Abstract
Chronic myelogenous leukemia (CML) is a malignant disease of the human hematopoietic stem cell caused by the BCR/ABL gene rearrangement. The only curative therapy is allogeneic transplantation. Although autologous transplants may prolong survival, most patients relapse because of disease persisting in the host and in the graft. Continued administration of chemotherapy after transplant could reduce the incidence of relapse provided that the autograft can be protected by transfer of a drug-resistance gene. However, CML autografts will almost certainly contain malignant stem cells that will also be rendered drug-resistant. The presence of the BCR/ABL oncoprotein is necessary and sufficient for malignant transformation seen in CML. We thus hypothesized that transfer of a vector that combines a drug-resistance gene with anti-BCR/ABL antisense (AS) sequences may allow for posttransplant chemotherapy to decrease persistent disease while rendering inadvertently transduced CML stem and progenitor cells functionally normal. We constructed a retroviral vector, LasBD, that combines the methotrexate (MTX)-resistant tyrosine-22 dihydrofolate-reductase (tyr22-DHFR) gene and AS sequences directed at the b3a2 BCR/ABL breakpoint. b3a2 BCR/ABL containing 32D and MO7e cells were transduced with LasBD and selected in MTX for 14 days. Expression of the AS sequences reduced BCR/ABL mRNA and p210BCR/ABL protein levels by 6- to 10-fold in most cells. This subsequently led to the restoration of normal function of BCR/ABL cDNA+ cells: they grew significantly slower in the presence of interleukin-3 (IL-3); they underwent apoptotic cell death when cultured without IL-3; and they had restored expression and function of adhesion receptors. These effects were specific, because LasBD-containing AS sequences directed at the b3a2 BCR/ABL breakpoint did not affect p190BCR/ABL-containing cells. LasBD also rendered 20% to 30% of primary Ph− and Ph+ CD34+ cells MTX-resistant and decreased BCR/ABL mRNA levels in MTX resistant Ph+ CD34+ cells by 10-fold. Expression of the MTX-resistant DHFR gene and the AS sequences has been stable for at least 1 year in vitro and for more than 70 days in vivo. Finally, LasBD decreased tumorigenicity of 32DBCR/ABL cells in vivo by 3 to 4 logs. In conclusion, the tyr22-DHFR gene in the LasBD vector can protect normal hematopoietic cells from MTX-mediated toxicity, whereas the AS sequences in LasBD can suppress expression of the BCR/ABL gene and restore normal function of BCR/ABL cDNA-containing cells. The LasBD vector may therefore prove to be an extremely useful adjunct in autologous transplantation for CML.
CHRONIC MYELOGENOUS leukemia (CML) is a malignant disease of the hematopoietic stem cell1 characterized by the Philadelphia chromosome (Ph)2 and the BCR/ABL gene rearrangement.3 A number of studies have shown that the BCR/ABL-derived oncoprotein, p210BCR/ABL, is necessary and sufficient for malignant transformation.4,5 The only curative treatment for CML is transplantation of stem cells from a related or unrelated donor.6,7 Although transplantation of autologous bone marrow or peripheral blood progenitors may restore Ph− hematopoiesis in a fraction of patients,8 most patients relapse within 1 year after transplantation. This is due in part to contamination of the graft with Ph+ cells9 and in part to disease persisting in the host after the preparative regimen.10 This finding shows the need for new approaches to eliminate minimal residual disease.
One approach to reduce relapse is to continue administration of chemotherapy after transplantation. However, these chemotherapeutic agents will not only affect Ph+ stem and progenitor cells but also normal stem cells. Transduction of normal stem cells with a drug-resistance gene may allow use of posttransplant chemotherapy that will suppress residual leukemia but selectively protect the genetically modified normal hematopoietic cells from the toxic effects of chemotherapy.11-14 However, the autograft that can be obtained from blood or marrow of CML patients will likely contain malignant, Ph+ stem and progenitor cells. It is likely, therefore, that a fraction of Ph+ stem cells in the graft will be transduced with the drug-resistance gene, protecting these cells from chemotherapy administered after transplantation.
The malignant phenotype of CML progenitors can be attributed to the presence of the BCR/ABL mRNA and p210BCR/ABL tyrosine kinase.4,5 Several studies have shown that elimination of the BCR/ABL mRNA with breakpoint-specific antisense (AS) oligodeoxynucleotides or anti-BCR/ABL AS-containing vectors results in normalization of the phenotypic characteristics of Ph+ progenitors.15 We hypothesized that cotransduction of a methotrexate (MTX)-resistance gene with anti-BCR/ABL antisense sequences may render normal hematopoietic cells MTX-resistant and restore normal function of drug-resistant Ph+ cells. This would then allow administration of MTX after transplantation to suppress the Ph+ clone while protecting dihydrofolate-reductase (DHFR)-containing normal hematopoietic cells from MTX toxicity and abrogating the malignant CML phenotype from MTX-resistant Ph+ progenitors.
MATERIALS AND METHODS
Vector Construction
The retroviral vector LasBD contains the murine tyr22DHFR gene16 transcriptionally regulated by an internal β-actin promoter and two 20-mer anti-b3a2 AS-modified sequences regulated by the Moloney murine leukemia virus (MoMuLV) long terminal repeat (LTR). The double copy modified AS unit (Table 1) was synthesized by overlapping polymerase chain reaction (PCR) using a 5′ oligonucleotide to incorporate both HindIII and Xho I restriction sites upstream (5′-GCAAGCTTCTCGAGGCTCTCTATAGAAGG-3′) and a 3′ oligonucleotide to incorporate a Pst I restriction site downstream -(5;pr-G C CA T CG A TC T GC A GG C AG A GT T CA A AA G CC C TT C GC-AGAGTTCAAAAGCCCTTCTATAGAGAGCCTC-3′; synthesized by National Biosciences, Inc, Plymouth, MN). The modified anti-b3a2 breakpoint double AS unit was amplified by 10 cycles of PCR (94°C for 50 seconds, 65°C for 30 seconds, and 72°C for 50 seconds). The Xho I/BamHI β-actin fragment from pgag β-actin (kindly provided by Dr James Wilson, University of Pennsylvania, Philadelphia, PA) was cloned into the Sal I/BamHI site of pUC19 (Promega, Madison, WI) and the AS fragment inserted between HindIII and Pst I, upstream of β-actin. The AS-β-actin sequence (Xho I/BamHI) was then cloned between the 5′LTR and the tyr22-DHFR gene in the retroviral vector construct pLDtyr22.17
Synthetic Oligonucleotides, Primers, and Probe for Amplification of BCR/ABL mRNA and cDNA, β-Actin mRNA, and AS mRNA
| . | Purpose . | . | Sequence . |
|---|---|---|---|
| Modified AS sequence | 5′-GCTCTCTATAGAAGGGCTTTTGAACTCTGCGAAGGGCTTTTGAACTCTGC-3′ | ||
| AS mRMA | RT-PCR | Primer A | 5′-GCAAGCTTCTCGAGGCTCTCTATAGAAGG-3′ |
| Primer B | 5′-CTGCAGGCAGAGTTCAA-3′ | ||
| Probe C | 5′-GAAGGGCTTTTGAACTCTGCGAAGGGG-3′ | ||
| b3a2 BCR/ABL mRNA | RT-PCR | Primer D | 5′-GGAGCTGCAGATGCTGACCAAC-3′ |
| Primer E | 5′-TCAGACCCTGAGGCTCAAAGTC-3′ | ||
| Probe F | 5′-GCTGAAGGGCTTTTGAACTCTGCTTA-3′ | ||
| ela2 BCR/ABL mRNA | RT-PCR | Primer G | 5′-GTTGTCGTGTCCGAGGCC-3′ |
| Primer H | 5′-TCAGACCCTGAGGCTCAAAGTC-3′ | ||
| Probe I | 5′-GGCCGCTGAAGGGCTTCTGCG-3′ | ||
| β-actin mRNA | RT-PCR | Primer J | 5′-TACCTCATGAAGATCCTCA-3′ |
| Primer K | 5′-TTCGTGGATGCCACAGGAC-3′ | ||
| Probe L | 5′-CCATCTCTTGCTCGAAGTC-3′ |
| . | Purpose . | . | Sequence . |
|---|---|---|---|
| Modified AS sequence | 5′-GCTCTCTATAGAAGGGCTTTTGAACTCTGCGAAGGGCTTTTGAACTCTGC-3′ | ||
| AS mRMA | RT-PCR | Primer A | 5′-GCAAGCTTCTCGAGGCTCTCTATAGAAGG-3′ |
| Primer B | 5′-CTGCAGGCAGAGTTCAA-3′ | ||
| Probe C | 5′-GAAGGGCTTTTGAACTCTGCGAAGGGG-3′ | ||
| b3a2 BCR/ABL mRNA | RT-PCR | Primer D | 5′-GGAGCTGCAGATGCTGACCAAC-3′ |
| Primer E | 5′-TCAGACCCTGAGGCTCAAAGTC-3′ | ||
| Probe F | 5′-GCTGAAGGGCTTTTGAACTCTGCTTA-3′ | ||
| ela2 BCR/ABL mRNA | RT-PCR | Primer G | 5′-GTTGTCGTGTCCGAGGCC-3′ |
| Primer H | 5′-TCAGACCCTGAGGCTCAAAGTC-3′ | ||
| Probe I | 5′-GGCCGCTGAAGGGCTTCTGCG-3′ | ||
| β-actin mRNA | RT-PCR | Primer J | 5′-TACCTCATGAAGATCCTCA-3′ |
| Primer K | 5′-TTCGTGGATGCCACAGGAC-3′ | ||
| Probe L | 5′-CCATCTCTTGCTCGAAGTC-3′ |
Vector Packaging
LasBD was shuttle-packaged through GP + E8618 cells into PG1319 or PA31720 cells. Transduced PG13 or PA317 cells were subcultured in RPMI containing 20% new born calf serum (Sigma, St Louis, MO) and 0.25 μmol/L MTX (Sigma). After 2 weeks, individual clones were harvested, expanded, and screened for high titer virus producers. The viral titer of PG13-LasBD was 8 × 106 cfu/mL (determined using HeLa cells) and that of PA317-LasBD 3 × 106 cfu/mL (determined using NIH-3T3 cells).
Hematopoietic Cell Lines
32Dp210 cells and 32Dp190 cells21 were maintained in Iscove's modified Dulbecco's medium (IMDM) supplemented with 10% fetal calf serum (FCS; Hyclone, Logan, UT) and L-glutamine (Sigma). LasBD-transduced 32Dp210 cells (LasBD-32Dp210) and LasBD-transduced 32Dp190 cells (LasBD-32Dp190) were maintained in IMDM supplemented with 20% FCS and 20% conditioned medium derived from a previously described recombinant murine (rMu) interleukin-3 (IL-3) producing cell line.22 MO7e (MYN) and MO7ep210 (MBA-4) cells were kindly provided by Dr John Dick (Hospital for Sick Children, Toronto, Ontario, Canada).23 MO7e and LasBD transduced MO7ep210 cells (LasBD-MO7ep210) were maintained in IMDM supplemented with 10% FCS and 5 ng/mL recombinant human (rh) IL-3 (R&D Systems, Minneapolis, MN). MO7ep210 cells were maintained in IMDM and 10% FCS.
Primary Marrow Progenitors
Heparinized bone marrow was obtained from normal individuals and patients with BCR/ABL mRNA-positive chronic-phase CML, after obtainining informed consent, using guidelines from the Committee on the use of Human Subjects for Clinical Research at the University of Minnesota. CD34+ HLA-DR+ and CD34+ HLA-DR− cells were selected by avidin-biotin column selection followed by fluorescence-activated cell sorting (FACS) as described.24 In CML, CD34+ HLA-DR+ cells are highly enriched in Ph+, BCR/ABL mRNA+ cells.24
MTX Sensitivity of Ph+ and Normal Colony-Forming Cells (CFC) and Long-Term Culture-Initiating Cells (LTC-IC)
CFC. CML and normal CD34+ HLA-DR+ cells (5 × 103) were plated in semisolid methylcellulose progenitor culture containing IMDM, 1.2% methylcellulose, 10−4 mol/L 2 mercaptoethanol, 20 mg/mL bovine serum albumin, 10 μg/mL insulin, 200 μg/mL transferrin (all from Sigma), 100 U/mL penicillin and streptomycin (GIBCO/BRL, Grand Island, NY), 5 ng/mL IL-3, 5 ng/mL granulocyte colony-stimulating factor (Neupogen; Amgen, Thousand Oaks, CA), 5 ng/mL stem cell factor (SCF; kindly provided by Amgen), and 3 U erythropoietin (Epogen; Amgen) with increasing MTX concentrations (10−10 to 10−6 mol/L MTX). After 14 days, colonies were enumerated as previously described.24
LTC-IC. Normal CD34+ HLA-DR− cells and CML CD34+ HLA-DR+ (containing largely Ph+ LTC-IC)24 cells (1 × 104) were cultured in IMDM with 20 mg/mL bovine serum albumin, 10 μg/mL insulin, 200 μg/mL transferrin (Sigma), 100 U/mL each penicillin and streptomycin, 5 ng/mL IL-3, 5 ng/mL IL-6 (Genetics Institute, Boston, MA), and 5 ng/mL SCF with increasing concentrations of MTX (10−10 to 10−6 mol/L MTX). After 1 week, cells were replated in complete long-term culture medium (IMDM with 12.5% FCS, 12.5% horse serum; Terry Fox Laboratories, Vancouver, British Columbia, Canada), 10−6 mol/L hydrocortisone (Upjohn, MI), and L-glutamine (GIBCO) for 5 weeks in contact with irradiated M2-10B4 feeders (kindly provided by Dr Connie Eaves, Terry Fox Laboratories) as described.24 The number of LTC-IC that survived the initial week of culture in the presence of MTX was determined by replating progeny from week 5 LTC in methylcellulose assay without MTX as described above.
Transduction of Cell Lines and Primary Progenitors
Cell lines. MO7ep210 and 32Dp210 cells were incubated in undiluted PG13 or PA317 retroviral stocks supplemented with 8 μg/mL polybrene (GIBCO/BRL) for 24 hours. Cells were then cultured in IMDM with 0.25 μmol/L MTX and 5 ng/mL rHuIL-3 or 20% rMuIL-3 containing NIH-3T3 conditioned medium to select for DHFR-expressing cells (bulk-selected cells). In addition, replicates of 1 × 103 transduced cells were cultured in wells of a 48-well plate in selective medium to isolate clones.
Primary bone marrow progenitors. Normal or CML CD34+ HLA-DR+ cells (5 × 104) were cultured in IMDM + 20% FCS + 5 ng/mL each of IL-3, IL-6, and SCF for 48 hours in contact with M2-10B4 feeders.24 After 48 hours, medium was replaced with 1 mL PG13-LasBD or PG13-LBD supernatant, 10 μg/mL protamine sulfate (Fujisawa Inc, Deerfield, IL), and 5 ng/mL each of IL-3, IL-6, and SCF for 24 hours. A total of 1 × 104 cells were then assayed for CFC as described above in the presence or absence of 5 × 10−8 mol/L MTX. The percent MTX-resistant colonies was calculated as [the number of CFC in MTX containing cultures of transduced cells divided by the number of CFC in MTX free cultures of transduced cells] × 100 − [the number of CFC in MTX containing cultures of mock-transduced cells divided by the number of CFC in MTX free cultures of mock-transduced cells].
Alternatively, 40,000 cells were cultured in a liquid culture system, similar to the methylcellulose cultures described above but without the addition of methylcellulose. After 14 days, cells were harvested and examined for the presence of BCR/ABL mRNA, using reverse transcription-PCR (RT-PCR).
Molecular Analysis of Retroviral Transduction and BCR/ABL mRNA
Northern blot. Total RNA was extracted from cells or tissue samples using RNeasy (Qiagen, Inc, Chatsworth, CA). RNA was treated with DNase1 (Ambion, Austin, TX) for 15 minutes at 37°C. Poly-A+ mRNA was isolated from 100 to 200 μg of total RNA (Oligotex mRNA procedure; Qiagen, Inc). For Northern blot analysis, 2 μg of poly(A)+ mRNA was electrophoresed in 1.2% agarose/formaldehyde gels25 and transferred to Nytran by downward capillary transfer (Turboblotter; Schleicher and Schuell, Keene, NH). Blots were fixed by UV cross-linking (Stratalinker; Stratagene, San Diego, CA) and hybridized overnight with a 32P-labeled β-actin probe and DHFR probe at 42°C in 50% formamide. After a final wash in 0.1× SSC/0.1% sodium dodecyl sulfate (SDS) for 20 minutes at 65°C, membranes were exposed to x-ray films at −70°C overnight.
Semiquantitative RT-PCR for antisense mRNA. To detect AS mRNA, total RNA was extracted from bulk-selected or cloned LasBD-32Dp210 cells. Total RNA was treated with DNase I and reverse transcribed using 200 U MoMLVRT (GIBCO/BRL) and 250 ng primer B for 1 hour at 37°C. Five hundred nanograms of cDNA was then amplified by adding 1 U Taq DNA polymerase (Promega) and 250 ng primer A (94°C for 1 minute, 55°C for 50 seconds, and 72°C for 1 minute for 30 cycles; Table 1). To show the semiquantitative nature of the reaction, the following sets of reactions were performed. In initial experiments, 500 ng of cDNA obtained after the RT step was subjected to 20 to 40 cycles of amplification. Because 30 cycles of amplification provided a semiquantitative measure of AS mRNA, we then performed RT-PCR (30 cycles) of threefold serial dilutions of AS cDNA. This showed a commensurate threefold decrease in PCR signal (not shown).
Semiquantitative RT-PCR for p210BCR/ABL and p190BCR/ABL mRNA. To detect BCR/ABL mRNA, total RNA was extracted from bulk-selected or cloned MO7ep210 and 32Dp210 or 32Dp190 cells that were mock-transduced or transduced with LasBD. Total RNA was digested with DNase I to degrade BCR/ABL cDNA, RNA was collected and reverse transcribed using 200 U MoMLVRT and 250 ng of primers E or H. PCR was performed after adding 1 U Taq DNA polymerase and 250 ng of the 5′ primers, D, J, or G (94°C for 1 minute, 60°C for 50 seconds, and 72°C for 1 minute; Table 1). The number of cycles needed to obtain semiquantitation of the BCR/ABL mRNA was determined initially by varying the number of cycles for a constant amount of cDNA. In initial experiments and throughout the evaluation of cell lines and primary cells, the semiquantitative nature was confirmed by serial dilution of cDNA amplified at a constant number of cycles. The sensitivity of the RT-PCR reaction for b3a2 BCR/ABL mRNA is 1/104 to 1/5 × 105 cells. In some experiments, the reverse transcription step was performed at 68°C for 20 minutes using the thermostabile Tth polymerase (Boehringer Mannheim, Indianapolis, IN).
PCR for p210 BCR-ABL cDNA. For detection of b3a2 BCR/ABL cDNA, PCR was used essentially as described above. The sensitivity for the PCR reaction is 1/104 to 1/105 cells.
Southern blot of RT-PCR and PCR products. PCR products were electrophoresed and transferred to a hybond membrane (Schleicher and Schuell) by overnight capillary transfer and fixed by UV irradiation. Membranes were hybridized with digoxigenin (Boehringer Mannheim) labeled p210BCR/ABL (F ), p190BCR/ABL (K), β-actin (I), or AS (C) (Table 1) probes per the manufacturer's instructions. X-ray films were exposed to membranes for 2 hours. Images captured using a GS-700 Imaging Densitometer (Bio-Rad, Hercules, CA) were quantitated using Molecular Analyst software.
Western Blot Analysis
A total of 105 viable cells was lysed directly in SDS sample buffer (50 mmol/L Tris-HCl pH 6.8, 2% SDS, 10% glycerol, and 5% β-mercaptoethanol). After boiling for 8 minutes, lysates were subjected to electrophoresis on 8% polyacrylamide gels and transferred to polyvinylidene difluoride (PVDF) membranes using a Semidry Transfer Apparatus (Bio-Rad). Blots were incubated with Blocker Blotto in TBS (Pierce, Rockford, IL) and 0.1 μg/mL anti-ABL antibody at 4°C overnight, washed four times in TBST (TBS supplemented with 0.05% tween 20), and incubated for 1 hour with a goat antimouse or antirabbit horseradish peroxidase-conjugated antibody (1:10,000 dilution). Protein bands were visualized using an ECL detection system (E.I. du Pont de Nemours & Co, Boston, MA). Blots were stripped (using stripping buffer: 2% SDS, 100 mmol/L 2-mercaptoethanol, 62.5 mmol/L Tris, pH 6.8) at 50°C for 30 minutes and reprobed with antibody against BCL-2, BAX, C-MYC, or P53. Antihuman and/or antimouse ABL, BCL-2, BAX, C-MYC, and P53 monoclonal mouse or rabbit antibodies were purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). Goat antimouse or antirabbit antibodies conjugated to horseradish peroxidase were from Jackson ImmunoResearch Laboratories, Inc (West Grove, PA).
Integrin Function and Expression
Adhesion receptor expression. MO7e, MO7ep210, and LasBD-MO7ep210 cells (bulk-selected and cloned) were stained with fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated monoclonal antibodies against CD29 (Pharmingen, San Diego, CA) and CD44 (Southern Biotechnology Associates, Birmingham, AL; 20 ng/105 cells) for 20 minutes at 4°C. Cells were washed and analyzed on a FACStarPLUS flow cytometer (Becton Dickinson Inc, Mountain View, CA) based on PE- and FITC-conjugated isotype-matched mouse control Igs.
Adhesion receptor capping. Aliquots of 5 to 10 × 104 MO7e, MO7ep210, and LasBD-MO7ep210 cells were incubated with an anti-b1 antibody (P4C10; GIBCO/BRL; 1:400 dilution of mouse ascites) for 1 hour at 4°C, washed with cold phosphate-buffered saline, and then incubated with 10 μg/mL goat-antimouse FITC antibody for 4 hours at 37°C. Cells were washed and fixed with 200 μL 2% paraformaldehyde, and then cytospin preparations were examined by immunofluorescence microscopy for the presence or absence of integrin caps. A minimum of 200 cells was counted.
In Vivo Tumorigenicity
A total of 104 to 107 cells was suspended in 0.2 mL phosphate-buffered saline and injected through the tail vein into C3H mice. All animals were housed and maintained according to institutional guidelines. Survival of animals was assessed daily for 100 days after inoculation. Seventy days after inoculation, 8 (107 cell dose), 16 (106 cell dose), and 17 (105 cell dose) animals that received differing numbers of LasBD-32Dp210 cells were killed by cervical dislocation. Spleen and marrow were examined for the presence of BCR/ABL cDNA, BCR/ABL mRNA, and AS mRNA as described above.
LasBD-32Dp210 cells. Four independent experiments were performed with LasBD-32Dp210 cells selected ex vivo with MTX. In each experiment, 8 to 12 animals per cell dose received injections. The number of animals that was evaluable between days 0 and 70 was 36 to 43 animals for each cell dose. Because a number of the surviving animals were killed on day 70, the number of animals evaluable between day 75 and 100 was 26 to 28 animals for each cell dose.
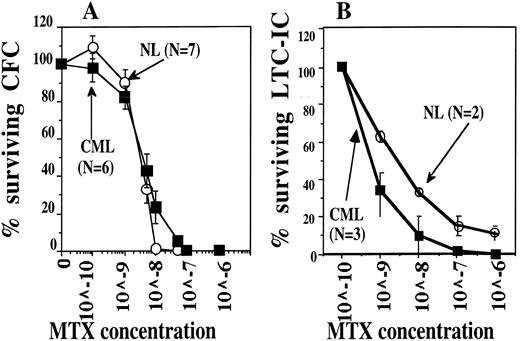
CML CFC (A) and LTC-IC (B) are at least as sensitive to MTX as their normal counterparts. Five thousand normal and CML CD34+HLA-DR+ cells were plated in serum-free methylcellulose assay with increasing MTX concentrations to determine the MTX sensitivity of CFC (left panel). Alternatively, 10,000 normal CD34+HLA-DR− cells (LTC-IC) and CML CD34+HLA-DR+ (Ph+ LTC-IC) cells were incubated for 1 week in serum-free medium, IL-3, IL-6, and SCF with increasing MTX concentrations. Cells were then plated in contact with M2-10B4 stromal feeders for 5 weeks. The number of MTX resistant LTC-IC was determined by replating LTC derived progeny in methylcellulose assay without MTX.
CML CFC (A) and LTC-IC (B) are at least as sensitive to MTX as their normal counterparts. Five thousand normal and CML CD34+HLA-DR+ cells were plated in serum-free methylcellulose assay with increasing MTX concentrations to determine the MTX sensitivity of CFC (left panel). Alternatively, 10,000 normal CD34+HLA-DR− cells (LTC-IC) and CML CD34+HLA-DR+ (Ph+ LTC-IC) cells were incubated for 1 week in serum-free medium, IL-3, IL-6, and SCF with increasing MTX concentrations. Cells were then plated in contact with M2-10B4 stromal feeders for 5 weeks. The number of MTX resistant LTC-IC was determined by replating LTC derived progeny in methylcellulose assay without MTX.
Untransduced 32Dp210 and 32Dp190 cells and LasBD-32Dp190 cells. Three independent experiments were performed with untransduced 32Dp210 and 32Dp190 cells and with LasBD-32Dp190 cells selected ex vivo with MTX. In each experiment, 8 to 10 animals per cell dose were injected. No animals were killed.
RESULTS AND DISCUSSION
Ph+ CML Progenitors Are Sensitive to MTX In Vitro
We constructed and tested a retroviral vector containing the mutant tyr22-DHFR gene and a double-copy AS sequence, termed LasBD. Our choice of the tyr22-DHFR gene as a drug-resistance gene rather than MDR-1 was based on the following premises. First, we and others have shown that transplantation of murine stem cells expressing an MTX-resistant DHFR gene can protect the recipient animals from lethal MTX toxicity.11-14 Several mutant forms of the DHFR gene have been described. The tyr22-DHFR variant gene was chosen because it has a favorable combination of marked drug resistance and retained catalytic activity.16 Second, even though MTX is not commonly used in the therapy of CML, we performed in vitro studies to evaluate its effects on normal and CML committed (CFC) and primitive (LTC-IC) progenitors. Culture of CML or normal CD34+ cells enriched for CFC or LTC-IC in the presence of increasing concentrations of MTX showed that Ph+ CML CFC and LTC-IC are at least as sensitive to MTX as NL progenitors (Fig 1). Thus, administration of MTX after transplantation may selectively eliminate the Ph+ CML progenitors. The third reason for choosing an MTX-resistant DHFR gene rather than the MDR-1 gene to protect the graft from chemotherapy is based on safety concerns. It is possible in theory that Ph+ stem and progenitor cells, rendered MTX-resistant by transfer of the DHFR gene, may escape antisense induced normalization of leukemic cell function. These progenitors may then continue to expand and acquire additional genetic abnormalities leading to blast crisis. Even though resistant to MTX, such cells would continue to be sensitive to the majority of antileukemic chemotherapeutic agents commonly used in the therapy of CML.
The LasBD-Encoded AS-mRNA Inhibits BCR/ABL Gene Expression in BCR/ABL-Containing Cell Lines
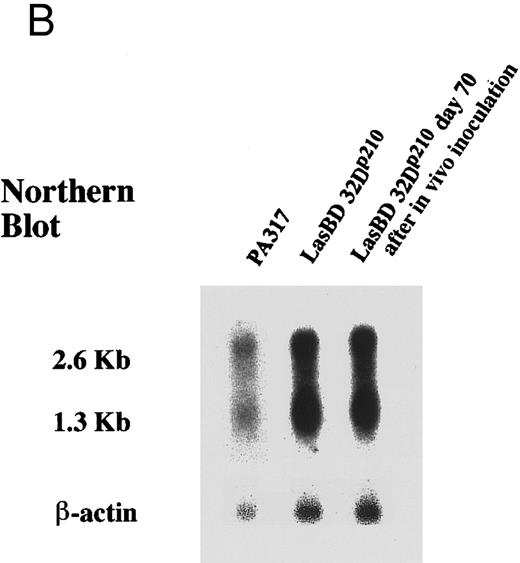
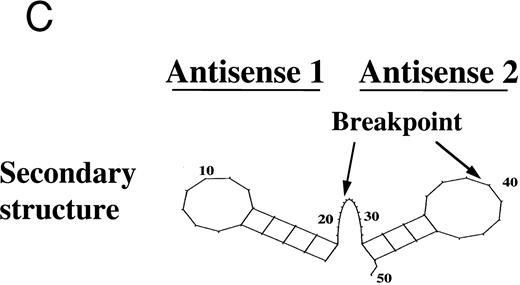
The LasBD vector incorporates the tyr22-DHFR gene as well as two 20-mer anti-b3a2 modified AS sequences (Fig 2A). The rationale for two copies of AS sequence was based on secondary structure analysis of the modified double-copy AS sequence within the retroviral vector RNA. Analysis of the secondary structure of AS based on minimal free energy indicates that both AS sequences have an open single-stranded region of at least 5 bases upstream and downstream from the b3a2 breakpoint to facilitate interaction with the target BCR/ABL mRNA. Results for the analysis of 50 contiguous basepairs are shown in Fig 2C. Similar results were obtained when the analyzed sequence was extended to 150 bp from the depicted sequence (not shown). In LasBD, the AS sequence is transcriptionally regulated by the 5′ LTR, whereas the tyr22-DHFR gene is transcriptionally regulated by an internal β-actin promoter. The presence of both mRNA species in PA317 virus producer cells was confirmed by Northern analysis (Fig 2B).
The LasBD retroviral vector. (A) Retroviral vector LasBD containing the tyr22-DHFR gene transcriptionally regulated by an internal β-actin promoter and two 20-mer anti-b3a2 AS modified sequences regulated by the MoMuLV-LTR. The retroviral vector, LBD, contains the tyr22-DHFR gene transcriptionally regulated by an internal β-actin promoter but no anti-BCR/ABL antisense sequences. (B) Northern blot analysis showing equal expression of the LTR-regulated AS-DHFR message (2.6 kb) and the internal β-actin promoter-regulated DHFR message (1.3 kb). Expression of both mRNAs is depicted for PA317 packaging cells, LasBD-32Dp210 cells recovered after ex vivo selection in MTX and IL-3, and LasBD-32Dp210 cells recovered 70 days after in vivo infusion of bulk-selected LasBD-32Dp210 cells into syngeneic C3H mice. (C) Secondary structure of the anti-b3a2 AS sequence, determined based on a dynamic program algorithm of the minimum free energy conformation of the RNA using the Program Manual for the Wisconsin Package (version 8, September 1994; Genetics Computer Group, Madison, WI).41 42 The optimal stable conformation of the modified double antisense mRNA indicates that 5 to 10 bases surrounding the b3a2 breakpoint are single-stranded.
The LasBD retroviral vector. (A) Retroviral vector LasBD containing the tyr22-DHFR gene transcriptionally regulated by an internal β-actin promoter and two 20-mer anti-b3a2 AS modified sequences regulated by the MoMuLV-LTR. The retroviral vector, LBD, contains the tyr22-DHFR gene transcriptionally regulated by an internal β-actin promoter but no anti-BCR/ABL antisense sequences. (B) Northern blot analysis showing equal expression of the LTR-regulated AS-DHFR message (2.6 kb) and the internal β-actin promoter-regulated DHFR message (1.3 kb). Expression of both mRNAs is depicted for PA317 packaging cells, LasBD-32Dp210 cells recovered after ex vivo selection in MTX and IL-3, and LasBD-32Dp210 cells recovered 70 days after in vivo infusion of bulk-selected LasBD-32Dp210 cells into syngeneic C3H mice. (C) Secondary structure of the anti-b3a2 AS sequence, determined based on a dynamic program algorithm of the minimum free energy conformation of the RNA using the Program Manual for the Wisconsin Package (version 8, September 1994; Genetics Computer Group, Madison, WI).41 42 The optimal stable conformation of the modified double antisense mRNA indicates that 5 to 10 bases surrounding the b3a2 breakpoint are single-stranded.
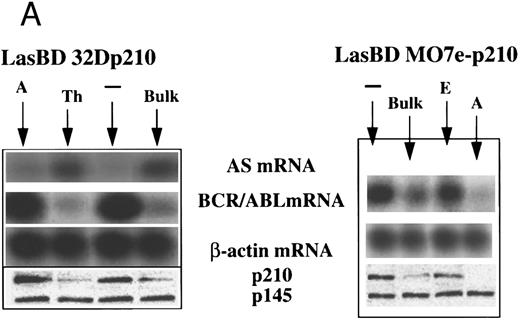
We transduced b3a2-BCR/ABL cDNA containing human MO7e (MO7ep210)23 and murine 32D (32Dp210)21 cells with the LasBD vector. Transduced cells were selected in the presence of 0.25 μmol/L MTX plus human or murine IL-3. In some experiments, clones of transduced cells were isolated. After 14 days, selective medium containing MTX was replaced with nonselective medium supplemented with IL-3. Transduced cells have now been stably maintained in vitro for more than 20 months without MTX. We first examined expression of the AS sequence in bulk-selected and cloned LasBD-32Dp210 cells. Northern blot analysis of bulk-selected LasBD-32Dp210 cells showed equal levels of the LTR-driven 2.6-kb transcript encoding the AS sequence and the DHFR gene and the β-actin–driven 1.3-kb mRNA encoding the DHFR gene alone (Fig 2B). We also performed semiquantitative RT-PCR on bulk-selected and cloned LasBD-32Dp210 cells. Significant levels of AS mRNA could be detected in bulk-selected LasBD-32Dp210 cells and most clones (Fig 3A). However, AS expression was somewhat variable, because some clones (eg, clone A) expressed only minimal amounts of AS mRNA.
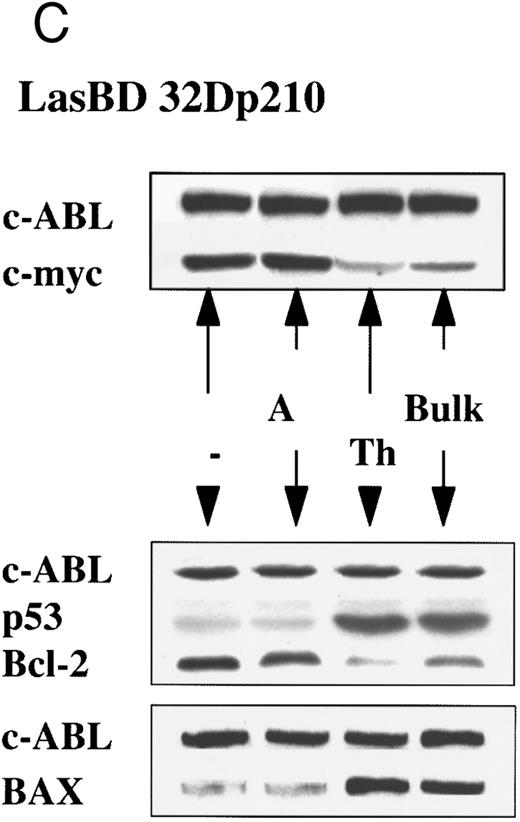
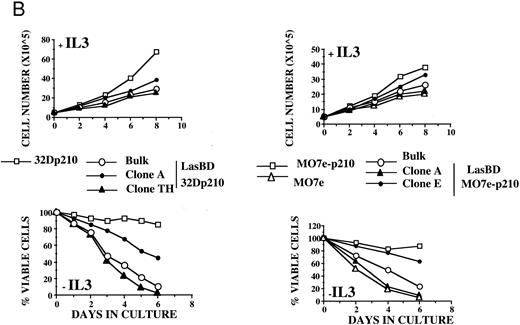
LasBD suppresses b3a2-mRNA and p210BCR/ABL expression and restores normal function. MO7ep210 or 32Dp210 cells were transduced with LasBD and cultured in IMDM+ 0.25 μmol/L MTX + rHuIL-3 or rMuIL-3 (Bulk) for 14 days. In addition, 1 × 103 transduced cells were cultured in wells of a 48-well plate in IMDM+ 0.25 μmol/L MTX + IL-3 (clones 32Dp210 A and TH and MO7ep210 A and E). (A) Molecular analysis of antisense and BCR/ABL expression: MTX-selected LasBD-32Dp210 and LasBD-M07ep210 cells and untransduced cells were analyzed by semiquantitative RT-PCR to detect AS mRNA, BCR/ABL mRNA, and β-actin mRNA and by Western blot to detect p210BCR/ABL and p145ABL protein, as described in the Materials and Methods. Expression of AS-mRNA and BCR/ABL mRNA as well as p210BCR/ABL protein for untransduced cells (indicated with a minus sign), bulk-selected (indicated as Bulk) and clonal cell populations (for 32Dp210 cells, Th and A; for M07ep210 cells, E and A) are shown. For Western blot analysis, the bands representing the p145ABL protein and the p210BCR/ABL protein are indicated with an arrow. Equal loading of the gels is shown by the presence of the p145ABL protein. (B) Effect of LasBD on growth in the presence and absence of IL-3. (Upper panels) Untransduced 32Dp210 and MO7ep210 cells and transduced, bulk-selected, or cloned 32Dp210 and MO7ep210 cells cultured for 6 days in IL-3–containing medium. On days 1, 3, 5, and 6, the number of viable cells was determined by enumerating cells stained with trypan blue in a hemocytometer. Total viable cell expansion is depicted. Bulk-selected LasBD-32Dp210 and LasBD-MO7ep210 cells and clones 32Dp210-Th and MO7ep210-A expanded significantly less than untransduced 32Dp210 and MO7ep210 cells or clones that continued to express BCR/ABL mRNA and protein (32Dp210-A and MO7ep210-E cells). (Lower panels) Untransduced 32Dp210 and MO7ep210 cells and transduced, bulk-selected, or cloned 32Dp210 and MO7ep210 cells cultured for 6 days without IL-3. On days 1, 3, 5, and 6, the number of viable cells was determined by enumerating cells stained with trypan blue in a hemocytometer. The percentage of viable cells is depicted. All bulk-selected LasBD-32Dp210 cells and LasBD-MO7ep210 cells and clones 32Dp210 -Th and MO7ep210-A died by day 6, whereas a significant proportion of untransduced 32Dp210 and MO7ep210 cells or clones that continued to express BCR/ABL mRNA and protein (32Dp210-A and MO7ep210-E cells) survived even in the absence of IL-3. (C) Untransduced 32Dp210 cells and transduced, bulk-selected, or cloned 32Dp210 cells cultured for 6 days without IL-3 (BCL-2, BAX, and p53) or with IL-3 (C-MYC). After 3 days, cells were recovered and protein extracts were subjected to Western analysis for expression of p145ABL, C-MYC, BCL-2, BAX, and p53. Decreased cell expansion of bulk-selected LasBD-32Dp210 cells and clone LasBD-32Dp210-Th that express only low levels of p210BCR/ABL in the presence of IL-3 was associated with decreased expression of C-MYC (upper panel). Cell death of bulk-selected LasBD-32Dp210 cells and clone LasBD-32Dp210-Th that express only low levels of p210BCR/ABL was associated with increased levels of p53 and BAX and decreased levels of BCL-2 (lower panel).
LasBD suppresses b3a2-mRNA and p210BCR/ABL expression and restores normal function. MO7ep210 or 32Dp210 cells were transduced with LasBD and cultured in IMDM+ 0.25 μmol/L MTX + rHuIL-3 or rMuIL-3 (Bulk) for 14 days. In addition, 1 × 103 transduced cells were cultured in wells of a 48-well plate in IMDM+ 0.25 μmol/L MTX + IL-3 (clones 32Dp210 A and TH and MO7ep210 A and E). (A) Molecular analysis of antisense and BCR/ABL expression: MTX-selected LasBD-32Dp210 and LasBD-M07ep210 cells and untransduced cells were analyzed by semiquantitative RT-PCR to detect AS mRNA, BCR/ABL mRNA, and β-actin mRNA and by Western blot to detect p210BCR/ABL and p145ABL protein, as described in the Materials and Methods. Expression of AS-mRNA and BCR/ABL mRNA as well as p210BCR/ABL protein for untransduced cells (indicated with a minus sign), bulk-selected (indicated as Bulk) and clonal cell populations (for 32Dp210 cells, Th and A; for M07ep210 cells, E and A) are shown. For Western blot analysis, the bands representing the p145ABL protein and the p210BCR/ABL protein are indicated with an arrow. Equal loading of the gels is shown by the presence of the p145ABL protein. (B) Effect of LasBD on growth in the presence and absence of IL-3. (Upper panels) Untransduced 32Dp210 and MO7ep210 cells and transduced, bulk-selected, or cloned 32Dp210 and MO7ep210 cells cultured for 6 days in IL-3–containing medium. On days 1, 3, 5, and 6, the number of viable cells was determined by enumerating cells stained with trypan blue in a hemocytometer. Total viable cell expansion is depicted. Bulk-selected LasBD-32Dp210 and LasBD-MO7ep210 cells and clones 32Dp210-Th and MO7ep210-A expanded significantly less than untransduced 32Dp210 and MO7ep210 cells or clones that continued to express BCR/ABL mRNA and protein (32Dp210-A and MO7ep210-E cells). (Lower panels) Untransduced 32Dp210 and MO7ep210 cells and transduced, bulk-selected, or cloned 32Dp210 and MO7ep210 cells cultured for 6 days without IL-3. On days 1, 3, 5, and 6, the number of viable cells was determined by enumerating cells stained with trypan blue in a hemocytometer. The percentage of viable cells is depicted. All bulk-selected LasBD-32Dp210 cells and LasBD-MO7ep210 cells and clones 32Dp210 -Th and MO7ep210-A died by day 6, whereas a significant proportion of untransduced 32Dp210 and MO7ep210 cells or clones that continued to express BCR/ABL mRNA and protein (32Dp210-A and MO7ep210-E cells) survived even in the absence of IL-3. (C) Untransduced 32Dp210 cells and transduced, bulk-selected, or cloned 32Dp210 cells cultured for 6 days without IL-3 (BCL-2, BAX, and p53) or with IL-3 (C-MYC). After 3 days, cells were recovered and protein extracts were subjected to Western analysis for expression of p145ABL, C-MYC, BCL-2, BAX, and p53. Decreased cell expansion of bulk-selected LasBD-32Dp210 cells and clone LasBD-32Dp210-Th that express only low levels of p210BCR/ABL in the presence of IL-3 was associated with decreased expression of C-MYC (upper panel). Cell death of bulk-selected LasBD-32Dp210 cells and clone LasBD-32Dp210-Th that express only low levels of p210BCR/ABL was associated with increased levels of p53 and BAX and decreased levels of BCL-2 (lower panel).
We next evaluated the molecular effects of AS expression on BCR/ABL mRNA and p210BCR/ABL protein levels. To ascertain the validity of the assays, RT-PCR reactions were also performed using Tth polymerase, which is resistant to higher temperatures. This allowed the reactions to be performed at 68°C for 20 minutes, therefore decreasing the likelihood of obtaining false-positive results due to persistent annealing of AS mRNA to the target BCR/ABL mRNA during the RT step. RT-PCR using either Tth or MoMLVRT showed a similar suppression of BCR/ABL mRNA in bulk versus clone A LasBD-32Dp210 cells (Fig 4B), which showed that assessment of BCR/ABL mRNA using Taq polymerase provides an accurate estimate of its suppression by LasBD. In addition, we performed RT-PCR reactions on mRNA recovered from untransduced 32Dp210 cells admixed with up to 10-fold higher amounts mRNA from LasBD-PA317 cells immediately before the RT-PCR reaction. The addition of 10× LasBD-PA317 mRNA, containing the double-copy AS mRNA, did not decrease our ability to detect BCR/ABL mRNA in 32Dp210 (Fig 4A). Semiquantitative RT-PCR of bulk-selected LasBD-MO7ep210 and LasBD-32Dp210 cells showed an eightfold reduction in BCR/ABL mRNA (Fig 3A). As observed for AS mRNA levels, some variability in elimination of BCR/ABL mRNA was detected in different clones of LasBD-32Dp210 and LasBD-MO7ep210 cells. It is noteworthy that BCR/ABL mRNA levels were low for those clones in which AS mRNA levels were high, whereas clones in which AS mRNA levels were lower continued to express higher levels of BCR-ABL mRNA.
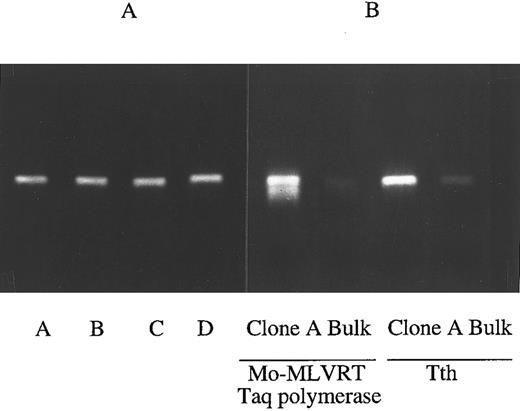
RT-PCR accurately detect BCR/ABL mRNA levels. (A) Presence of AS mRNA does not impede RT-PCR reaction for BCR/ABL mRNA. mRNA from untransduced 32Dp210 cells was admixed with 0 (lane A), onefold (lane B), fivefold (lane C), or 10-fold (lane D) excess mRNA from LasBD-PA317 cells immediately before the RT-PCR reaction. After reverse transcription with MoMLVRT, cDNAs were amplified for 30 cycles. The addition of up to 10-fold excess LasBD-PA317 mRNA containing the double-copy AS mRNA did not decrease our ability to assess the level of BCR/ABL mRNA in 32Dp210 cells. (B) RT-PCR using Tth or MoMLVRT/Taq polymerase. RT-PCR reaction was performed either at 68°C for 20 minutes using the Tth polymerase or at 37°C for 60 minutes using MoMLVRT/Taq polymerase. mRNA obtained from LasBD-32Dp210 clone A and bulk-selected LasBD-32Dp210 cells was amplified for 30 cycles, and the degree of BCR/ABL mRNA suppression detected using either MoMLVRT or Tth was similar.
RT-PCR accurately detect BCR/ABL mRNA levels. (A) Presence of AS mRNA does not impede RT-PCR reaction for BCR/ABL mRNA. mRNA from untransduced 32Dp210 cells was admixed with 0 (lane A), onefold (lane B), fivefold (lane C), or 10-fold (lane D) excess mRNA from LasBD-PA317 cells immediately before the RT-PCR reaction. After reverse transcription with MoMLVRT, cDNAs were amplified for 30 cycles. The addition of up to 10-fold excess LasBD-PA317 mRNA containing the double-copy AS mRNA did not decrease our ability to assess the level of BCR/ABL mRNA in 32Dp210 cells. (B) RT-PCR using Tth or MoMLVRT/Taq polymerase. RT-PCR reaction was performed either at 68°C for 20 minutes using the Tth polymerase or at 37°C for 60 minutes using MoMLVRT/Taq polymerase. mRNA obtained from LasBD-32Dp210 clone A and bulk-selected LasBD-32Dp210 cells was amplified for 30 cycles, and the degree of BCR/ABL mRNA suppression detected using either MoMLVRT or Tth was similar.
Results from RT-PCR reactions were extended by examining the effect of AS expression on p210BCR/ABL protein levels observed on Western blot analysis. For bulk-selected LasBD-MO7ep210 and LasBD-32Dp210 cells, a sixfold decrease in p210BCR/ABL level was seen compared with mock-transduced cells. LasBD did not affect p145ABL protein levels (Fig 3A). As for BCR/ABL mRNA levels, some variability between clones was seen. A strict correlation was seen between the degree of suppression of the BCR/ABL mRNA and p210BCR/ABL protein levels, further indicating that the semiquantitative RT-PCR reaction provided an accurate measure of the AS-mediated suppression of target mRNA. As observed for BCR/ABL mRNA levels, the level of expression of the BCR/ABL AS sequence was inversely correlated with p210BCR/ABL protein levels, again suggesting a causal relationship between AS mRNA levels and inhibition of BCR/ABL oncogene expression.
Differences in expression of the AS mRNA may be due to differences in copy number of the retroviral gene present per cell. Alternatively, differences in the location of the viral integrant in the genome may affect the LTR-regulated transcription of the AS sequence. However, the bulk-selected LasBD-32Dp210 cells contained similar amounts of AS-mRNA as LasBD-32Dp210 clone Th. Likewise, suppression of BCR/ABL mRNA and protein was similar in bulk-selected LasBD-32Dp210 and LasBD-M07ep210 cells as in LasBD-32Dp210 clone Th and LasBD-MO7ep210 clone A. This indicates that the fraction of LasBD-32Dp210 and M07e-32Dp210 cells in which the AS mRNA is poorly expressed, resulting in minimal suppression of the BCR/ABL oncoprotein, is small.
LasBD Restores Normal Phenotype of BCR/ABL-Containing Cell Lines
We next evaluated the effect of transduction with LasBD on the functional behavior of BCR/ABL-containing cell lines. LasBD-mediated suppression of BCR/ABL mRNA and p210BCR/ABL protein (bulk-selected LasBD-32Dp210 cells and clone LasBD-32Dp210 Th; bulk-selected LasBD-MO7ep210 cells and clone LasBD-MO7ep210 A) resulted in a significantly lower expansion of cells when maintained with rMuIL-3 or rHuIL-3 (Fig 3B and C). However, expansion of LasBD-32Dp210 clone A or LasBD-MO7ep210 clone E (clones that continued to express BCR/ABL mRNA and p210BCR/ABL protein) was only minimally reduced in comparison with mock-transduced 32Dp210 or MO7ep210 cells. Because decreased growth was associated with a threefold decrease in C-MYC protein levels in the LasBD-32Dp210 clone Th or bulk-selected LasBD-32Dp210 cells (Fig 3C), these results suggest that the AS sequence inhibits p210BCR/ABL-dependent activation of the RAS-MAPK-MYC pathway.26-28
32D and MO7e cells are growth factor dependent in vitro and undergo apoptotic cell death when cultured in the absence of IL-3. However, upon transduction with BCR/ABL cDNA, these cells become IL-3 independent and no longer undergo apoptosis once IL-3 is removed.21,23 We therefore examined survival of mock- and LasBD-transduced MO7ep210 and 32Dp210 cells when cultured for 6 days in the absence of IL-3. Mock-transduced MO7ep210 and 32Dp210 cells continued to expand, even in the absence of IL-3. In contrast to untransduced cells, bulk-selected LasBD-MO7ep210 and LasBD-32Dp210 died after 2 to 4 days of culture without IL-3. Clones of either cell line in which BCR/ABL mRNA and p210BCR/ABL protein was almost completely eliminated (LasBD-32Dp210 clone Th and LasBD-MO7ep210 clone A) died after 2 to 4 days of culture without IL-3. Clones that expressed some p210BCR/ABL protein (LasBD-32Dp210 clone A and LasBD-MO7ep210 clone E) died at a slower rate. Cells died by apoptosis, demonstrated by staining cells on day 3 after IL-3 withdrawal with the DNA dye 7AAD (not shown).29 This was associated with significantly increased expression of p53 and BAX and decreased expression of BCL-2 (Fig 3C).30 31
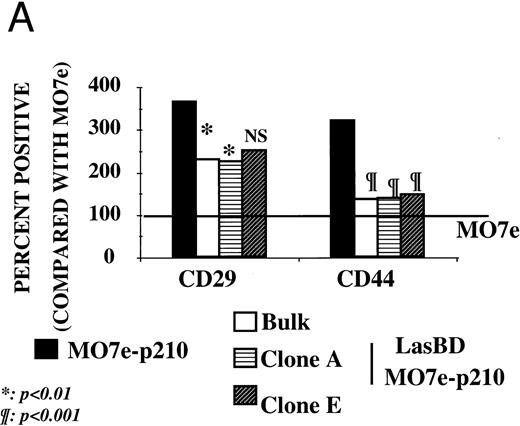
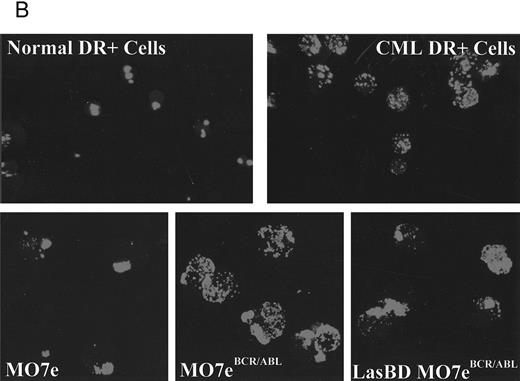
We and others have previously shown that adhesion of CML progenitors to stroma32,33 or the ECM component, fibronectin, is defective.34,35 Decreased adhesion of CML cells occurs even though increased levels of adhesion receptors responsible for interaction with fibronectin (such as β1 integrins34,35 and CD4436 ) are present on CML CD34+ cells. Because decreased adhesion to the BM microenvironment may cause the abnormal circulation of primitive progenitors in the blood and may in part be responsible for the massive uncontrolled proliferation of CML progenitors,33,37,38 we next examined if suppression of p210BCR/ABL with LasBD restores adhesion receptor expression and function. As is seen for primary CML Ph+ CD34+ cells,34-36 MO7ep210 cells expressed significantly more β1 integrins (CD29) and CD44 (Fig 5A) than did parent MO7e cells. After transduction of MO7ep210 cells with LasBD, levels of β1 integrin and CD44 decreased significantly. We have also shown that integrins are functionally defective in CML and do not, for instance, form caps when CD34+ cells are incubated with anti-β1 integrin antibodies.37 A similar effect was seen for MO7ep210 cells: compared with parent MO7e cells, incubation with anti-β1 antibodies did not induce receptor capping on MO7ep210 cells (Fig 5B). However, capping was restored in LasBD-MO7ep210 cells.
LasBD restores normal integrin function and expression. (A) MO7e, MO7ep210, and LasBD-MO7ep210 bulk-selected and cloned cells were stained with FITC- or PE-conjugated monoclonal antibodies against CD29 or CD44 and analyzed on a FACStarPLUS flow cytometer. PE- and FITC-conjugated isotype matched mouse IgGs were used as negative controls. Results are presented as the mean ± SEM of four individual experiments. Statistics: Paired Student's t-test: P < .001. (B) Aliquots of 5 to 10 × 104 normal CD34+ cells, primary CML CD34+ HLA-DR+ cells or MO7e, MO7ep210 cells and LasBD-MO7ep210 bulk-selected cells were incubated with an adhesion blocking anti-β1 antibody followed by a goat-antimouse FITC antibody, as described in the Materials and Methods. Cytospin preparations were examined by immunofluorescence microscopy for the presence or absence of integrin caps.
LasBD restores normal integrin function and expression. (A) MO7e, MO7ep210, and LasBD-MO7ep210 bulk-selected and cloned cells were stained with FITC- or PE-conjugated monoclonal antibodies against CD29 or CD44 and analyzed on a FACStarPLUS flow cytometer. PE- and FITC-conjugated isotype matched mouse IgGs were used as negative controls. Results are presented as the mean ± SEM of four individual experiments. Statistics: Paired Student's t-test: P < .001. (B) Aliquots of 5 to 10 × 104 normal CD34+ cells, primary CML CD34+ HLA-DR+ cells or MO7e, MO7ep210 cells and LasBD-MO7ep210 bulk-selected cells were incubated with an adhesion blocking anti-β1 antibody followed by a goat-antimouse FITC antibody, as described in the Materials and Methods. Cytospin preparations were examined by immunofluorescence microscopy for the presence or absence of integrin caps.
Suppression of BCR/ABL mRNA and Protein by LasBD Is Breakpoint Specific
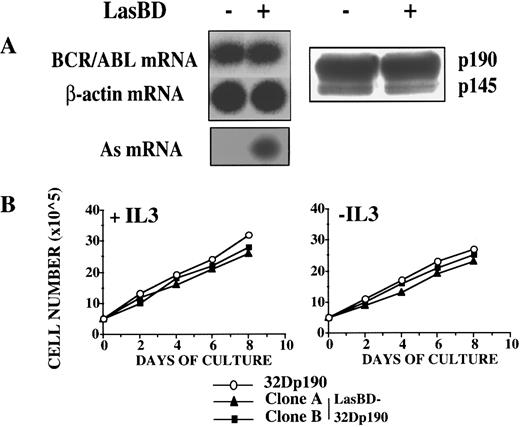
To demonstrate specificity of the AS sequence, we transduced 32D cells that contained the p190BCR/ABL cDNA (32Dp190) with LasBD. RT-PCR and Western analysis indicated that LasBD did not suppress p190BCR/ABL mRNA or protein, even though there was substantial AS mRNA expression (Fig 6A). In contrast to LasBD-32Dp210 cells, which underwent apoptosis once IL-3 was withdrawn, LasBD-32Dp190 cells continued to expand in the absence of rMuIL-3 (Fig 6B).
LasBD does not suppress p190BCR/ABL expression and does not affect the function of 32Dp190 cells. (A) 32Dp190 cells were transduced with LasBD and selected with MTX and rMuIL-3. Untransduced (in lanes with minus sign) and MTX-selected, transduced cells (in lanes with plus sign) were analyzed by semiquantitative RT-PCR to detect AS mRNA, BCR/ABL mRNA, and β-actin mRNA (left panel) and by Western blot to detect p190BCR/ABL and p145ABL protein (right panel), as described in the Materials and Methods. (B) LasBD-transduced (clones A and B) and untransduced 32Dp190 cells were cultured for 6 days in IL-3–containing (left panel) or IL-3–free (right panel) cultures and viable cell expansion was measured.
LasBD does not suppress p190BCR/ABL expression and does not affect the function of 32Dp190 cells. (A) 32Dp190 cells were transduced with LasBD and selected with MTX and rMuIL-3. Untransduced (in lanes with minus sign) and MTX-selected, transduced cells (in lanes with plus sign) were analyzed by semiquantitative RT-PCR to detect AS mRNA, BCR/ABL mRNA, and β-actin mRNA (left panel) and by Western blot to detect p190BCR/ABL and p145ABL protein (right panel), as described in the Materials and Methods. (B) LasBD-transduced (clones A and B) and untransduced 32Dp190 cells were cultured for 6 days in IL-3–containing (left panel) or IL-3–free (right panel) cultures and viable cell expansion was measured.
We thus conclude that LasBD, which contains a double-copy AS sequence specific for the b3a2 BCR/ABL breakpoint, suppresses expression of b3a2-BCR/ABL mRNA and protein in a sequence-specific manner. Furthermore, transduction with the LasBD vector renders the majority of 32Dp210 and MO7ep210 cells functionally normal: they grow slower in the presence of IL-3, undergo apoptosis in the absence of IL-3, and express normal levels of adhesion receptors that are functionally normal.
LasBD Suppresses BCR/ABL mRNA in Primary Ph+ Cells and Renders CD34+ Progenitors MTX-Resistant
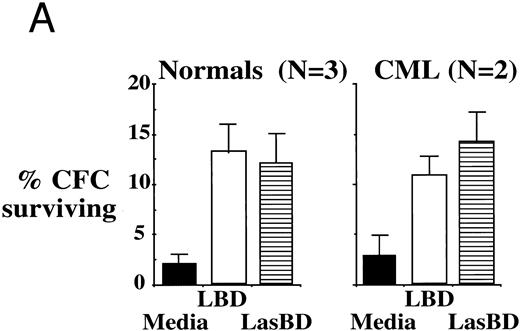
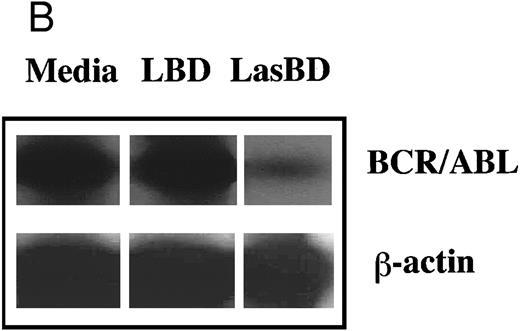
To extend these studies to more clinically relevant target cell populations, we next transduced primary CML Ph+ or normal CD34+ HLA-DR+ progenitor cells with the LBD or LasBD vector. CML sample were from patients with a known b3a2 splice site. The LBD vector contains the tyr22-DHFR gene transcriptionally regulated by an internal β-actin promoter, but does not contain the anti-BCR/ABL AS sequence (Fig 2A). Semiquantitative RT-PCR analysis of BCR/ABL mRNA levels in CML CD34+ HLA-DR+ cells selected with MTX showed a 10-fold reduction in BCR/ABL mRNA levels after transduction with LasBD. In contrast, transduction with the LBD vector, which does not contain the anti-BCR/ABL AS sequence, did not affect BCR/ABL mRNA levels (Fig 7B). Transduction of normal and CML CD34+ HLA-DR+ cells with either the LBD vector or the LasBD vector with subsequent plating in methylcellulose culture showed that an equal fraction became resistant to 5 × 10−8 mol/L MTX (Fig 7A). These results show that the AS sequence as well as the tyr22-DHFR gene are functional when transduced into primary CML or normal hematopoietic progenitors.
LasBD suppresses BCR/ABL mRNA in primary CML cells and renders normal and CML progenitors MTX-resistant. (A) 1 × 104 normal or CML CD34+ HLA-DR+ cells were transduced with LasBD or the control vector, LBD containing PA317 supernatants, protamine sulfate, IL-3, IL-6, and SCF in contact with M2-10B4 stromal feeders for 24 hours. Cells were subsequently cultured in serum-free semisolid methylcellulose culture in the presence or absence of 5 × 10−8 mol/L MTX to determine the percentage of MTX-resistant progenitors. Results are represented as the percentage of CFC surviving 5 × 10−8 mol/L MTX from 2 patients with CML (with known b3a2 breakpoint) and 3 normal individuals. (B) 5 × 104 LasBD and LBD-transduced CML CD34+ HLA-DR+ cells were cultured for 14 days in liquid serum-free culture containing 5 × 10−8 mol/L MTX and cells examined by RT-PCR for the presence of the BCR/ABL mRNA.
LasBD suppresses BCR/ABL mRNA in primary CML cells and renders normal and CML progenitors MTX-resistant. (A) 1 × 104 normal or CML CD34+ HLA-DR+ cells were transduced with LasBD or the control vector, LBD containing PA317 supernatants, protamine sulfate, IL-3, IL-6, and SCF in contact with M2-10B4 stromal feeders for 24 hours. Cells were subsequently cultured in serum-free semisolid methylcellulose culture in the presence or absence of 5 × 10−8 mol/L MTX to determine the percentage of MTX-resistant progenitors. Results are represented as the percentage of CFC surviving 5 × 10−8 mol/L MTX from 2 patients with CML (with known b3a2 breakpoint) and 3 normal individuals. (B) 5 × 104 LasBD and LBD-transduced CML CD34+ HLA-DR+ cells were cultured for 14 days in liquid serum-free culture containing 5 × 10−8 mol/L MTX and cells examined by RT-PCR for the presence of the BCR/ABL mRNA.
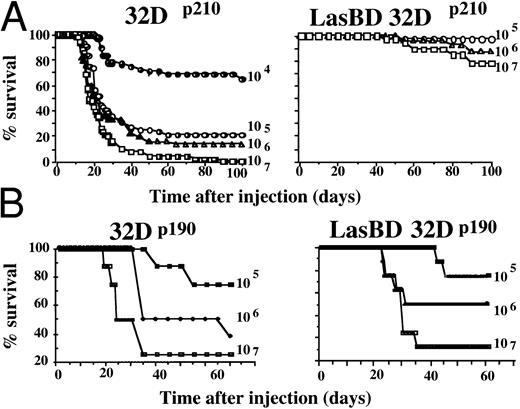
LasBD Decreases Tumorigenicity of 32Dp210 Cells In Vivo by 3 to 4 Logs in a Sequence-Specific Manner
Finally, we examined if transduction with LasBD impacts on survival of animals that receive 32Dp210 or 32Dp190 cells. Infusion of 106 and 107 32Dp210 (Fig 8A) or 32Dp190 cells (Fig 8B) per animal resulted in the death of greater than 70% of animals by day 40 and 90% to 100% of animals by day 100. In addition, greater than 30% of animals that received 104 32Dp210 cells succumbed by day 50. When LasBD-transduced ex vivo MTX-selected 32Dp190 cells were injected, no improvement in survival of animals was noted (Fig 8B). In contrast, transduction of 32Dp210 cells with LasBD and selection with MTX before inoculation significantly decreased in vivo tumorigenicity. Only 10% of animals receiving 107 LasBD-32Dp210 cells succumbed between days 40 and 75, and an additional 15% died between days 75 and 100. However, no animal receiving less than 106 LasBD-32Dp210 cells died. Interestingly, LasBD-32Dp210 cells persisted in the animals but did not seem to cause lethality. BCR/ABL cDNA, but not BCR/ABL mRNA, could be detected by PCR in the spleens and marrow of greater than 50% of animals that received 105 to 107 LasBD-32Dp210 cells when examined 70 days after inoculation, indicating that the LasBD-32Dp210 cells survived in vivo but were no longer tumorigenic. Marrow cells from animals inoculated with 105 to 107 LasBD-32Dp210 cells 70 days earlier were cultured in the presence of 0.25 μmol/L MTX for 2 weeks and surviving cells expanded in MTX-free, IL-3–containing medium. Northern analysis indicated that messages initiated by the 5′LTR promoter and the internal β-actin promoter continued to be expressed to the same extent as was seen for LasBD-32Dp210 cells analyzed before inoculation (Fig 2B). These reselected cells exhibited normal function, because withdrawal of IL-3 in vitro resulted in the death of reselected cells, similar to what we observed before transfer into C3H animals (not shown). This shows that the DHFR gene and the AS sequence continue to be expressed in cells that have been maintained without selective pressure in vivo for 70 days and that the LasBD vector decreases tumorigenicity of 32Dp210 cells in vivo by at least 3 logs. Survival of cells expressing neither BCR/ABL mRNA nor protein in vivo is consistent with the observation that LasBD-transduced 32Dp210 cells survive in vitro when maintained in the presence of serum and rMuIL-3, but that their growth is suppressed compared with that of untransduced 32Dp210 cells.
LasBD reduces tumorigenicity of 32Dp210 cells but not 32DP190 cells in vivo. A total of 104 to 107 untransduced (left panels) or LasBD transduced (right panels) 32Dp210 (A) and 32Dp190 (B) cells, selected ex vivo with MTX, were injected through the tail vein into C3H mice (4 independent experiments, 8 to 12 animals/group/experiment). Survival of animals was assessed daily for 100 days after inoculation. After 70 days, some animals that received 107, 106, and 105 LasBD-32Dp210 cells were killed, and the spleen and marrow were examined for the presence of BCR/ABL cDNA, BCR/ABL mRNA, and AS mRNA.
LasBD reduces tumorigenicity of 32Dp210 cells but not 32DP190 cells in vivo. A total of 104 to 107 untransduced (left panels) or LasBD transduced (right panels) 32Dp210 (A) and 32Dp190 (B) cells, selected ex vivo with MTX, were injected through the tail vein into C3H mice (4 independent experiments, 8 to 12 animals/group/experiment). Survival of animals was assessed daily for 100 days after inoculation. After 70 days, some animals that received 107, 106, and 105 LasBD-32Dp210 cells were killed, and the spleen and marrow were examined for the presence of BCR/ABL cDNA, BCR/ABL mRNA, and AS mRNA.
Conclusion
We conclude that the LasBD vector containing the tyr22-DHFR gene as well as AS sequences directed at the b3a2 BCR/ABL breakpoint renders normal and CML progenitors MTX-resistant and suppresses BCR/ABL mRNA and protein from b3a2 BCR/ABL-containing CML cell lines and primary Ph+ CML progenitors. This leads to the functional normalization of CML cells, because they exhibit normal proliferative behavior in response to cytokines, undergo apoptotic cell death when cultured under unfavorable conditions, have restored integrin function and expression, and decreased tumorigenicity in vivo by 3 to 4 logs. Both the MTX-resistant DHFR gene and the AS sequence are expressed stably in vitro and in vivo, even in the absence of selective pressure. Studies are ongoing to evaluate whether or not the LasBD vector will, like other mutant DHFR containing vectors,11-14 protect transduced normal hematopoietic cells from the toxic effects of MTX at concentrations that will suppress BCR/ABL+ cells in vivo. The LasBD vector may prove extremely useful in an autologous transplant setting: in protecting normal hematopoiesis from MTX toxicity, LasBD may allow administration of MTX after transplantation to suppress leukemia that persists in the graft or in the host after myeloablation while rendering transduced, leukemic stem cells and progenitors functionally normal. Finally, a similar approach could be contemplated in the treatment of other hematologic malignancies associated with novel fusion oncoproteins, such as Ph+ acute lymphocytic leukemia or acute promyelocytic leukemia.39 40
ACKNOWLEDGMENT
The authors acknowledge the excellent technical help of Kirk Van Overbeke and Todd R. Lenvik.
Supported in part by National Institutes of Health Grants No. RO1-HL-54039, RO1-CA-60803, and PO1-CA-65493 and Leukemia Society of America Grant No. LSA-6377. Also supported by the University of Minnesota Bone Marrow Transplant Research Fund and the University of Minnesota Hospitals and Clinics. C.M.V. is a Scholar of the Leukemia Society of America.
Address reprint requests to Catherine M. Verfaillie, MD, Department of Medicine, University of Minnesota, Box 480 UMHC, 422 Delaware St SE, Minneapolis, MN 55455.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal