | 49 × 106 | 22.5 | 70.0 |
| ALL-20 | 0.9 × 106 | 1.2 × 106 | 26 × 106 | 82 × 106 | 28.9 | 68.3 |
| ALL-21 | 1.1 × 106 | 0.6 × 106 | 22 × 106 | 50 × 106 | 19.6 | 84.0 |
| ALL-25 | 1.3 × 106 | 0.7 × 106 | 11 × 106 | 18 × 106 | 8.2 | 26.1 |
| ALL-28 | 1.1 × 106 | 0.9 × 106 | 33 × 106 | 66 × 106 | 29.9 | 73.3 |
| ALL-30 | 1.3 × 106 | 1.9 × 106 | 0 | 0 | 0 | 0 |
| ALL-31 | 2.7 × 106 | 1.8 × 106 | 19 × 106 | 28 × 106 | 7.1 | 15.8 |
| ALL-33 | 1.5 × 106 | 1.7 × 106 | 17 × 106 | 51 × 106 | 11.0 | 30.0 |
| ALL-36 | 2.0 × 106 | 1.3 × 106 | 0 | 0 | 0 | 0 |
| ALL-38 | 0.6 × 106 | 1.2 × 106 | 0 | 0 | 0 | 0 |
| ALL-39 | 0.8 × 106 | 1.0 × 106 | 0 | 0 | 0 | 0 |
| ALL-41 | 0.9 × 106 | 1.0 × 106 | 11 × 106 | 49 × 106 | 12.1 | 48.8 |
| CML-1 | 0.4 × 106 | 1.1 × 106 | 2.8 × 106 | 6.3 × 106 | 7.0 | 5.7 |
| CML-2 | 1.3 × 106 | 1.0 × 106 | 16 × 106 | 26 × 106 | 12.2 | 26.4 |
| CML-3 | 0.9 × 106 | 0.7 × 106 | 0 | 0 | 0 | 0 |
| Patient . | Day 0 (T-cell number) . | Day 30 (T-cell number) . | T-Cell Expansion . | |||
|---|---|---|---|---|---|---|
| . | CD4+ . | CD8+ . | CD4+ . | CD8+ . | (fold increase) . | |
| . | . | . | . | . | CD4+ . | CD8+ . |
| ALL-17 | 0.8 × 106 | 0.7 × 106 | 18 × 106 | 49 × 106 | 22.5 | 70.0 |
| ALL-20 | 0.9 × 106 | 1.2 × 106 | 26 × 106 | 82 × 106 | 28.9 | 68.3 |
| ALL-21 | 1.1 × 106 | 0.6 × 106 | 22 × 106 | 50 × 106 | 19.6 | 84.0 |
| ALL-25 | 1.3 × 106 | 0.7 × 106 | 11 × 106 | 18 × 106 | 8.2 | 26.1 |
| ALL-28 | 1.1 × 106 | 0.9 × 106 | 33 × 106 | 66 × 106 | 29.9 | 73.3 |
| ALL-30 | 1.3 × 106 | 1.9 × 106 | 0 | 0 | 0 | 0 |
| ALL-31 | 2.7 × 106 | 1.8 × 106 | 19 × 106 | 28 × 106 | 7.1 | 15.8 |
| ALL-33 | 1.5 × 106 | 1.7 × 106 | 17 × 106 | 51 × 106 | 11.0 | 30.0 |
| ALL-36 | 2.0 × 106 | 1.3 × 106 | 0 | 0 | 0 | 0 |
| ALL-38 | 0.6 × 106 | 1.2 × 106 | 0 | 0 | 0 | 0 |
| ALL-39 | 0.8 × 106 | 1.0 × 106 | 0 | 0 | 0 | 0 |
| ALL-41 | 0.9 × 106 | 1.0 × 106 | 11 × 106 | 49 × 106 | 12.1 | 48.8 |
| CML-1 | 0.4 × 106 | 1.1 × 106 | 2.8 × 106 | 6.3 × 106 | 7.0 | 5.7 |
| CML-2 | 1.3 × 106 | 1.0 × 106 | 16 × 106 | 26 × 106 | 12.2 | 26.4 |
| CML-3 | 0.9 × 106 | 0.7 × 106 | 0 | 0 | 0 | 0 |
To assess the capacity of the autologous T-cell lines to lyse leukemia cells, T cells were examined at various effector-to-target ratios and tested against the following targets: (1) unstimulated leukemia cells; (2) CD40-stimulated leukemia cells; (3) autologous nonmalignant cells (when available); (4) allogeneic CD40-B cells; and (5) K562 cells. As shown in Table 3, the T-cell lines were capable of lysing both the unstimulated and the CD40-stimulated leukemia cells, demonstrating that our methodology was successful in generating antileukemia cytotoxic T cells. To attempt to show antileukemia specificity, when available, autologous nonmalignant cells, including phytohemagglutinin (PHA)-activated T-cell blasts (ALL-28 and CML-1) and non-T, non-B (ALL-17 and ALL-21) were tested. In all cases, the majority of these autologous nonmalignant cells expressed both MHC class I and class II (data not shown). As shown in Table 3, cytolysis was significantly lower or absent on these nonmalignant cells compared with autologous unstimulated or CD40-stimulated leukemia cells. In addition, these CTL did not lyse other targets such as allogeneic CD40-B cells or K562 cells, confirming their antileukemia specificity (Table 3). Patient ALL-25 was an exception in which cytolysis of 38% of allogeneic CD40-B cells was observed. The capacity of antileukemia CTL to lyse pre-B leukemia cells from other patients was studied in 3 patients and no cytolysis against the allogeneic pre-B tumor cells was observed (data not shown). The absence of cross-reactivity by CTL against the allogeneic tumor cells provides support for the conclusion that CTL were anti–leukemia-specific and not simply directed against components of the allogeneic human serum.
Cytotoxicity Mediated by Autologous Antileukemia T-Cell Lines Generated From Bulk Cultures of Bone Marrow Stimulated With sCD40L
| Patient . | E:T Ratio . | Cytotoxicity (% of specific lysis) . | ||||
|---|---|---|---|---|---|---|
| . | . | Leukemia . | CD40 Leukemia . | Autologous Nonmalignant . | Allogeneic CD40-B . | K562 . |
| ALL-17 | 20:1 | 45% | 61% | 10% | 7% | <5% |
| ALL-20 | 40:1 | 37% | 56% | ND | <5% | ND |
| ALL-21 | 40:1 | 63% | 71% | 14% | 11% | 8% |
| ALL-25 | 20:1 | 51% | 92% | ND | 38% | ND |
| ALL-28 | 40:1 | 48% | 69% | <5% | <5% | <5% |
| ALL-30 | — | — | — | — | — | — |
| ALL-31 | 20:1 | 28% | 43% | ND | <5% | <5% |
| ALL-33 | 30:1 | 55% | 76% | ND | <5% | 10% |
| ALL-36 | — | — | — | — | — | — |
| ALL-38 | — | — | — | — | — | — |
| ALL-39 | — | — | — | — | — | — |
| ALL-41 | 30:1 | 32% | 60% | ND | 9% | <5% |
| CML-1 | 40:1 | 35% | 52% | <5% | <5% | <5% |
| CML-2 | 30:1 | 40% | 44% | ND | <5% | ND |
| CML-3 | — | — | — | — | — | — |
| Patient . | E:T Ratio . | Cytotoxicity (% of specific lysis) . | ||||
|---|---|---|---|---|---|---|
| . | . | Leukemia . | CD40 Leukemia . | Autologous Nonmalignant . | Allogeneic CD40-B . | K562 . |
| ALL-17 | 20:1 | 45% | 61% | 10% | 7% | <5% |
| ALL-20 | 40:1 | 37% | 56% | ND | <5% | ND |
| ALL-21 | 40:1 | 63% | 71% | 14% | 11% | 8% |
| ALL-25 | 20:1 | 51% | 92% | ND | 38% | ND |
| ALL-28 | 40:1 | 48% | 69% | <5% | <5% | <5% |
| ALL-30 | — | — | — | — | — | — |
| ALL-31 | 20:1 | 28% | 43% | ND | <5% | <5% |
| ALL-33 | 30:1 | 55% | 76% | ND | <5% | 10% |
| ALL-36 | — | — | — | — | — | — |
| ALL-38 | — | — | — | — | — | — |
| ALL-39 | — | — | — | — | — | — |
| ALL-41 | 30:1 | 32% | 60% | ND | 9% | <5% |
| CML-1 | 40:1 | 35% | 52% | <5% | <5% | <5% |
| CML-2 | 30:1 | 40% | 44% | ND | <5% | ND |
| CML-3 | — | — | — | — | — | — |
The autologous nonmalignant cells were non-T, non-B (majority MHC I+/MHC II+) or PHA blasts (majority MHC I+/MHC II+). The cytotoxic assay was performed as described in the Materials and Methods.
Abbreviations: E:T, effector to target; ND, not determined.
Anti–pre-B–cell leukemia-specific T cells can be generated from bone marrow but not from peripheral blood.Using the identical methodologic approach, we attempted to generate autologous antileukemia T-cell lines from the peripheral blood of these patients. Priming of peripheral blood MNC fraction (>75% blasts) was performed using the protocol depicted in Fig 2. Phenotypic analysis of peripheral blood MNC fraction showed that T cells represented 1.9% to 11.5% of the cells (1.1% to 7.1% CD4+ cells and 0.8% to 4.4% CD8+ cells). Surprisingly, no T-cell expansion was observed and no T-cell lines could be generated from the peripheral blood of any of these patients (Table 4). These results show that anti–leukemia-specific CTL can be generated from the bone marrow but not from the peripheral blood of patients with pre-B–cell leukemias, despite a higher percentage of T cells and the high tumor cell infiltration of the peripheral blood in all of the patients tested. It is important to note that, in all cases, the peripheral blood leukemic cells expressed CD40 and responded to stimulation by CD40L as measured by proliferation and induction or upregulation of adhesion molecules and B7 family members (data not shown).
Generation of Autologous Antileukemia T-Cell Lines From the Peripheral Blood of Patients With Pre-B–Cell Leukemias: Comparison With Bone Marrow
| Patient . | Peripheral Blood . | ||||||
|---|---|---|---|---|---|---|---|
| . | Day 0 (T-cell number) . | Day 30 (T-cell expansion) . | Bone Marrow . | . | |||
| . | CD4+ . | CD8+ . | CD4+ . | CD8+ . | Day 30 (T-cell expansion) . | CD8+ . | . |
| . | . | . | . | . | CD4+ . | . | . |
| ALL-17 | 5.0 × 106 | 1.7 × 106 | 0 | 0 | 22.5 | 70.0 | |
| ALL-20 | 7.1 × 106 | 4.4 × 106 | 0 | 0 | 28.9 | 68.3 | |
| ALL-30 | 3.2 × 106 | 2.0 × 106 | 0 | 0 | 0 | 0 | |
| ALL-31 | 1.1 × 106 | 0.8 × 106 | 0 | 0 | 7.1 | 15.8 | |
| ALL-36 | 3.7 × 106 | 2.1 × 106 | 0 | 0 | 0 | 0 | |
| ALL-41 | 4.3 × 106 | 3.5 × 106 | 0 | 0 | 12.1 | 48.8 | |
| CML-1 | 4.6 × 106 | 1.9 × 106 | 0 | 0 | 7.0 | 5.7 | |
| Patient . | Peripheral Blood . | ||||||
|---|---|---|---|---|---|---|---|
| . | Day 0 (T-cell number) . | Day 30 (T-cell expansion) . | Bone Marrow . | . | |||
| . | CD4+ . | CD8+ . | CD4+ . | CD8+ . | Day 30 (T-cell expansion) . | CD8+ . | . |
| . | . | . | . | . | CD4+ . | . | . |
| ALL-17 | 5.0 × 106 | 1.7 × 106 | 0 | 0 | 22.5 | 70.0 | |
| ALL-20 | 7.1 × 106 | 4.4 × 106 | 0 | 0 | 28.9 | 68.3 | |
| ALL-30 | 3.2 × 106 | 2.0 × 106 | 0 | 0 | 0 | 0 | |
| ALL-31 | 1.1 × 106 | 0.8 × 106 | 0 | 0 | 7.1 | 15.8 | |
| ALL-36 | 3.7 × 106 | 2.1 × 106 | 0 | 0 | 0 | 0 | |
| ALL-41 | 4.3 × 106 | 3.5 × 106 | 0 | 0 | 12.1 | 48.8 | |
| CML-1 | 4.6 × 106 | 1.9 × 106 | 0 | 0 | 7.0 | 5.7 | |
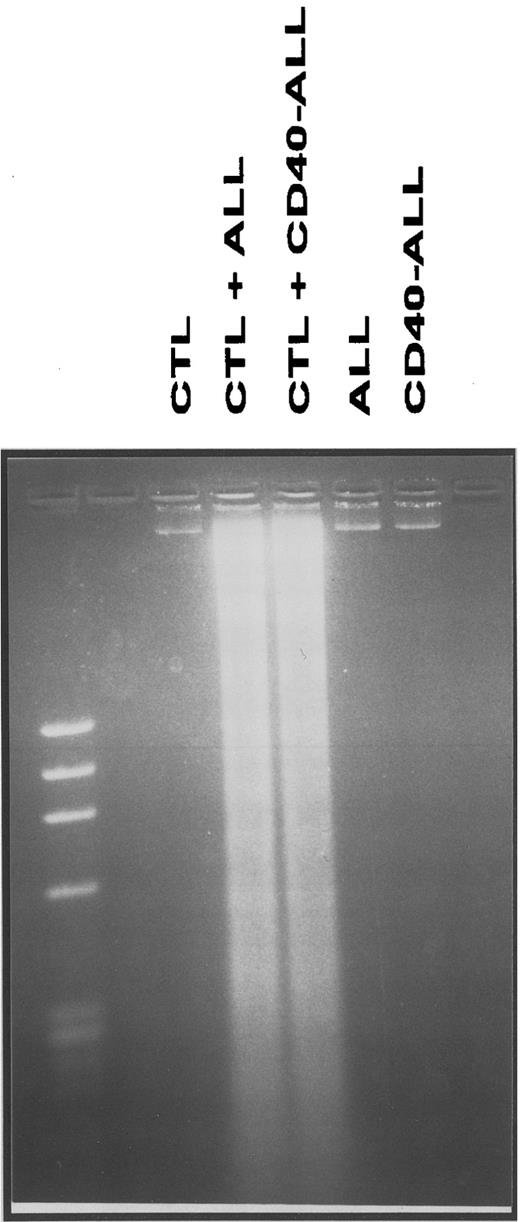
Antigen-specific cytolysis of pre-B ALL cells is mediated by CD8+ T cells that induce apoptosis of leukemic cells via the granzyme/perforin pathway.We next sought to determine the mechanism(s) by which anti–pre-B ALL-specific T cells induce lysis of the leukemia cells. Tumor cells were incubated with T cell lines at an optimal effector-to-target ratio, harvested after 6 to 8 hours, and analyzed for DNA fragmentation. As shown in Fig 4, CTL induce apoptosis of either unstimulated and CD40-stimulated pre-B ALL cells. In contrast, no DNA fragmentation was observed when either leukemic cells or T cells were cultured alone under the same conditions (Fig 4).
DNA fragmentation of pre-B ALL cells following coculture with T-cell lines (CTL) generated and expanded from bone marrow bulk cultures. Cultures were performed as described in the Materials and Methods, and the effector:target ratio used was 40:1. This figure depicts case ALL-17 and is representative of three independent experiments (3 different patients).
DNA fragmentation of pre-B ALL cells following coculture with T-cell lines (CTL) generated and expanded from bone marrow bulk cultures. Cultures were performed as described in the Materials and Methods, and the effector:target ratio used was 40:1. This figure depicts case ALL-17 and is representative of three independent experiments (3 different patients).
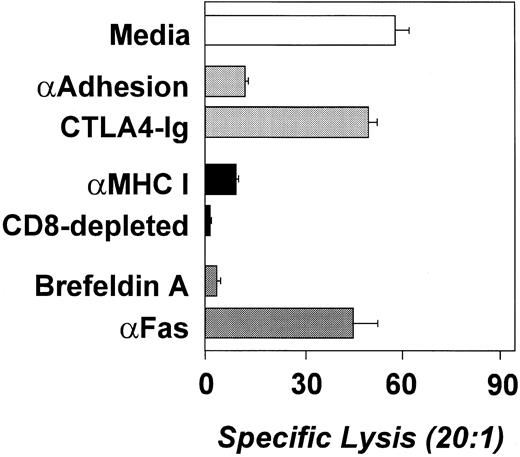
To determine which T-cell populations were mediating the lysis of the leukemia cells, the cytotoxic assays were performed in the presence of blocking antibodies. Figure 5 depicts an experiment representative of the 4 patients tested. The addition of an anti-MHC I MoAb resulted in significant reduction or elimination of the cytotoxicity mediated by the T-cell lines, suggesting that CD8+ cells were responsible for the cytolysis (Fig 5). These results were confirmed by the depletion of CD8+ T cells using magnetic bead depletion because the CD8-depleted cell fraction was incapable to induce apoptosis of the leukemia cells (Fig 5).
Antileukemia cytotoxicity is mediated by CD8+ cells and involves the secretory pathway (perforin/granzyme) rather than the FAS/FAS-L pathway. Cytotoxicity is represented as the percentage of specific lysis. Blocking antibodies (anti–LFA-3, anti–ICAM-1, anti-MHC I, and anti-Fas) were used at 10 mg/mL. CD8 depletion was performed by magnetic bead depletion and the depleted population was greater than 97% CD4+ T cells. Brefeldin A was used at 5 mg/mL. This experiment was performed using leukemia cells and CTL from ALL-17 and is representative of four independent experiments (4 different patients).
Antileukemia cytotoxicity is mediated by CD8+ cells and involves the secretory pathway (perforin/granzyme) rather than the FAS/FAS-L pathway. Cytotoxicity is represented as the percentage of specific lysis. Blocking antibodies (anti–LFA-3, anti–ICAM-1, anti-MHC I, and anti-Fas) were used at 10 mg/mL. CD8 depletion was performed by magnetic bead depletion and the depleted population was greater than 97% CD4+ T cells. Brefeldin A was used at 5 mg/mL. This experiment was performed using leukemia cells and CTL from ALL-17 and is representative of four independent experiments (4 different patients).
Lastly, we attempted to determine which molecules and lytic pathways are involved in the cytolysis of the leukemia cells by the antileukemia CTL. Significant reduction of the leukemia cell cytotoxicity was observed when blocking antibodies against the adhesion molecules LFA-3/CD58 and ICAM-1/CD50 (Fig 5) were added to the cytotoxic assay. The addition of CTLA4-Ig did not affect cytolysis, showing that, despite its critical role in the generation of the antileukemia CTL, B7 costimulation is not required at the effector phase. Importantly, in the 4 patients studied, the addition of an anti-FAS blocking antibody did not affect the lysis of the tumor cells, demonstrating that cytolysis is not mediated via the FAS/FAS-L pathway. Moreover, the addition of Brefeldin A, a protein synthesis inhibitor, resulted in the abrogation of the leukemia cell cytolysis (Fig 5). These results suggest that, in these patients, the antileukemia cytotoxicity is mediated preferentially by a granzyme/perforin pathway (secretory pathway) rather than a FAS/FAS-L pathway.
DISCUSSION
In the present report, we have developed a technique to generate and expand anti–pre-B leukemia-specific autologous cytolytic T cells. T-cell costimulation through CD28 by B7 family members is an absolute requirement for the generation of anti–pre-B leukemia autologous CTL. Although CD28-mediated costimulation can be delivered by bystander cells (B7-family transfectants or B7-expressing professional APCs), optimal priming and CTL expansion occurs when the costimulatory signal is presented in cis with the TCR signal (CD40-activated pre-B leukemia cells) or by cross-linking CD28 directly with anti-CD28 MoAb. By direct costimulation with CD40-stimulated pre-B leukemia cells and repetitive priming and cytokine-driven expansion, anti–leukemia-specific CTL could be generated from the unfractionated pre-B leukemia-infiltrated bone marrows from 10 of 15 patients evaluated. This technique resulted in variable expansion of both CD4+ and CD8+ T cells, although the expansion of CD8+ T cells was consistently greater than that observed for CD4+ T cells. Surprisingly, using the identical techniques, we were unable to generate anti–leukemia-specific CTL from the peripheral blood of any of these patients, suggesting that the frequency of the antileukemia CTL precursors is likely to be much lower in the peripheral blood than in the marrow. In 4 patients tested, cytolysis of the leukemia cells is mediated by CD8+ T cells capable of inducing tumor cell apoptosis through the secretory perforin/granzyme pathway rather than the FAS/FAS-L pathway. These studies provide an in vitro model system to characterize the molecular mechanisms that control tumor antigen recognition and clonal expansion of tumor-specific T cells. More importantly, this approach should facilitate our capacity to develop strategies to adoptively transfer anti–pre-B leukemia-specific CTL in an attempt to treat minimal residual leukemic cells after induction, consolidation, maintenance, or salvage therapy.
Previously, we showed that pre-B ALL cells were either inefficient or ineffective allo-APCs. Moreover, we demonstrated that this defect was due to the lack of B7-mediated costimulation and could be repaired by CD40 stimulation of the pre-B ALL cells.8 This resulted in upregulation of MHC and adhesion molecules and the induction of B7 family costimulatory molecules. We therefore sought to determine whether CD40 stimulation was necessary to repair this defect or, alternatively, whether costimulation could be delivered by bystander APC, including B7 family transfectants or professional APCs (indirect costimulation). When bystander cells expressing high levels of B7-1 and/or B7-2 were used as costimulators, allogeneic T cells proliferated only modestly, yet demonstrated alloantigen specificity. The induction of proliferation required B7 family-mediated costimulation because it was completely abrogated by B7 blockade. These results are in accordance with previous reports in both murine and human systems that showed that optimal T-cell activation required both the expression of the MHC/antigen complex and the costimulatory molecule(s) by the same cell.26-28 Moreover, analysis of the T-cell–APC interaction sites during the initiation of an immune response demonstrated that recruitment and spatial reorganization of these molecules on both the T cell and the APC were essential. Specifically, interactions between MHC/antigen complex and the B7 family molecules on the APC and the TCR/CD3 complex and CD28 on the T cells are critical for maximal signaling resulting in T-cell clonal expansion (A. Kupfer, communication in Keystone Symposium, 1995). These findings confirm the considerable advantage of the presentation of both antigen and costimulation by the tumor cell itself rather than these signals independently delivered by the tumor cell and a second cell capable of delivering a B7-mediated costimulatory signal.
In light of our ability to generate anti–pre-B leukemia-specific allogeneic T-cell proliferation, we next sought to generate autologous, anti–leukemia-specific T-cell–mediated immunity. As we did for allogeneic T cells, we again chose to compare direct versus indirect CD28 costimulation to determine whether autologous anti–pre-B leukemia CTL could be generated and, if so, how could they be optimally expanded. Moreover, we hoped that indirect costimulation would be comparable because it would permit us to have simple and reproducible method to generate autologous anti–pre-B leukemia-specific T cells in vitro. For these experiments, we reasoned that both priming and expansion of precursor CTL would be essential. The first step consisted of priming, which we hoped would result in the selection of antigen-specific T cells. During priming, TCR signal was delivered by the tumor cell and costimulation was provided by either CD40-stimulated (by sCD40L) tumor cells, anti-CD28 MoAb, or B7 family transfectants. The second step consisted of T-cell expansion induced by the addition of IL-2 and followed by repetitive stimulation of the primed anti–leukemia-specific T cells with CD40-activated leukemia cells (see Fig 2). During priming, the addition of sCD40L to the bone marrow bulk culture proved to be superior to anti-CD28 MoAb and significantly superior to B7 family transfectants. The superiority of sCD40L over the anti-CD28 MoAb in the priming of antileukemia T cells is likely due to the induction/upregulation of MHC and adhesion molecules following the CD40 ligation on leukemia cells that contribute to an increased APC function of these cells. Although the addition of IL-2 during the priming step resulted in a consistent increase in the expansion of both CD4+ and CD8+ T cells, a proportional decrement in the efficiency of leukemia cell specific cytolysis was observed. Moreover, the addition of IL-2 during priming resulted in the generation/expansion of T cells capable of proliferating to and lysing allogeneic CD40-B cells, suggesting that IL-2 also expanded T cells of other antigenic specificities. Taken together, these results show that the optimal method to generate anti–leukemia-specific CTL in vitro appears to be the addition of sCD40L followed by repetitive stimulations with CD40-stimulated ALL cells and IL-2.
Using the above-mentioned methodology, anti–leukemia-specific CTL could be efficiently generated from the bone marrow of 10 of 15 patients. The expansion of both CD4+ and CD8+ bone marrow T cells was observed in all cases, although, in general, the expansion of CD8+ cells was twofold higher. It is likely that the expansion of CD4+ cells was important for the CTL generation, because proliferation of CD8+ T cells is often dependent on the IL-2 provided by accessory cells, usually CD4+ T cells.29-32 It is presently unknown why we failed to generate antileukemia T cells in 5 patients, because their tumor cells uniformly expressed CD40 and were responsive to stimulation by CD40L. Moreover, alternative priming strategies, such as the addition of anti-CD28 MoAb, were also ineffective (data not shown). Whether this is due to the lack or defective presentation of immunogenic tumor-associated antigens by the leukemia cells or the absence of T cells with TCR specificities for these antigens in the T-cell repertoire of these patients, remains to be determined.
An important issue in this study is the formal demonstration of the antileukemia specificity of the CTLs generated using this methodology. Because the leukemia samples used in these studies were highly infiltrated by tumor cells (>90%), only very small numbers of nonmalignant cells could be isolated from the bone marrow of these patients. Therefore, we were able to test the ability of the CTLs to lyse autologous nonmalignant targets in only 4 patients. Neverthless, in all of them, the generated CTLs did not significantly lyse autologous non-T, non-B cells (2 patients) or autologous PHA T-cell blasts (2 patients). In all 4 of these cases, the majority of these target cells express both MHC class I and MHC class II, and these non-T, non-B, nonmalignant autologous cells were also capable of inducing an allogeneic MLR (data not shown). Moreover, the absence of CTL cross-reactivity against allogeneic pre-B leukemia cells provides support for the conclusion that these CTL responses were not simply directed against components of the allogeneic human serum (or MHC products).
The bone marrow CTL generated in 10 of 15 patients were capable of lysing both unstimulated and CD40-stimulated leukemia cells, although the efficiency of lysis was variable between patients. Our results also show that antileukemia CTL lysed more efficiently CD40-stimulated than unstimulated tumor cells. An explanation for this observation is that CD40-ALL are more actively cycling (A.A.C., unpublished observations), and it has been previously shown that cycling cells are more susceptible to CTL-induced apoptosis.33 Moreover, the observation, in all the patients tested, that the antileukemia cytolysis is mediated by CD8+ cells using the perforin/granzyme B secretory pathway rather than the FAS/FAS-L pathway is in accordance with the demonstration by Ju et al34 that the FAS/FAS-L pathway is essential for the cytolysis mediated by CD4+ CTL but not by CD8+ CTL.
The primary objective of this study was the generation of anti–leukemia-specific CTL for adoptive immunotherapy. We therefore reasoned that the generation of antileukemia CTL from the peripheral blood of these patients would represent a simple alternative source of T cells with which to translate this experimental procedure to a clinical trial. Surprisingly, in all of the cases studied, no T-cell expansion was observed from peripheral blood using the identical priming conditions, even if the leukemia cell content was always higher than 75% and the absolute number of T cells was greater than that in marrow. Moreover, the peripheral blood leukemic cells analyzed expressed CD40 and were responsive to CD40L stimulation, exhibiting a similar phenotype and allo-APC capacity to the bone marrow leukemia cells from the same patients (data not shown). Therefore, the failure to generate CTL is most likely due to the very low frequency or even absence of antileukemia CTL precursors in the peripheral blood. The relative higher frequency of antigen-specific CTL precursors in the marrow might represent in vivo priming in the bone marrow, because the marrow microenvironment might represent a more efficient site to prime CTL precursors compared with the peripheral blood.
These results show for the first time that anti–pre-B–cell leukemia-specific T-cell–mediated immunity can be generated in vitro. Moreover, these studies present evidence for the existence of autologous CTL with reactivity against the leukemia cells, the requirements for their optimal generation from the bone marrow, and provide valuable information for the design of clinical protocols for the treatment of B-cell precursor malignancies. Such anti–pre-B–cell specific T cells should permit us to begin to identify tumor associated and specific antigens expressed by pre-B ALL cells. Moreover, these results provide a methodology to assess whether patients with pre-B cell ALL would experience long-term disease-free intervals have existing anti–pre-B–cell leukemia-specific immunity. Most importantly, this methodology may allow us to ultimately improve the therapeutic index for children who are presently treated with pre-B cell ALL. Although the cure rate is rather high, the toxicity to the heart and brain are far from acceptable. If the induction or enhancement of antileukemia immunity provides us with novel treatment strategies to either treat minimal residual leukemia following conventional therapy and/or reduce the intensity of conventional therapy, we may be able to increase the cure rate while decreasing nonspecific end organ toxicity.
Supported by National Institutes of Health Grants No. P01-CA68484-02 and P01-CA66996-01 to L.M.N. A.A.C. was a recipient of a fellowship from Junta Nacional de Investigação Cientı́fica e Tecnológica (Portugal). H.M.A. is supported by a joint program of Protech (Boston, MA) and the Dana-Farber Cancer Institute. P.G. is supported by the American-Italian Cancer Foundation.
Address reprint requests to Angelo A. Cardoso, MD, Dana Farber Cancer Institute, D-740, 44 Binney St, Boston, MA 02115.

![Fig. 1. (A) Expression of B7-1 and B7-2 molecules by CHO-transfectants, professional APCs, and pre-B ALL cells (unstimulated and CD40-stimulated). The histograms shown for DCs and macrophages are from a single donor and are representative of five donors. Open areas represent fluorescence distribution of the B7-1 or B7-2 molecules and solid areas represent that of isotype-matched control antibodies. The cell number is shown on the Y-axis. / (B) Proliferative responses of purified allogeneic CD3+ T cells to irradiated pre-B ALL cells in the presence or absence of direct or bystander costimulation. Proliferation was assessed by measuring [3H] thymidine incorporation for the last 18 hours of the 6-day assay. The SI were calculated for each individual experiment as follows: SI = cpm(T cells + ALL cells)/cpm(T cells) . Bars represent mean ± SEM of the SI from the MLRs using 4 different patients and 5 different allogeneic T-cell donors. Allogeneic DCs and monocytes were syngeneic with the T cells. Control-Ig (C-Ig) and CTLA4-Ig fusion proteins were used at 10 μg/mL. Anti-CD28 MoAb (CD28) was used at 2.5 μg/mL. (C) Secondary proliferative responses of T cells primed by pre-B ALL cells in the presence of bystander costimulation. Proliferation was assessed by measuring [3H] thymidine incorporation for the last 18 hours of the 6-day assay. Bars represent mean ± SEM of the SI.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/2/10.1182_blood.v90.2.549/4/m_bl_0057f1a.jpeg?Expires=1767765879&Signature=2J-0QUrakoIp0qNYrHWUKZEWA0UnNPh~WxMG2A8EIxKSwDPHn7h~4G~z6yLgrXjLnrVQyniOO7fJHcSaZ1Hu7ZrTwqa1KNdwiBcjBp8~ffO21a3KtFZjXWA4fhYvq2RTGHKYG-tt-KdPYmNF-J-gGwkbf33yc8zKPPWg8sz6x~JAvYYcuY~7Ho18yrlAOVgiFrVdESqxFSNaN4P1JRcURK7Ijwwalfu2ZDkLQV3umP4VxuYuMCu3CtJnkJI~UaQlOJXULSoidc5b4m8ImR4tcPVhmoAj6R6QatV7As3aPyAdt-AOZoTa0t5c5AyjiiKxRkSS1PhWQLDVhtkWxKix2g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. (A) Expression of B7-1 and B7-2 molecules by CHO-transfectants, professional APCs, and pre-B ALL cells (unstimulated and CD40-stimulated). The histograms shown for DCs and macrophages are from a single donor and are representative of five donors. Open areas represent fluorescence distribution of the B7-1 or B7-2 molecules and solid areas represent that of isotype-matched control antibodies. The cell number is shown on the Y-axis. / (B) Proliferative responses of purified allogeneic CD3+ T cells to irradiated pre-B ALL cells in the presence or absence of direct or bystander costimulation. Proliferation was assessed by measuring [3H] thymidine incorporation for the last 18 hours of the 6-day assay. The SI were calculated for each individual experiment as follows: SI = cpm(T cells + ALL cells)/cpm(T cells) . Bars represent mean ± SEM of the SI from the MLRs using 4 different patients and 5 different allogeneic T-cell donors. Allogeneic DCs and monocytes were syngeneic with the T cells. Control-Ig (C-Ig) and CTLA4-Ig fusion proteins were used at 10 μg/mL. Anti-CD28 MoAb (CD28) was used at 2.5 μg/mL. (C) Secondary proliferative responses of T cells primed by pre-B ALL cells in the presence of bystander costimulation. Proliferation was assessed by measuring [3H] thymidine incorporation for the last 18 hours of the 6-day assay. Bars represent mean ± SEM of the SI.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/2/10.1182_blood.v90.2.549/4/m_bl_0057f1b.jpeg?Expires=1767765879&Signature=y9g-515jjWISBIT-4U8Alr-ohSkxLlo4Tb5vg1yn9LgfkT6owDj83~Dpaq3bBRL9FNv5zo-Ely3Fs~OdQGG~ERiu1d8NTwpv5lQ1gWDSWglXcX7vd1UFPxE9A751F5349cuYcdY91fuLQIw7iYbc3BudEynjgAJAp~qgvUsw~MgSYc19xpbpdVzgS6pfBj489FfZkYaLVobz8JmFVSnhbs44vRbIMdXx-41opvQlxK9qJRvG6XpRhWfWZPaAv8erqV66jDG2rQwR5Vj9IXjopD3wfRoX6XSSyMV5mhom8d6Ex6Kf9~DANKKf-5Z4Vn0eknhDQhcOdFvlzeUTBZTObg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. (A) Expression of B7-1 and B7-2 molecules by CHO-transfectants, professional APCs, and pre-B ALL cells (unstimulated and CD40-stimulated). The histograms shown for DCs and macrophages are from a single donor and are representative of five donors. Open areas represent fluorescence distribution of the B7-1 or B7-2 molecules and solid areas represent that of isotype-matched control antibodies. The cell number is shown on the Y-axis. / (B) Proliferative responses of purified allogeneic CD3+ T cells to irradiated pre-B ALL cells in the presence or absence of direct or bystander costimulation. Proliferation was assessed by measuring [3H] thymidine incorporation for the last 18 hours of the 6-day assay. The SI were calculated for each individual experiment as follows: SI = cpm(T cells + ALL cells)/cpm(T cells) . Bars represent mean ± SEM of the SI from the MLRs using 4 different patients and 5 different allogeneic T-cell donors. Allogeneic DCs and monocytes were syngeneic with the T cells. Control-Ig (C-Ig) and CTLA4-Ig fusion proteins were used at 10 μg/mL. Anti-CD28 MoAb (CD28) was used at 2.5 μg/mL. (C) Secondary proliferative responses of T cells primed by pre-B ALL cells in the presence of bystander costimulation. Proliferation was assessed by measuring [3H] thymidine incorporation for the last 18 hours of the 6-day assay. Bars represent mean ± SEM of the SI.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/2/10.1182_blood.v90.2.549/4/m_bl_0057f1c.jpeg?Expires=1767765879&Signature=4OlvvMNlxJxMxSLJExICSAT07gXC8IiIxaRwS61-~~fHwyhaDUt5kQve8MrAXPUSh~Zr4HAivjZRLmnXB5Nj-4Jg2Whbep1DMkJHs6vmHlWSoJmAeKDu1j9h~kn6azENB-F8tnnqaSHplfOpO906yAKW10uEC1SStUjbZKISkUD805c~v8~5MLFp3dnEj0YCcGzalJQqBXpXVu3Hcz6WEN8sW7Aai76vlwhoArJP7CiBVVfyDGNpPW8Pmbq1OWb4bt4LzcSkZmch6Bq-66Bx2YSeisBxLUJX7i3AWzvrHgVaf7NiHKDDas6PIwCAF42VJ8O1Ipzxsr~zjNPd5dxOXQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. (A) T-cell lines generated from the bone marrow of leukemia patients recognize and proliferate when challenged by unstimulated and CD40-stimulated leukemia cells: Comparison of direct or bystander costimulation. Proliferation was assessed by measuring [3H] thymidine incorporation for the last 18 hours of the 3-day assay. The results shown are from one experiment and are representative of three independent experiments (3 different patients). / (B) T-cell lines generated from the bone marrow of leukemia patients are capable of lysing both unstimulated and CD40-stimulated leukemia cells: Comparison of direct or bystander costimulation. Cytotoxicity was assessed as described in the Materials and Methods. The results shown are from one experiment and are representative of three independent experiments (3 different patients).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/2/10.1182_blood.v90.2.549/4/m_bl_0057f3a.jpeg?Expires=1767765879&Signature=egoVJpL9xMNCg9Tk0NGBQpGfE3tXnidBgIGXJZmscR-w~Flz6qBK~D1yK~~-BZNtIfNM2p~MY81IouTGeE9MSIwrbAoRet6Vl5o1MB1ci2mCwr5xZqbsh3sghO7J2m6KFHB~XBQhVUX9dcCZ8KEPQ9FYVc0jomhloIMuITvnEH7tqR0VnSV~QHUHMX3z3gddH8MEIgmkI0CyEKmUildnYugqTlnS7e6uFrzlC7gjzXenQnfGqSuRUYf8gRzurEeYc5XMVPv78tf353suv9krdEunlyYIR4IxB6eZ-CqRSj~KMnlSTmqgXsIjxSbGVqrYs2fcfASuLtAxAqGn9v2xyA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. (A) T-cell lines generated from the bone marrow of leukemia patients recognize and proliferate when challenged by unstimulated and CD40-stimulated leukemia cells: Comparison of direct or bystander costimulation. Proliferation was assessed by measuring [3H] thymidine incorporation for the last 18 hours of the 3-day assay. The results shown are from one experiment and are representative of three independent experiments (3 different patients). / (B) T-cell lines generated from the bone marrow of leukemia patients are capable of lysing both unstimulated and CD40-stimulated leukemia cells: Comparison of direct or bystander costimulation. Cytotoxicity was assessed as described in the Materials and Methods. The results shown are from one experiment and are representative of three independent experiments (3 different patients).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/2/10.1182_blood.v90.2.549/4/m_bl_0057f3b.jpeg?Expires=1767765879&Signature=b4bRnoouTujuBldAX-F9UmEZQgq-jb4~37l8yiYZn4WuF3sS8vA2Dvs-GJTwKKaXJcSa3mEksXIQBgJm-FT2g3~xvmXBK8Clsmh-kqWgFkmZ4Nz~zZjuC74BwGAXqmg-IyIYLlx64KDBTeAF1mWQ4W9VFcLLzL9f9Rrej-uU9g8kkeUp1ZjpNvZqg9AsBMwzW2V5kbT03YwYomJO7neDJzKeZN4uo3~rwGkik1axOBEYkUFI1UEEMbUZlNdb0ZkltHuq~zzNFq1QJaK-qxib-Wylq2Hvfb64n01eHOGftInX6ownqwZ7fra-KoDDKqLnqXfLsGb2dV81Nf2hetuEOw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal