Abstract
Primary effusion lymphoma (PEL) represents a novel B-cell non-Hodgkin's lymphoma (NHL) type associated with Kaposi's sarcoma-associated herpesvirus infection and typically growing as lymphomatous effusions in the body cavities. The precise B-cell subset from which PEL originates as well as the biologic mechanisms responsible for its peculiar growth pattern are unclear. In this study, we have analyzed PEL for the expression status of CD138/syndecan-1, a molecule selectively associated with late stages of B-cell differentiation and implicated in cell-to-cell and cell-to-extracellular matrix interactions. PEL patient samples (n = 7) and cell lines (n = 5) were investigated by multiple approaches, including immunocytochemistry, flow cytometry, RNA analysis, and Western blot studies. For comparison, lymphomatous effusions other than PEL (n = 13) and tissue-based NHL (n = 103) were also tested. Expression of CD138/syndecan-1 associates at high frequency with PEL (5 of 7 patient samples and 5 of 5 cell lines), whereas it is consistently absent among other lymphomatous effusions (n = 13). The CD138/syndecan-1 isoform expressed by PEL has an average molecular weight of 420 kD, which is substantially different from that of CD138/syndecan-1 molecules generally expressed by plasma cells. These data, along with previous immunophenotypic evidence, unequivocally define that PEL cells represent a preterminal stage of B-cell differentiation and may bear implications for the peculiar growth pattern of this lymphoma.
PRIMARY EFFUSION lymphoma (PEL), otherwise termed body cavity-based lymphoma, is a peculiar type of B-cell non-Hodgkin's lymphoma (NHL) that has been recently recognized as an individual clinico-pathologic category based on the consistent infection by Kaposi's sarcoma-associated herpesvirus (KSHV; also known as HHV-8) and the distinctiveness of its clinical and biologic features.1-11 At the clinical level, PEL displays a marked preference for liquid growth in the serous cavities of the body.1-11 However, pathologically, the basic feature of PEL is a net predilection for diffuse spreading along the serous membranes without infiltrative or destructive growth patterns.11 PEL consistently originates from B cells.1-11 However, the overwhelming majority of cases exhibit a non-B, non-T (ie, indeterminate) phenotype, lacking expression of surface Igs and B-cell–associated antigens.1-11 Rare cases of PEL displaying a B-cell phenotype have also been reported, suggesting that PEL may be characterized by a certain degree of phenotypic heterogeneity.2,4,5,8 10
Because of the recent identification of PEL, several issues concerning the nature of the disease await clarification. Previous phenotypic evidence has suggested that PEL may derive from preterminal B cells differentiating toward plasma cells.3,7-11 However, despite these suggestions, the precise stage of B-cell maturation of PEL has not been formally established. Further refinement of the detailed B-cell origin of PEL cells may be provided by the study of phenotypic markers specifically associated with late stages of B-cell differentiation, such as the plasma cell-specific CD138 antigen recognized by the B-B4 monoclonal antibody (MoAb).12
Another issue that awaits clarification concerns the growth peculiarities of PEL cells. Indeed, it has been observed that the growth pattern of PEL is characterized by the concomitance of liquid phase accumulation of tumor cells in the body cavities combined to a diffuse, noninvasive spread of the tumor along the serous membranes.1-11 Elucidation of the biologic basis of the PEL growth pattern may be derived from studies of molecules thought to regulate cell-to-cell and cell-to-extracellular matrix (ECM) interactions in the B-cell lineage. One such molecule is represented by syndecan-1, a member of the syndecan family.13-16 Syndecan-1 has been shown to bind several ECM molecules via its heparan sulphate chains, including fibrillar collagens, fibronectin, thrombospondin, and tenascin.17-19 Among B cells, syndecan-1 is selectively expressed by a subset of pre-B cells and by plasma cells and has been proposed to mediate the adhesion of these B-cell populations to collagen.12,20 Recently, it has been defined that syndecan-1 identifies with the plasma cell-specific CD138 antigen recognized by the B-B4 MoAb.12
In an attempt to contribute to the elucidation of the nature of PEL, we have analyzed a panel of these lymphomas for the expression of CD138/syndecan-1 by multiple approaches. Our results show that PEL frequently and selectively associates with expression of CD138/syndecan-1 throughout the spectrum of lymphomatous effusions. These data indicate that PEL is constituted by a preterminally differentiated B-cell population and suggest potential implications for the peculiar growth pattern of this lymphoma type.
MATERIALS AND METHODS
Patient samples. The present study is based on 20 cases of high-grade B-cell NHL presenting as lymphomatous effusions at diagnosis. All cases were consecutively observed at the Centro di Riferimento Oncologico (CRO; Aviano, Italy) between September 1984 and February 1997. In 10 cases (6 acquired immunodeficiency syndrome [AIDS]-related and 4 AIDS-unrelated), the malignant effusion was the exclusive or the predominant localization of the lymphoma. These cases were classified as primary lymphomatous effusions. In the remaining 10 cases (3 AIDS-related and 7 AIDS-unrelated), the effusion was associated with solid lymphomatous masses and was considered secondary to a tissue-based lymphoma. Samples of lymphomatous effusions were collected under sterile conditions during standard diagnostic procedures.4 21
All samples included in this study were subjected to immunophenotypic and immunogenotypic characterization, as well as determination of tumor infection by Epstein-Barr virus (EBV) and KSHV, according to previously reported strategies.4 The immunophenotypic and molecular results of part of the cases have been described previously.4 All lymphomatous effusions were B-cell monoclonal proliferations. Based on the combination of morphologic and biologic features, cases were assigned to one of the following categories: small noncleaved cell lymphoma (SNCCL), diffuse large-cell lymphoma (DLCL), and PEL. On these grounds, samples of primary lymphomatous effusions included 7 PEL, 2 DLCL, and 1 SNCCL (Table 1). Conversely, samples of secondary lymphomatous effusions included 8 DLCL and 2 SNCCL (Table 1). As a control, cryopreserved cell samples from 103 tissue-based B-cell NHL and 41 plasma cell tumors (36 multiple myelomas and 5 extramedullary plasmacytomas) were also studied.
Immunocytochemical Detection of the CD138/Syndecan-1 Antigen and Characterization of Morphologic, Phenotypic, and Viral Features of Lymphomatous Effusions
| Sample* . | CD138† . | Diagnosis . | Phenotype . | HIV‡ . | EBVρ . | KSHVρ . |
|---|---|---|---|---|---|---|
| Primary lymphomatous effusions | ||||||
| 1 | + | PEL | Non-B, non-T | + | + | + |
| 2 | + | PEL | Non-B, non-T | + | − | + |
| 3 | + | PEL | Non-B, non-T | + | + | + |
| 4 | + | PEL | Non-B, non-T | − | − | + |
| 5 | + | PEL | Non-B, non-T | + | + | + |
| 6 | − | PEL | B | − | − | + |
| 7 | − | PEL | B | + | + | + |
| HBL-6 | + | PEL | Non-B, non-T | + | + | + |
| BC-1 | + | PEL | Non-B, non-T | + | + | + |
| BC-2 | + | PEL | Non-B, non-T | + | + | + |
| BCBL-1 | + | PEL | Non-B, non-T | + | − | + |
| CROAP-2 | + | PEL | Non-B, non-T | + | + | + |
| 8 | − | DLCL | Non-B, non-T | − | − | − |
| 9 | − | DLCL | Non-B, non-T | + | + | − |
| 10 | − | SNCCL | B | − | + | − Secondary lymphomatous effusions |
| 11 | − | SNCCL | B | − | − | − |
| 12 | − | SNCCL | B | + | − | − |
| 13 | − | DLCL | B | − | − | − |
| 14 | − | DLCL | B | − | − | − |
| 15 | − | DLCL | B | − | − | − |
| 16 | − | DLCL | B | + | + | − |
| 17 | − | DLCL | B | + | + | − |
| 18 | − | DLCL | B | − | − | − |
| 19 | − | DLCL | B | − | − | − |
| 20 | − | DLCL | B | − | − | − |
| Sample* . | CD138† . | Diagnosis . | Phenotype . | HIV‡ . | EBVρ . | KSHVρ . |
|---|---|---|---|---|---|---|
| Primary lymphomatous effusions | ||||||
| 1 | + | PEL | Non-B, non-T | + | + | + |
| 2 | + | PEL | Non-B, non-T | + | − | + |
| 3 | + | PEL | Non-B, non-T | + | + | + |
| 4 | + | PEL | Non-B, non-T | − | − | + |
| 5 | + | PEL | Non-B, non-T | + | + | + |
| 6 | − | PEL | B | − | − | + |
| 7 | − | PEL | B | + | + | + |
| HBL-6 | + | PEL | Non-B, non-T | + | + | + |
| BC-1 | + | PEL | Non-B, non-T | + | + | + |
| BC-2 | + | PEL | Non-B, non-T | + | + | + |
| BCBL-1 | + | PEL | Non-B, non-T | + | − | + |
| CROAP-2 | + | PEL | Non-B, non-T | + | + | + |
| 8 | − | DLCL | Non-B, non-T | − | − | − |
| 9 | − | DLCL | Non-B, non-T | + | + | − |
| 10 | − | SNCCL | B | − | + | − Secondary lymphomatous effusions |
| 11 | − | SNCCL | B | − | − | − |
| 12 | − | SNCCL | B | + | − | − |
| 13 | − | DLCL | B | − | − | − |
| 14 | − | DLCL | B | − | − | − |
| 15 | − | DLCL | B | − | − | − |
| 16 | − | DLCL | B | + | + | − |
| 17 | − | DLCL | B | + | + | − |
| 18 | − | DLCL | B | − | − | − |
| 19 | − | DLCL | B | − | − | − |
| 20 | − | DLCL | B | − | − | − |
Abbreviations: +, positive; −, negative; DLCL, diffuse large-cell lymphoma; SNCCL, small noncleaved cell lymphoma; PEL, primary effusion lymphoma.
Pathologic samples derived from patients are indicated by numbers; cell lines are indicated by their conventional denomination.
As assesseed by immunostaining with the B-B4 MoAb.
Host HIV status as defined by standard serologic assays.
ρ Tumor cell infection as defined by a combination of in situ hybridization, Southern blot analysis, and PCR studies.
Cell lines. The following PEL cell lines were included in this study: HBL-6, BC-1 (purchased from American Type Culture Collection [ATCC], Rockville, MD), BC-2 (purchased from ATCC), BCBL-1, and CROAP-2.22-25
Immunocytochemistry. Cytospin preparations of lymphomatous effusions were fixed in acetone-chloroform at room temperature for 10 minutes before immunostaining with the anti-CD138/syndecan-1 B-B4 MoAb (Immuno Quality Products, Lagitre, Milan, Italy).12 Immunocytochemistry was performed with the alkaline phosphatase antialkaline phosphatase (APAAP) method as described.26 In addition to lymphomatous effusions, anti–B-B4 MoAb was also applied to frozen tissues from 22 representative cases of tissue-based B-cell NHL and 5 cases of extramedullary plasmacytomas. Cell lineage of lymphomatous effusions and tissue-based lymphomas was determined by immunocyto-histochemical staining.26 Immunohistochemistry on frozen tissue complemented the analysis in lymphoma tissue sections.
RNA isolation and reverse transcriptase-polymerase chain reaction (RT-PCR). Expression of syndecan-1–specific mRNA was analyzed as previously described.27 Total RNA (1 μg), extracted by the guanidium thiocyanate method,28 was reverse-transcribed by avian myeloblastosis virus (AMV) reverse transcriptase (Promega Co, Madison, WI) in a 20-μL reaction mix containing hexadeoxyribonucleotides random primers (0.5 μg) for 1.0 hours at 42°C. Two microliters of the same cDNA preparations was amplified in a 50-μL volume of final reaction mix in a Perkin Elmer 9600 thermal cycler (Perkin Elmer Cetus, Emeryville, CA) with 25 pmol/L of primers specific for syndecan-1 (sense, 5′-GAT GGC TCT GGG GAT GAC TC-3′, region 308-327; antisense, 5′-TGT TTG GTG GGC TTC TGG TAG-3′, region 1099-1119)29 and β-actin (Clontech Laboratories Inc, Palo Alto, CA; sense, region 578-609; antisense, region 1415-1384). Ten microliters of amplified cDNAs was run in 1.5% agarose gels, blotted onto nylon membranes (Boehringer Mannheim, Mannheim, Germany), and hybridized with 2 × 106 cpm/mL of 5′-end-labeled oligoprobes specifically designed to recognize PCR products.
Distribution of CD138/Syndecan-1 Expression as Assessed by the B-B4 MoAb Reactivity Among Lymphomatous Effusions, Tissue-Based Lymphomas, and Plasmacytoma/Myeloma
| Diagnosis . | Positive/Tested . |
|---|---|
| Primary lymphomatous effusions | |
| DLCL | 0/2 |
| SNCCL (Burkitt's lymphoma) | 0/1 |
| PEL | 10/12* |
| Secondary lymphomatous effusions | |
| DLCL | 0/8 |
| SNCCL (Burkitt's lymphoma) | 0/2 |
| Tissue-based lymphomas | |
| SLL | 0/58 |
| LPL | 3/4 |
| MCL | 0/7 |
| FCCL | 0/10 |
| DLCL | 2/18† |
| SNCCL (Burkitt's lymphoma) | 0/6 |
| Plasmacytoma/myeloma | 41/41 |
| Diagnosis . | Positive/Tested . |
|---|---|
| Primary lymphomatous effusions | |
| DLCL | 0/2 |
| SNCCL (Burkitt's lymphoma) | 0/1 |
| PEL | 10/12* |
| Secondary lymphomatous effusions | |
| DLCL | 0/8 |
| SNCCL (Burkitt's lymphoma) | 0/2 |
| Tissue-based lymphomas | |
| SLL | 0/58 |
| LPL | 3/4 |
| MCL | 0/7 |
| FCCL | 0/10 |
| DLCL | 2/18† |
| SNCCL (Burkitt's lymphoma) | 0/6 |
| Plasmacytoma/myeloma | 41/41 |
Reactivity with the anti-CD138/syndecan-1 MoAb B-B4 was assessed by flow cytometry (FC) and frozen sections immunohisto-cytochemistry (IHC). Cells from 22 B-cell tissue-based lymphoma cases encompassing the different histotypes (10 small lymphocytic lymphoma, 3 mantle cell lymphoma, 6 follicular center cell lymphoma, and 3 diffuse large B-cell lymphoma) were analyzed by both FC and IHC.
Abbreviations: DLCL, diffuse large-cell lymphoma; SNCCL, small noncleaved cell lymphoma; PEL, primary effusion lymphoma; SLL, small lymphocytic lymphoma; LPL, lymphoplasmacytoid lymphoma; MCL, mantle cell lymphoma; FCCL, follicular center cell lymphoma.
Positive cases included 5 of 7 pathologic samples and 5 of 5 cell lines.
The 2 DLCL cases staining positive for CD138/syndecan-1 expression displayed immunoblastic plasmacytoid features.
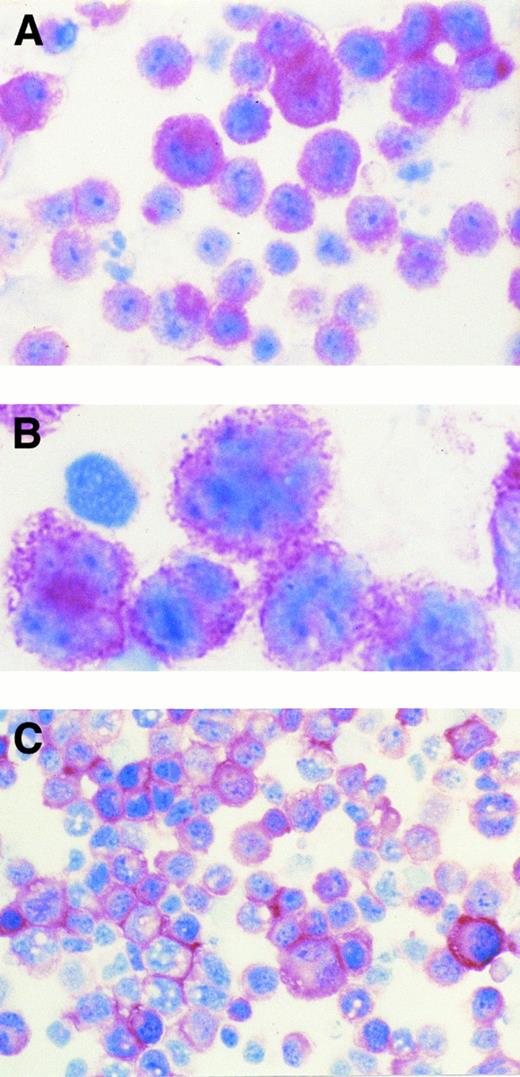
(A) AIDS-related KSHV+ PEL (patient no. 5). The majority of tumor cells show strong cytoplasmic and membrane staining for B-B4 antibody that recognizes the plasma cell-specific CD138/syndecan-1 antigen. Cytospin preparation, APAAP immunostaining, hematoxylin counterstain. (Original magnification × 400.) (B) AIDS-related KSHV+ PEL (patient no. 5). High power photograph showing pleomorphic tumor cells with intense membrane staining for B-B4 antibody. Cytospin preparation, APAAP immunostaining, hematoxylin counterstain. (Original magnification × 1,000 oil immersion.) (C) AIDS-related KSHV+ PEL-derived cell line (HBL-6). Cultured cells show a dominant membrane staining for B-B4 antibody. Cytospin preparation, APAAP immunostaining, hematoxylin counterstain. (Original magnification × 320.)
(A) AIDS-related KSHV+ PEL (patient no. 5). The majority of tumor cells show strong cytoplasmic and membrane staining for B-B4 antibody that recognizes the plasma cell-specific CD138/syndecan-1 antigen. Cytospin preparation, APAAP immunostaining, hematoxylin counterstain. (Original magnification × 400.) (B) AIDS-related KSHV+ PEL (patient no. 5). High power photograph showing pleomorphic tumor cells with intense membrane staining for B-B4 antibody. Cytospin preparation, APAAP immunostaining, hematoxylin counterstain. (Original magnification × 1,000 oil immersion.) (C) AIDS-related KSHV+ PEL-derived cell line (HBL-6). Cultured cells show a dominant membrane staining for B-B4 antibody. Cytospin preparation, APAAP immunostaining, hematoxylin counterstain. (Original magnification × 320.)
Flow cytometry. Flow cytometry studies of the CD138/syndecan-1 antigen were performed for selected cases of lymphomatous effusions as well as for 103 cases of tissue-based B-cell NHL and for 36 multiple myelomas. Indirect immunofluorescence of cell lines and patient samples was performed by incubating cells with the same saturating concentration of anti-CD138/syndecan-1 MoAb B-B4 (Immuno Quality Products), as previously described.27 Nonspecific binding of MoAbs was assessed by labeling cells with isotype-matched control mouse Igs (Jackson ImmunoResearch Laboratories, West Grove, PA). Viable, antibody-labeled cells were identified according to their forward and right angle scattering, electronically gated, and analyzed for surface fluorescence on a FACScan flow cytometer (Becton Dickinson Immunocytometry Sytems, San Jose, CA).
For selected PEL cases expressing the CD138/syndecan-1 antigen, fluorescence values were converted into the number of molecules of equivalent soluble fluorochrome per cell (MESF ) by comparing the results of anti-CD138/syndecan-1 (B-B4) staining with calibrated fluorescence reference standard microbeads (Flow Cytometry Standards Corporation, Inc, Research Triangle Park, NC), as previously described.30 31 Fluorescence intensity achieved with an isotype matched unreactive antibody was subtracted from that obtained with anti-CD138/syndecan-1 (B-B4) to calculate the net MESF values. Results represent mean ± SEM of MESF values derived from three different experiments.
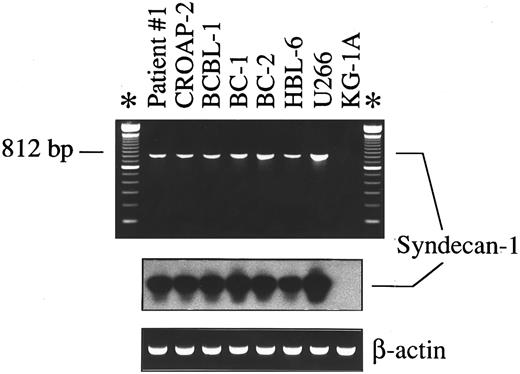
Expression of mRNA encoding for CD138/syndecan-1 in primary tumor cells from a patient with PEL (patient no. 1), human PEL-derived cell lines (CROAP-2, BCBL-1, BC-1, BC-2, and HBL-6), the human myeloma cell line U266, and myeloid leukemia cells KG-1A, as detected by RT-PCR analysis. cDNA bulks were prepared and amplified with specific primers for human syndecan-1 (upper panel) and β-actin (lower panel). Ten microliters of amplified cDNAs was also run on 1.5% agarose gels, blotted onto nylon membranes, and hybridized with a syndecan-1–specific cDNA probe (center panel).
Expression of mRNA encoding for CD138/syndecan-1 in primary tumor cells from a patient with PEL (patient no. 1), human PEL-derived cell lines (CROAP-2, BCBL-1, BC-1, BC-2, and HBL-6), the human myeloma cell line U266, and myeloid leukemia cells KG-1A, as detected by RT-PCR analysis. cDNA bulks were prepared and amplified with specific primers for human syndecan-1 (upper panel) and β-actin (lower panel). Ten microliters of amplified cDNAs was also run on 1.5% agarose gels, blotted onto nylon membranes, and hybridized with a syndecan-1–specific cDNA probe (center panel).
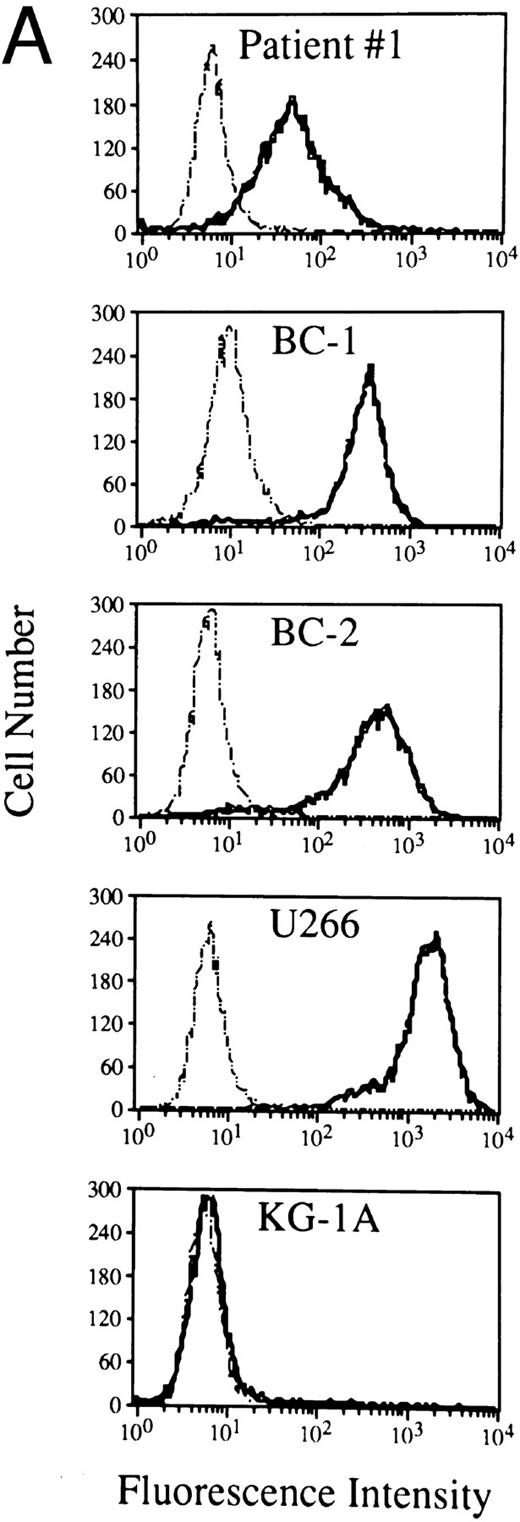
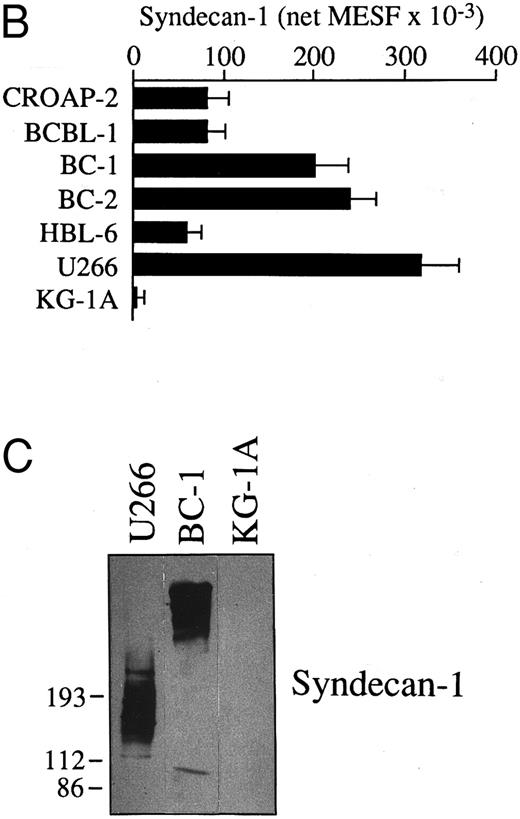
Expression and molecular characterization of the CD138/syndecan-1 antigen in cells from PEL. (A) Flow cytometry profiles showing CD138/syndecan-1 surface expression in primary tumor cells from patient no. 1, human PEL-derived cell lines (BC1 and BC2), the human myeloma cell line U266, and myeloid leukemia cells KG-1A. Cells were incubated with the anti-CD138/syndecan-1 MoAb BB-4 (solid lines) and an irrelevant isotype-matched mouse antibody (dotted lines), followed by fluorescein isothiocyanate (FITC)-labeled goat antimouse Ig. The X- and Y-axes, respectively, indicate the logarithm of relative green fluorescence intensity and the relative cell numbers. (B) Relative differences in CD138/syndecan-1 surface expression among PEL-derived cell lines (CROAP-2, BCBL-1, BC-1, BC-2, and HBL-6), human myeloma cells U266, and myeloid leukemia cells KG-1A. Fluorescence values were converted into MESF by comparing the results of anti-CD138/syndecan-1 staining with calibrated reference microbeads containing measured numbers of FITC molecules. Results are expressed as the mean ± SEM of net MESF values derived from three different experiments. (C) Western blot analysis of cell lysates from the human myeloma cell line U266 (5.0 × 106/100 μL), the PEL cell line BC-1 (5.0 × 106/100 μL), and the acute myelogenous leukemia cell line KG-1A (2.5 × 106/100 μL). The blot was incubated with 1.5 μg/mL of the anti-CD138/syndecan-1 MoAb BB-4 and shown by chemiluminescence. The position of molecular weight markers is indicated.
Expression and molecular characterization of the CD138/syndecan-1 antigen in cells from PEL. (A) Flow cytometry profiles showing CD138/syndecan-1 surface expression in primary tumor cells from patient no. 1, human PEL-derived cell lines (BC1 and BC2), the human myeloma cell line U266, and myeloid leukemia cells KG-1A. Cells were incubated with the anti-CD138/syndecan-1 MoAb BB-4 (solid lines) and an irrelevant isotype-matched mouse antibody (dotted lines), followed by fluorescein isothiocyanate (FITC)-labeled goat antimouse Ig. The X- and Y-axes, respectively, indicate the logarithm of relative green fluorescence intensity and the relative cell numbers. (B) Relative differences in CD138/syndecan-1 surface expression among PEL-derived cell lines (CROAP-2, BCBL-1, BC-1, BC-2, and HBL-6), human myeloma cells U266, and myeloid leukemia cells KG-1A. Fluorescence values were converted into MESF by comparing the results of anti-CD138/syndecan-1 staining with calibrated reference microbeads containing measured numbers of FITC molecules. Results are expressed as the mean ± SEM of net MESF values derived from three different experiments. (C) Western blot analysis of cell lysates from the human myeloma cell line U266 (5.0 × 106/100 μL), the PEL cell line BC-1 (5.0 × 106/100 μL), and the acute myelogenous leukemia cell line KG-1A (2.5 × 106/100 μL). The blot was incubated with 1.5 μg/mL of the anti-CD138/syndecan-1 MoAb BB-4 and shown by chemiluminescence. The position of molecular weight markers is indicated.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting. Cells were washed twice in ice-cold phosphate-buffered saline (PBS; 80 mmol/L Na2HPO4 , 20 mmol/L NaH2PO4 , 100 mmol/L NaCL, pH 7.4) and lysed on ice for 30 minutes in a buffer containing 10 mmol/L Tris-HCl, pH 8.0, 1% Nonidet P-40, 150 mmol/L NaCl, 1.0 mmol/L EDTA, 1.0 mmol/L phenylmethylsulphonyl fluoride, and 10 mg/mL leupeptin. After centrifugation for 5 minutes at 14,000 rpm (4°C), the supernatant was collected and samples were separated on a 5.0% SDS-PAGE after boiling in reduced Laemmli sample buffer. Proteins were blotted onto Zeta Probe membrane (Bio Rad Laboratories, Hercules, CA) and blocked with PBS containing 0.5% casein (Sigma Chemical Co, St Louis, MO) and 0.1% Tween 20 for 2 hours at room temperature under shaking. The membrane was then incubated for 1 hour at room temperature with PBS-0.5% casein containing 1.5 μg/mL of the anti-CD138/syndecan-1 MoAb B-B4 (Serotec, Oxford, UK) or an irrelevant mouse IgG1 MoAb, washed three times with PBS-0.5% casein-0.1% Tween 20, incubated with peroxidase-conjugated goat antimouse Ig (Amersham Life Science, Amersham, UK), and shown by an enhanced chemiluminescence (ECL) system (Amersham) following the manufacturer's instructions.
RESULTS
Characterization of lymphomatous effusions. The main morphologic, immunophenotypic, and viral features of lymphomatous effusions are summarized in Table 1. Among PEL, most cases lacked antigens restricted to the B-cell and T-cell lineage (Table 1). Thus, their phenotype was classified as non-B, non-T (indeterminate). Only 2 PEL (cases no. 6 and 7) expressed B-cell lineage markers and their phenotype was classified as B.
Expression of CD138/syndecan-1 antigen among lymphomatous effusions. A positive immunostaining for the CD138/syndecan-1 antigen was observed in tumor cells of 10 of 12 PEL, including 5 of 7 PEL patient samples and 5 of 5 PEL cell lines (Tables 1 and 2 and Fig 1). In contrast, neoplastic cells from primary lymphomatous effusions other than PEL (n = 3) as well as from all cases of secondary lymphomatous effusions (n = 10) were consistently negative for CD138/syndecan-1 expression (Table 2). The pattern of CD138/syndecan-1 immunostaining in positive cases was consistent with antigen expression on both cell membrane and cytoplasm (Fig 1).
Among PEL, expression of the CD138/syndecan-1 antigen occurred in both AIDS-related and AIDS-unrelated cases and appeared to be independent of infection of the tumor clone by EBV (Table 1). Conversely, it was noted that all cases of PEL that had been scored positive for CD138/syndecan-1 expression displayed a non-B, non-T phenotype, whereas the 2 cases scored negative for CD138/syndecan-1 expressed a B-cell phenotype (Table 1).
High frequency of CD138/syndecan-1 expression appears to be a feature virtually specific for PEL throughout the whole spectrum of B-cell NHL, because expression of CD138/syndecan-1 was absent in 98 of 103 cases of tissue-based B-cell NHL (Table 2). The 5 cases of tissue-based B-cell NHL scored positive for CD138/syndecan-1 expression were represented by 3 of 4 tested cases of lymphoplasmacytoid lymphoma and 2 of 18 tested cases of DLCL.
Expression of syndecan-1 mRNA and characterization of surface CD138 in PEL cells. We have analyzed selected PEL by mRNA studies, flow cytometry, and Western blotting. As shown in Fig 2, a 812-bp amplified cDNA product specific for CD138/syndecan-1 was detectable in all PEL cell lines (CROAP-2, BCBL-1, BC-1, BC-2, and HBL-6) as well as in primary PEL cells from patient no. 1. These same PEL samples were found to express surface CD138/syndecan-1 antigen by flow cytometry with the MoAb BB-4 (Fig 3A and B). BC-1 and BC-2 cells displayed a very high cellular density of CD138, ie, 204,000 and 243,000 MESF, respectively, whereas in other PEL, CD138 staining intensity ranged from 58,000 to 83,000 MESF (Fig 3B). The relative amount of surface CD138/syndecan-1 in BC-1 and BC-2 was only slightly lower than that of U266 plasma cells.
To further characterize the molecular structure of the syndecan-1 isoform expressed by PEL, we have analyzed BC-1 tumor cells by SDS-PAGE and Western blotting (Fig 3C). Immunoreactive syndecan-1 from BC-1 migrated as a broad smear with an estimated average molecular weight of 420 kD. This pattern was strikingly different from that observed in cell lysates from U266 myeloma cells, which show a predominant broad smear starting at about 200 kD. Given the presence of amplified cDNA products of comparable size after RT-PCR analysis of syndecan-1 RNA in BC-1 and U266 cells (Fig 2), it is likely that the differences observed between PEL and multiple myeloma are due to posttranslational changes. Conceivably, PEL cells express syndecan-1 molecules with different amounts and/or size of their glycosaminoglycan chains. The signal detected by the MoAb B-B4 in Western blot experiments of PEL cells was considered to be specific for syndecan-1, because no syndecan-1–related molecular components were immunodetected by MoAb BB-4 in cell lysates from negative control cells KG-1A (Fig 3C) or when the blot was incubated with an irrelevant isotype-matched control mouse MoAb (not shown).
DISCUSSION
Previous studies have indicated that the morphologic and immunophenotypic features associated with PEL are consistent with an origin from a late stage of B-cell maturation.2,3,7-11 The consistent expression of CD138/syndecan-1 by PEL, as demonstrated by this study, unequivocally defines that PEL reflect a preterminal stage of B-cell differentiation, because the CD138/syndecan-1 antigen is regarded as the most reliable surface marker of plasma cells.12,27,32 33 The association between CD138/syndecan-1 expression and PEL appears to be specific for this lymphoma among lymphomatous effusions and, with few exceptions, also among tissue-based B-cell NHL. Notably, expression of CD138/syndecan-1 was absent in 2 PEL cases included in this report. In contrast to all other PEL samples, which expressed non-B, non-T phenotypes, the 2 CD138/syndecan-1 negative PEL expressed several B-cell–associated antigens.
Very recently, KSHV has been detected in bone marrow stromal cells of multiple myeloma and has been proposed to exert a pathogenetic role through paracrine production of viral interleukin-6 by infected stromal cells.34 These data further reinforce the link between KSHV and neoplastic disorders of preterminal and terminal B cells, although the precise pathogenetic mechanism exploited by the virus may vary in different diseases.
Beside providing an additional phenotypic marker for the diagnosis of PEL, the expression of CD138/syndecan-1 may explain in part the unique biologic behavior of this lymphoma. Typically, PEL displays a confined spreading along the serous membranes without invasive or destructive growth patterns.7,11 It is possible that the high levels of CD138/syndecan-1 expressed by PEL may cause enhanced adhesiveness to ECM components and that, in turn, these may be responsible for the peculiar growth pattern of PEL cells. In this regard, several studies have shown that cells expressing CD138/syndecan-1 can bind a number of ECM molecules, including fibronectin, thrombospondin, tenascin, and type I, III, or V collagen.14,15,19,20,35 Furthermore, syndecan-1 downregulation or loss is associated with ECM invasion and metastatic behavior of different types of normal and tumor cell,14,15,35-38 whereas its overexpression is able to restore anchorage-dependent growth and reduce the motility of human transformed cells.39
Quantitative phenotypic analysis showed that PEL cells may associate with surface levels of CD138/syndecan-1 as elevated as those detected in multiple myeloma plasma cells, which use this surface proteoglycan to remain tightly anchored to the bone marrow stroma.13,14,20 However, curiously, PEL cells express CD138/syndecan-1 molecules displaying a larger molecular weight than that found in plasma cells, probably reflecting a different size and/or composition of their glycosaminoglycan chains.14,35 The basis for the difference in the glycosylation pattern of CD138/syndecan-1 between PEL and multiple myeloma cells is unclear. According to one scenario, the specific CD138/syndecan-1 isoforms expressed by PEL cells might entail peculiar binding properties and functions that, consequently, might influence the interactions between tumor cells and their microenvironment. Notably, differences in the size and composition of glycosaminoglycan chains have been previously suggested to reflect different ligand binding properties and functions of syndecan-1 molecules in various cell types.14-16,35 40
The full elucidation of the growth peculiarities of PEL and their relationship to CD138/syndecan-1 is beyond the scope of this study and requires investigations specifically addressing this issue. In particular, future studies will have to provide a detailed biochemical characterization of the CD138/syndecan-1 isoforms expressed by PEL and will need to define the interactions occurring between PEL and purified ECM components both in the presence and in the genetically induced suppression of CD138/syndecan-1 expression.
ACKNOWLEDGMENT
The following reagent was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: BCBL-1 SP from Drs Michael McGrath and Don Ganem.
G.G., A.C., and A.P. have contributed equally to this work.
Supported by grants from IX Progetto AIDS, ISS (Rome, Italy; Grants No. 9404-33 and 9404-04); the Associazione Italiana per la Ricerca sul Cancro (Milan, Italy); the “Fondazione Piera, Pietro e Giovanni Ferrero” (Alba, Italy); the Associazione Italiana contro le Leucemie (AIL) “Trenta ore per la vita” (Rome, Italy); and the Ministero della Sanità, Ricerca Finalizzata IRCCS (Rome, Italy).
Address reprint requests to A. Carbone, MD, Division of Pathology, INRCCS-Centro di Riferimento Oncologico, Via Pedemontana Occidentale 12, 33081 Aviano (PN), Italy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal