Abstract
p300, which was originally cloned as a nuclear binding target of the adenovirus E1A oncoprotein, forms a family with cyclic-AMP response element binding protein (CREB)-binding protein (CBP). p300/CBP are considered to be transcriptional coactivators that connect the basal transcriptional machinery to various DNA-binding transcriptional factors. p300/CBP are implicated in both cell differentiation and regulation of cell-cycle. We identify here that the p300 gene is fused to the MLL gene and that in-frame MLL-p300 fusion protein is generated in acute myeloid leukemia (AML) with t(11; 22)(q23; q13). These findings suggest that the basis for the leukemogenesis of t(11; 22)-AML is the inability of p300 to regulate cell-cycle and cell differentiation after fusion with MLL.
THE TRANSFORMING activity of adenovirus E1A protein resides in two distinct domains. Targets of the protein include p300/cyclic-AMP response element binding protein (CREB)-binding protein (CBP) and products of the retinoblastoma protein (RB) susceptibility gene family, suggesting that p300/CBP as well as RB are involved in the regulation of cell cycle.1 p300/CBP contain three separate cysteine/histidine (C/H)-rich regions and a bromodomain and functions, at least in part, by linking the basal transcriptional machinery to various signal-responsive transcriptional factors such as CREB,2-4 nuclear hormone receptors,5,6 STAT1,7 STAT2,8 c-jun,9 c-fos,10 myb,11,12 NF-κB,13 and MyoD.14 In numerous cell types, elevation of intracellular cAMP levels is associated with an arrest of proliferation in G1 and the promotion of cell differentiation.15,16 In this context, p300/CBP also play a key role in cell differentiation, because p300/CBP specifically bind to CREB, which is phosphorylated by cAMP-dependent protein kinases.4 It was reported that p300/CBP can cooperate with tissue-specific bHLH proteins and are essential for muscle and B-cell differentiation.17 Moreover, it was shown that the induction of a cyclin-dependent kinase (CDK) inhibitor, p21, depends on p300 during both keratinocyte differentiation18 and neuronal differentiation.19 In addition, phosphorylation and activation of p300/CBP occur during differentiation of cells such as embryonal carcinoma cells.20 Recently, it was found that p300/CBP act as a novel type of acetyltransferase and acetylate nucleosomes in accord with p300/CBP-associated factor (P/CAF ).21 22
Chromosomal translocations associated with hematopoietic malignancies often result in the creation of chimeric proteins containing transcriptional factors, some of which are regulators of hematopoietic development. Aberrant transcription is considered to interfere with normal maturation and thereby plays a major role in tumorigenesis.23 Recently, it was found that the CBP gene located on 16p13 was fused with MOZ in acute myeloid leukemia (AML) patients with t(8; 16)(p11; p13)24,25 and with MLL in myelodysplastic syndrome (MDS) patients with t(11; 16)(q23; p13).26 p300 displays striking homology to CBP3; moreover, the gene encoding p300 maps to 22q13.27 We identify here that the p300 gene is fused to the MLL gene in an AML patient with t(11; 22)(q23; q13). These findings suggest that the basis for the leukemogenesis of t(11; 22)-AML is the inability of p300 to regulate cell cycle and cell differentiation after fusion with MLL.
MATERIALS AND METHODS
Patient. A 4-year-old boy was initially diagnosed as having non-Hodgkin lymphoma. A complete remission was achieved by the conventional chemotherapy including etoposide (total dose of etoposide, 5,200 mg/m2). Sixty-seven months after diagnosis, he developed secondary AML, which was cytogenetically characterized as t(11; 22)(q23; q13). He obtained a complete remission, but relapsed 20 months later. The leukemic cells in this study were obtained from peripheral blood at relapse.
Cytogenetic studies. The chromosomes of bone marrow samples were analyzed using the regular trypsin-Giemsa- or Q-banding method, as described previously.28 29
Isolation of genomic p300 clones. The λ human genomic library was screened using human p300 cDNA27 as a probe and 11 clones were isolated. Two of the clones (λhp300-2 and λhp300-9) were hybridized to the 1.4-kb BamHI fragment of 5′-region of p300 cDNA and were used for fluorescence in situ hybridization (FISH) analysis.
FISH analysis. Chromosomal mapping of the genomic clones (λhp300-2 and λhp300-9) was performed using the FISH method.30 The phage clones were labeled by the standard nick translation method using biotin-16-dUTP (Boehringer Mannheim, Mannheim, Germany). To confirm the origin of cloned DNAs, we also mapped these genomic clones to leukemic cells together with whole chromosome painting probe for chromosome 22 (WCP22; Coatasome 22, digoxigenin-labeled; Oncor, Gaithersburg, MD).
Southern and Northern blot analyses. Southern and Northern blot analyses were performed as described previously28,31 with some modifications. Briefly, high molecular weight DNA was extracted from frozen mononuclear cells of the patient carrying t(11; 22)-leukemic cells and normal peripheral blood as a control by proteinase K digestion and phenol/chloroform extraction. Ten micrograms of DNA was digested with BamHI or HindIII, subjected to electrophoresis on 0.8% agarose gels, and transferred onto charged nylon filters (Pall BioSupport, Tokyo, Japan) in 20× SSC. The filters were hybridized with 32P-labeled random-primed probes under the described conditions. Total RNA from frozen cells was extracted using the acid guanidine isothiocyanate-phenol-chloroform method.26 Ten micrograms of total RNA was electrophoresed and transferred onto nylon filters. The filters were hybridized with 32P-labeled random-primed probes. A 1.5-kb BamHI fragment of p300 cDNA27 and a 0.9-kb BamHI fragment (designated probe x) that spans exons 5 through 11 of MLL cDNA32 were used.
Reverse transcriptase-polymerase chain reaction (RT-PCR). Four micrograms of total RNA was reverse transcribed to cDNA in a total volume of 20 μL with random hexamers and 20 U of reverse transcriptase (AMV; Boehringer Mannheim). One microliter of cDNA was amplified by PCR in a total volume of 100 μL with 50 mmol/L KCl, 1.5 mmol MgCl2 , 10 mmol/L Tris-HCl (pH 9.0 at room temperature), 25 pmol/L of each primer, 75 μmol/L of each dNTP, and 2.5 U of Taq polymerase (Boehringer Mannheim). Samples were overlaid with 100 μL of mineral oil (Sigma, St Louis, MO). After 30 rounds of PCR (30 seconds at 94°C, 30 seconds at 55°C, and 1 minute at 72°C), 8 μL of PCR product was electrophoresed in a 1% agarose gel. Primers used were as follows: MLL-7S, 5′-TCCTCAGCACTCTCTCCAAT-3′; MLL-9S, 5′-GGTGTTGTCGTCGTTGCAAA-3′; MLL-11A, 5′-TTTGCCTGGAGTTGTGGATC-3′; p300-2S, 5′-CATCAGAATTCACCCTCGCC-3′; p300-7.5A, 5′-GCTGAAGTACTTGGCTGGTC-3′; p300-8A, 5′-GGCTCCTGATACTGTCCAGT-3′; and p300-11A, 5′-ACCTGTCCTTCAATGCTTAC-3′.
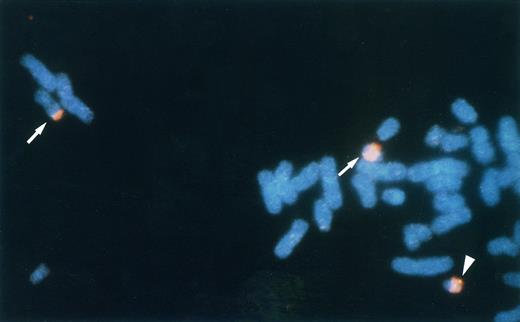
FISH analysis of the leukemic metaphase chromosomes. Chromosome 22 and a phage clone λhp300-2 were detected with TRITC and FITC, respectively. Split signals of λhp300-2 are observed on the boundary between WCP22-painted and unpainted regions of der(11)t(11; 22) and der(22)t(11; 22) (arrows). The intact signal of λhp300-2 was observed on the normal chromosome 22 (arrowhead).
FISH analysis of the leukemic metaphase chromosomes. Chromosome 22 and a phage clone λhp300-2 were detected with TRITC and FITC, respectively. Split signals of λhp300-2 are observed on the boundary between WCP22-painted and unpainted regions of der(11)t(11; 22) and der(22)t(11; 22) (arrows). The intact signal of λhp300-2 was observed on the normal chromosome 22 (arrowhead).
Sequencing of PCR products. PCR products were cloned into the TA cloning vector (Invitrogen, Carlsbad, CA). Nucleotide sequences were determined by the fluorometric method (Dye Terminator Cycle Sequencing Kit; Applied Biosystems, Urayasu, Japan).
Western blot analysis. Protein extraction and Western blotting were performed as described.20 Proteins were resolved on sodium dodecyl sulfate-5% polyacrylamide gels and transferred to filters (Hybond-ECL; Amersham, Buckinghamshire, UK) by electroblotting as described elsewhere.20 Filters were blocked in 5% nonfat dried milk, dissolved in phosphate-buffered saline plus 0.1% Tween-20 (PBS-T). After extensive washing in PBS-T, the filters were incubated for 1 hour at room temperature with p300-specific monoclonal antibody (RW105; UBI, Lake Placid, NY). After further washes in PBS-T, the filters were incubated with horseradish peroxidase-conjugated antibodies raised in goat against rabbit IgG (Amersham) and then washed with PBS-T. The immune complexes were visualized with the ECL detection system (Amersham).
RESULTS
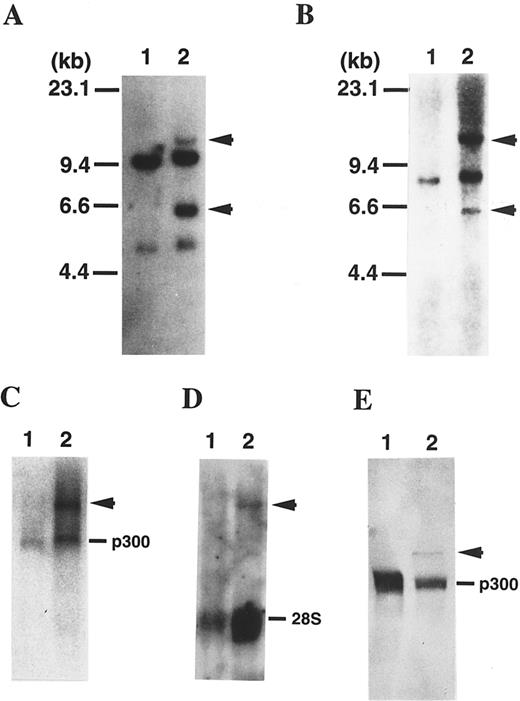
Rearrangement of the p300 gene in t(11; 22)-AML. FISH analysis using human genomic DNA clones spanning the p300 gene showed that two of the clones (λhp300-2 and λhp300-9) containing the 5′ end of the coding region of the p300 gene hybridized to both der(11) and der(22) as well as to normal chromosome 22 in metaphase chromosomes of the leukemic cells (Fig 1). These results suggest that the p300 gene is disrupted in t(11; 22) and that the breakpoint is located in the 5′ region of the p300 gene. To determine the breakpoint in the p300 gene, we performed Southern blot analysis using several fragments of the p300 cDNA as probes. Using a 1.4-kb BamHI-fragment spanning nucleotides 2987 to 4405 in the 5′-region of p300 bromodomain, we detected aberrant bands, suggesting that the breakpoint is located in this region of the p300 gene (Fig 2A). Northern analysis showed a 9.5-kb germline band of p300 and one abnormally sized band (11.5 kb; Fig 2C). On the other hand, the transcript of 11.5 kb in size was also detected by the MLL probe in leukemic cells of the patient, but the normal MLL transcript was not detected, possibly because the quality of mRNAs was poor (Fig 2D).
(A) Southern blot analysis of DNA digested with BamHI and probed with a 1.4-kb BamHI fragment of p300 cDNA probe. As a control, normal peripheral lymphocytes were used (lane 1). The patient exhibits rearranged bands (arrowhead), suggesting that a 1.4-kb BamHI fragment of p300 cDNA spans the translocation breakpoint (lane 2). (B) The same blot was rehybridized with the 0.9-kb BamHI fragment of MLL cDNA (probe x). The patient exhibits rearranged bands the same size as the bands detected with a BamHI fragment of p300 cDNA (arrowhead), suggesting that probe × also spans the breakpoint and that the p300 gene is translocated to the MLL gene. (C) Northern blot of RNAs using a 1.5-kb BamHI fragment of p300 cDNA probe. As a control, normal peripheral lymphocytes were used (lane 1). The patient showed an additional transcript (arrowhead) of approximately 11.5 kb (lane 2). (D) The same blot was rehybridized with the 0.9-kb BamHI fragment of MLL cDNA (probe x). The patient exhibits an aberrant band the same size as the band detected with a BamHI fragment of p300 cDNA (arrowhead). (E) Western blot analysis using p300-specific antibody against the C-terminal of p300. As a control, Namalwa, a Burkitt lymphoma cell line, was used (lane 1). The patient showed an additional polypeptide (arrowhead) of approximately 350 kD (lane 2).
(A) Southern blot analysis of DNA digested with BamHI and probed with a 1.4-kb BamHI fragment of p300 cDNA probe. As a control, normal peripheral lymphocytes were used (lane 1). The patient exhibits rearranged bands (arrowhead), suggesting that a 1.4-kb BamHI fragment of p300 cDNA spans the translocation breakpoint (lane 2). (B) The same blot was rehybridized with the 0.9-kb BamHI fragment of MLL cDNA (probe x). The patient exhibits rearranged bands the same size as the bands detected with a BamHI fragment of p300 cDNA (arrowhead), suggesting that probe × also spans the breakpoint and that the p300 gene is translocated to the MLL gene. (C) Northern blot of RNAs using a 1.5-kb BamHI fragment of p300 cDNA probe. As a control, normal peripheral lymphocytes were used (lane 1). The patient showed an additional transcript (arrowhead) of approximately 11.5 kb (lane 2). (D) The same blot was rehybridized with the 0.9-kb BamHI fragment of MLL cDNA (probe x). The patient exhibits an aberrant band the same size as the band detected with a BamHI fragment of p300 cDNA (arrowhead). (E) Western blot analysis using p300-specific antibody against the C-terminal of p300. As a control, Namalwa, a Burkitt lymphoma cell line, was used (lane 1). The patient showed an additional polypeptide (arrowhead) of approximately 350 kD (lane 2).
Rearrangement of the MLL gene in t(11; 22)-AML. The breakpoint in the 11q23 region was identified within the breakpoint cluster region of the MLL gene by Southern blotting using an MLL cDNA probe (probe x; Fig 2B). We detected additional bands, the same size as the bands detected with the BamHI-fragment of p300 cDNA, suggesting that the p300 gene is fused to the MLL gene.
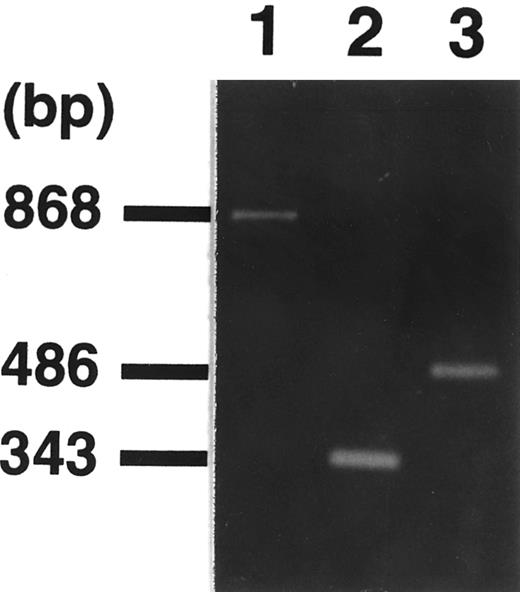
Isolation of the MLL-p300 fusion transcript. We performed RT-PCR and sequence analysis to confirm the fusion transcript. First, we set an antisense primer on the p300 gene in reference to the breakpoint on the CBP gene in t(8; 16)(p11; p13)24,25 and t(11; 16)(q23; p13).26 However, we could not detect the PCR product using sense primer sets for various positions in the breakpoint cluster region of the MLL gene. Using a sense primer on MLL exon 7 (MLL-7S) and an antisense primer (p300-8A) on the 5′-region of bromodomain of p300, we obtained PCR products of 868 bp (Fig 3). Furthermore, we performed RT-PCR assay by using inner sets of primers (sense primer, MLL-9S; antisense primer, p300-7.5A) and detected an amplified fragment of an expected size (Fig 3). Sequence analysis of the amplified fragments showed that exon 9 on the MLL gene was juxtaposed to the p300 cDNA sequence with an in-frame junction (Fig 4). The breakpoint on the MLL gene was expected to be located on intron 9. The reciprocal PCR product of p300-MLL fusion transcript was detected by RT-PCR assay using a sense primer on p300 (p300-2S) and an antisense primer on MLL exon 11 (MLL-11A; Fig 3). We also performed genomic PCR assay by using a set of primers (sense primer, MLL-9S; antisense primer, p300-11A) and detected a 1.6-kb fragment containing MLL and p300 genomic breakpoints (Fig 5).
Identification of fusion transcripts by RT-PCR. Primers used were MLL-7S and p300-8A (lane 1), MLL-9S and p300-7.5A (lane 2), and p300-2S and MLL-11A (lane 3), respectively. Sequencing analysis of the amplified fragment (lane 1) showed a 344-bp fragment of MLL exons 7 through 9 in the 5′ region and 524 bp of p300 exon in the 3′ region. Primers (p300-2S and MLL-11A) were designed for the detection of reciprocal p300-MLL transcript, resulting in the detection of amplified fragment (lane 3).
Identification of fusion transcripts by RT-PCR. Primers used were MLL-7S and p300-8A (lane 1), MLL-9S and p300-7.5A (lane 2), and p300-2S and MLL-11A (lane 3), respectively. Sequencing analysis of the amplified fragment (lane 1) showed a 344-bp fragment of MLL exons 7 through 9 in the 5′ region and 524 bp of p300 exon in the 3′ region. Primers (p300-2S and MLL-11A) were designed for the detection of reciprocal p300-MLL transcript, resulting in the detection of amplified fragment (lane 3).
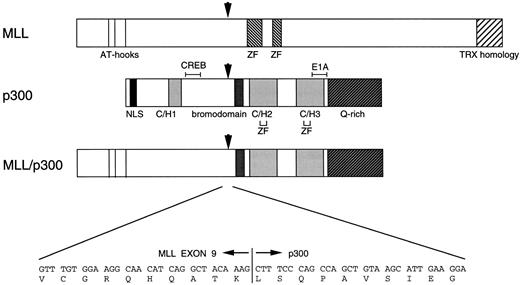
Schematic representation of the MLL, p300, and putative MLL-p300 chimeric proteins. The functional domains of MLL and p300 are shown beneath the figure. The nt and single-letter amino acid sequences surrounding the MLL-p300 breakpoint are shown at the bottom of the figure. NLS, nuclear localization signal; C/H, cysteine/histidine-rich region; Q rich, glutamine-rich region; ZF, Zinc finger domain; CREB, CREB-binding site; E1A, E1A binding site. Arrows indicate the breakpoint of each gene and the fusion point of chimeric protein. MLL-p300 fusion junction is in-frame, and putative chimeric protein loses part of the C/H-rich region and the CREB-binding site located in p300.
Schematic representation of the MLL, p300, and putative MLL-p300 chimeric proteins. The functional domains of MLL and p300 are shown beneath the figure. The nt and single-letter amino acid sequences surrounding the MLL-p300 breakpoint are shown at the bottom of the figure. NLS, nuclear localization signal; C/H, cysteine/histidine-rich region; Q rich, glutamine-rich region; ZF, Zinc finger domain; CREB, CREB-binding site; E1A, E1A binding site. Arrows indicate the breakpoint of each gene and the fusion point of chimeric protein. MLL-p300 fusion junction is in-frame, and putative chimeric protein loses part of the C/H-rich region and the CREB-binding site located in p300.
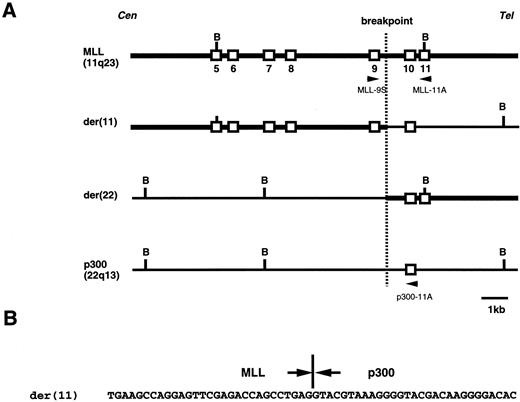
Genomic analysis of the t(11; 22)(q23; q13) chromosome translocation. (A) Physical map of the t(11; 22) junction in the patient, as well as the corresponding regions from chromosomes 11 and 22. (B) Sequence of the t(11; 22) breakpoint region in the patient. Cen and Tel denote the direction of the centromeres and telomeres of the two chromosomes. Open vertical boxes represent defined exons. Restriction sites: B, BamHI. kb, kilobase.
Genomic analysis of the t(11; 22)(q23; q13) chromosome translocation. (A) Physical map of the t(11; 22) junction in the patient, as well as the corresponding regions from chromosomes 11 and 22. (B) Sequence of the t(11; 22) breakpoint region in the patient. Cen and Tel denote the direction of the centromeres and telomeres of the two chromosomes. Open vertical boxes represent defined exons. Restriction sites: B, BamHI. kb, kilobase.
Expression of the MLL-p300 fusion protein. To examine the expression of the fusion protein that resulted from t(11; 22), Western blot analysis was performed with a p300-specific monoclonal antibody. As shown in Fig 2E, a band (∼350 kD) was observed in addition to the germline band (∼270 kD). Because the antibody specifically recognizes the C-terminal region of p300, this extra band probably corresponds to MLL-p300 fusion protein. Further study using MLL-specific antibody is required to substantiate the identity of the extra band.
DISCUSSION
To date, the t(11; 22) has been so far reported in patients with de novo acute myelomonocytic leukemia (AMMoL).33 34 A chromosomal breakpoint in 11q23 was detected within the MLL breakpoint cluster region by Southern blotting and the partner gene was shown to be localized in a region centromeric to BCR gene on 22q11. Therefore, the partner gene may differ from the p300 gene. In the present study, we found that the p300 gene was involved in leukemia and that the MLL-p300 fusion protein was generated in a secondary AML patient with t(11; 22).
We detected transcribed chimeric mRNAs from both derivative chromosomes by RT-PCR assay. Previous studies of variant 11q23 translocations involving three-way rearrangement showed that the conserved junction arises from translocation of chromosomal material to the der(11) chromosome.35 Furthermore, in a case with t(4; 11), expression of der(4) product was not detectable.36 These results suggest that the der(11) MLL-p300 fusion protein is a pathogenetically important fusion product.
A schematic representation of the predicted MLL-p300 fusion protein is shown in Fig 4. The predicted MLL-p300 fusion transcript encodes a protein of 3,006 amino acids, 1,531 amino acids from MLL (amino acids 1 to 1,531) and 1,475 amino acids from p300 (amino acids 940 to 2,414). The AT-hook domains, putative methyltransferase, and a transcriptional repression domain of MLL are retained in the MLL-p300 chimeric protein. On the other hand, the chimeric protein retains the acetyltransferase domain and the TFIIB-binding domain of p300, but it lacks most of the N-terminal C/H-rich region and the putative CREB-binding region of p300. However, almost all of the parts of CBP are retained in the MOZ-CBP and MLL-CBP fusion proteins,24 26 which are created by t(8; 16) and t(11; 16), respectively. These findings suggest that a simple truncation of the region of p300 is unlikely to be sufficient to promote leukemogenesis and that the basis for the leukemogenesis of t(11; 22)-AML may be altered function of p300 by structural changes through fusion with MLL.
It is noted that the MLL gene is fused with various partner genes by 11q23 chromosome translocations. To date, 12 partner genes for MLL have been cloned from leukemia cells with various types of reciprocal translocations.37 However, the normal functions of MLL and the fusion protein remain unknown. We identified here one of the partner genes of MLL involved in both cell differentiation and regulation of cell cycle. Our results suggest that the basis for the leukemogeneses induced by MLL gene translocations may be altered function of the chimeric partner gene product. Functional analysis of the MLL-p300 fusion protein will provide new insights into leukemogenesis.
ACKNOWLEDGMENT
The authors thank M. Yanagisawa (Professor, Department of Pediatrics, Faculty of Medicine, University of Tokyo, Tokyo, Japan) for his advice and review. We also thank M. Seto (Laboratory of Chemotherapy, Aichi Cancer Center Research Institute, Aichi, Japan) and D.M. Livingston (Dana-Farber Cancer Institute, Boston, MA) for providing the MLL cDNA probe (probe x) and p300 cDNA, respectively. We express appreciation to S. Sohma for her technical assistance.
Supported by a Grant-in-Aid for Cancer Research from the Ministry of Health and Welfare of Japan, a Grant-in-Aid for Scientific Research on Priority Areas, and Grant-in-Aid for Scientific Research (B) and (C) from the Ministry of Education, Science, Sports and Culture of Japan.
Address reprint requests to Yasuhide Hayashi, MD, Department of Pediatrics, Faculty of Medicine, University of Tokyo, 7-3-1 Hongo Bunkyo-ku, Tokyo 113, Japan.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal