Abstract
To identify essential molecules capable of inducing terminal morphologic maturation and cell death of myeloid progenitor cells, we isolated cDNA clones by functional expression cloning using a library constructed from all-trans retinoic acid (ATRA)-treated human promyelocytic HL-60 cells. Clones which induced morphologic changes in HL-60 cells from blastic cells to mature neutrophilic granulocytes were selected. The isolated positive cDNA clone was demonstrated to encode an antisense RNA for cytochrome c oxidase/serine tRNA derived from a mitochondrial gene (MARCO). When MARCO was expressed in HL-60 cells with the lac switch system, blastic cell morphology became neutrophilic after 48-hour incubation with IPTG, and cell death was observed after 3 days. Also, high molecular weight DNA fragmentation was observed after 36 hours in culture. Similar results were observed using transformants from human K562 cells and CMK cells. RT-PCR analysis revealed that MARCO was transcribed in both ATRA and TNF-α systems, and also in human blood neutrophilic granulocytes. Following transfection with cytochrome c oxidase expression plasmids, TNF-α–induced high molecular weight DNA fragmentation in U937 cells and HL-60 cells was inhibited in these transformants. These results indicate that maturational changes in hematopoietic cells and the process of cell death may be induced by mitochondrial respiratory insufficiency, and also that the mitochondrial gene MARCO may be used as one of the candidates for gene supplementation therapy for the acute leukemias.
HEMATOPOIETIC myeloid progenitor cells can proliferate with blastic features, and, when differentiation is induced, cell division stops and both morphologic and enzymatic maturation are triggered toward cell death.1 To clarify the mechanism of this maturation process, several in vitro systems have been developed. Of these, tumor necrosis factor-α (TNF-α)–induced morphologic changes as well as cell death and those induced by ATRA provide useful systems, because they resemble the physiologic myeloid maturation process and cell death. It was reported that HL-60 cells showed morphologic changes as well as high molecular weight DNA fragmentation following administration of TNF-α.2-6 In this system, HL-60 cells showed no cell death, while U937 cells showed cell death as well as DNA fragmentation.2,6 HL-60 cells have also been reported to undergo morphologic changes to mature neutrophilic granulocytes in the presence of ATRA, which was followed by cell death.5 7-9 Elucidation of the mechanisms of these differentiation systems would facilitate better understanding of physiologic myeloid cell death process.
There have been many reports on cell death mechanisms in which interleukin 1-β converting enzyme (ICE) families and cell cycle modulators have been shown to take part in cell death.10 Also, mitochondrial function has been reported to be linked to cell death. It was reported that the product of Bcl-2, which acts as an apoptosis escape gene, was located in the mitochondrial membrane.10-12 Recently, bax13 and bad14 were reported to form heterodimers with bcl-2 to co-ordinately regulate cellular survival as well as cell death. Also, some reports revealed that drugs with toxic effects on mitochondria, especially on the electron transfer, induced cell death.15,16 Based on these results, mitochondria seems to be important in induction of, as well as escape from, cell death. A role of mitochondria has also been demonstrated in cytokine-induced cell death, eg, in TNF-α–mediated cytotoxicity, which is characterized by necrosis as well as apoptosis.17,18 It was reported that TNF-α–induced intra-mitochondrial production of superoxide anions (reactive oxygen intermediates) after suppression of the mitochondrial respiratory chain system inducing cell death in TNF-α–sensitive L929 cells.18-21 Recently, Jia et al reported TNF-α–induced cell death mediated through its effect on mitochondrial function using drug-resistant cell lines. In their study, the sensitivity to TNF-α increased with increasing mitochondrial respiration, and cell death was triggered when the activities of respiratory enzymes, presumably cytochrome c oxidase, were suppressed by TNF-α.22 These observations indicate that suppression of mitochondrial respiration is the principal event in the induction of cell death as well as morphologic maturation in this system.
We report here isolation of a cDNA expressed during the differentiation of HL-60 cells after ATRA stimulation. This cDNA was responsible for a change in the morphology of HL-60 cells from blastic to mature neutrophilic appearance with the segmented nucleus. The isolated cDNA clone was demonstrated to encode an antisense RNA for mitochondrial cytochrome c oxidase/serine tRNA. To elucidate its physiologic significance, the isolated clone was characterized using transformants obtained with the lac switch system, and effects on other cell lines were also investigated. Interestingly, this antisense transcript was detected by RT-PCR analysis. When transformants overexpressing cytochrome c oxidase were treated with TNF-α, cytotoxic effects were suppressed. The morphologic changes and cell death that occur in the maturation of myeloid progenitor cells will be discussed with reference to mitochondrial cytochrome c oxidase. The physiologic implications of the inducible MARCO transcript are also discussed.
MATERIALS AND METHODS
Cells.HL-60, K562, CMK, and U937 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) (GIBCO-BRL, Gaithersburg, MD) supplemented with 10% fetal calf serum (FCS) (Flow Laboratories, North Ryde, Australia) in a humidified 7.5% CO2 incubator. NFS-60 cells were maintained as described previously.23 TF-1 cells were cultured with 10 ng/mL recombinant human granulocyte-macrophage colony-stimulating factor (rhGM-CSF) (Hoechst Japan, Tokyo, Japan) in DMEM supplemented with 10% FCS.24 Bone marrow cells were obtained from a healthy human volunteer with informed consent. These cells were further fractionated using Lymphoprep (SG. 1077; Nycomed, Oslo, Norway) and cultured in Petri dishes (Falcon; Becton Dickinson, Lincoln Park, NJ) for 90 minutes to eliminate adherent cell fractions, and nonadherent mononuclear cells were prepared. Human blood neutrophilic granulocytes were obtained as reported previously.25 The prepared neutrophils were cultured at 2 × 105 cells/mL in RPMI 1640 (GIBCO-BRL) with 1% FCS with or without rhG-CSF (50 ng/mL; Chugai, Tokyo, Japan) for the indicated periods in a humidified 7.5% CO2 incubator.
cDNA library construction.All-trans retinoic acid (Wako Chemical, Osaka, Japan) was added to the HL-60 cell cultures at 5 × 10−7 mol/L for 48 hours, following which total RNA was extracted by the acid guanidium isothiocyanate-phenol-chloroform (AGPC) method,26 and poly (A) RNA was selected by oligo (dT) cellulose column chromatography (type 7; Pharmacia, Uppsala, Sweden). cDNA was synthesized (Superscript; GIBCO-BRL) using 5 μg of poly (A)-rich RNA with oligo (dT) primers, and ligated with an EcoRI-Not I-BamHI adapter (Takara ligation kit; Takara, Ohtsu, Japan).27 The synthesized double-stranded cDNA was electrophoresed through 1.2% agarose gels (Seakem GTG agarose; Takara), fractionated above 1.0 kb, and ligated to EcoRI-digested, CIAP-treated pBK-CMV vector (Stratagene, La Jolla, CA). After electroporation into Escherechia coli DH 10b cells (GIBCO-BRL) at 1.6 kV, 200 Ω, 25 μF in a 0.1 cm gap chamber (GIBCO-BRL), an expression library was prepared.28
Expression cloning.The constructed library was further divided into subpools (500 independent colonies/subpool, total 600 subpools), plasmids were prepared from each subpool by the alkaline lysis method29 and were electroporated into HL-60 cells by the K-PBS method.30 Briefly, cells were washed three times with K-PBS− and suspended in 800 μL of K-PBS+ with the prepared plasmids on ice for 10 minutes. After electroporation (0.23 kV, 960 μF) in a 0.4 cm gap electrode chamber (GIBCO-BRL), cells were incubated on ice for 10 minutes, followed by further incubation at room temperature for 30 minutes with 5 mL of serum-free DMEM. Ten milliliters of DMEM supplemented with 10% FCS was then added, followed by culture in a humidified atmosphere of 7.5% CO2 for 2 to 3 days. Half of each culture was collected after 2 days and Hirt's medium was added (final concentrations of 0.6 mol/L NaCl and 0.1% sodium dodecyl sulfate [SDS]) and stocked at 4°C.31 Aliquots of the remainder of each culture were further incubated for 1 day (for 3-day morphology) or collected (for 2-day morphology), and smear slides were prepared using Cytospin II (Shandon, Pittsburgh, PA) and stained with Wright-Giemsa staining. The positive subpools were determined microscopically, and extrachromosomal DNA was recovered from the stocked materials by phenol/chloroform extraction. After electroporation into E coli DH 10b, transformants were obtained from which the plasmids were recovered and further subpools (100 independent colonies/subpool) were prepared, and screened to isolate single clones. The positive subpools were also recovered from E coli glycerol stocks and screened to isolate single clones. The nucleotide sequences of the obtained cDNA clones were determined using a DNA sequencer (model 370A; Applied Biosystems, Perkin Elmer Japan, Urayasu, Japan) and a Taq DyeDeoxy Primer cycle sequencing kit from Applied Biosystems.
Obtaining transformants with the lac switch system.The lac switch vector system was purchased form Stratagene, in which pOPRSVI-CAT was digested with Not I to remove the CAT gene and further ligated with the entire cDNA (pOPRSVI-MARCO). HL-60 cells were washed three times with K-PBS−, suspended in 750 μL of K-PBS+, and then suspended in 50 μL of K-PBS+ containing 3′SS eukaryotic lac-repressor protein expression plasmid linearized with BamHI, and pOPRSVI-MARCO or pOPRSVI without insert (mock-transfection) linearized with Sac I. Cells were electroporated at 0.23 kV, 960 μF (0.4 cm, gap), and aliquots of 100 μL were dispensed into 96-well plates (Costar, Cambridge, MA). After 24-hour incubation, 50 μg/mL of Hygromycin B (Sigma, St Louis, MO) and 200 μg/mL of G418 (Sigma) were added to HL-60 and CMK cells. For K562 cells, Hygromycin B was added at the same concentration but G418 was added at 500 μg/mL. Cells were further cultured for 10 days, and transformants were obtained.
Northern blotting analysis.Northern blotting was carried out as described.29 Briefly, total RNA was extracted by the AGPC method, and each 10 μg was electrophoresed through 0.8% agarose gels in 1X MOPS buffer supplemented with 2.2 mol/L formaldehyde. After electrophoresis gels were treated with 10X SSC for 1 hour and RNA was transferred onto nylon membranes (Screen plus; NEN-Dupont, Boston, MA). The next day, the membranes were washed, dried, baked for 2 hours in a vacuum oven, and stored until use. The probe for northern blotting was prepared as follows; MARCO fragment from the PvuII site to the 3′ end of the cDNA was ligated into pBluescript (SK+) in which 5′ and 3′ direction were determined by nucleotide sequencing, and digested with Xba I for T7 primer or Xho I for T3 primer. The linearized plasmids were treated with 0.2 mol/L NaOH at room temperature for 5 minutes to make single-stranded DNA, and labeled with 32P-dCTP and Klenow in the presence of each primer. Prehybridization was performed in 2.5X SSC, 50 mmol/L Na-P (6.5), 5X Denhardt's solution, 0.1% SDS and 100 μg/mL denatured ssDNA at 42°C for 4 hours. Hybridization proceeded with 2.5X SSC, 20 mmol/L Na-P (6.5), 1X Denhardt's solution, 0.1% SDS, 10% dextran sulfate, and 25 μg/mL denatured ssDNA at 42°C. The next day, the membranes were washed with 2X SSC, 0.1% SDS for 30 minutes twice at room temperature, then 0.1X SSC, 0.1% SDS for 30 minutes four times at 50°C. Membranes were subjected to autoradiography at −80°C for 2 days (Fuji Film, Minami-Ashigara, Japan). cDNA fragment from human β-actin gene was prepared from human blood lymphocytes with RT-PCR as reported before,32 with which washed membranes were rehybridized and autoradiographed.
Characterization of the transformants.The obtained transformants were cultured with or without 5 mmol/L Isopropyl β-D-Thiogalactopyranoside (IPTG) (Sigma) for the indicated periods, and after washing, smear slides were prepared and the morphology of the cells was estimated microscopically with Wright-Giemsa staining. A cell counting kit was purchased from Dojindo (Tokyo, Japan), and cells were stained and monitored according to the manufacturer's protocol with an ELISA monitor (Model 450; Bio-Rad, Richmond, CA). Viable cell number was determined by Trypan blue dye exclusion assay. Also, cell counting was performed with microscopical determination. High molecular weight DNA was extracted with a Sepa-gene kit (Sanko-Junyaku, Tokyo, Japan), and electrophoresed at 10 μg/lane through 2% agarose gel in TBE and photographed with a Polaroid camera. For DNA preparation from the HL-60 transformant NF-1 cells incubated for 72 or 84 hours with IPTG, DNA was extracted from 1 × 108 cultured cells and electrophoresed because of low yield of DNA.
Flow cytometry analysis.HL-60 cells or transformant cells were incubated in DMEM supplemented with 10% FCS with or without IPTG for 48 hours and cells were treated with CD11β antibody (Becton Dickinson) for 1 hour on ice. Antibody binding was detected with a fluoresceinated F(ab′)2 rabbit antimouse antibody (Dako, Glostrup, Denmark). Flow cytometry was performed on a Becton Dickinson FACScan.
Reverse transcriptase-polymerase chain reaction (RT-PCR).Total RNA was extracted from each cell population by the AGPC method, and treated with DNaseI (RNase free; Takara) at 37°C for 15 minutes. After two cycles of phenol/chloroform extraction, first strand cDNA was synthesized with Molony murine reverse transcriptase (GIBCO-BRL) using 5 μg of the prepared total RNA with 10 pmol antisense primer as shown in Fig 1.33 After RNase treatment (37°C 30 min), PCR was performed under the following conditions: 94°C for 30 seconds for denaturation, 51°C for 2 minutes for annealing, and 72°C for 2 minutes for extension for 30 cycles with sense and antisense primers (50 pmol each) as shown in Fig 1 (sense primer; 5′-TGGGTGCCCAAAGAATCAGAA-3′ and antisense primer; 5′-AGCATCATCAATAGTAGAAGC-3′) with hot starting (adding the primer mixture at 80°C). After chloroform extraction and ethanol precipitation, PCR products were electrophoresed through 3% agarose gels in TBE buffer.
Nucleotide sequence of MARCO cDNA. The entire nucleotide sequence of the isolated cDNA clone, MARCO, is shown in (a). The nucleotide sequences of murine mitochondrial DNA fragment and rat mitochondrial cytochrome c oxidase/serine tRNA cDNA are shown in (b) and (c), respectively (from 3′ to 5′ direction) with the corresponding complementary sequence of MARCO. The nucleotide of MARCO is numbered from 5′ terminal. In each sequence profile, mismatched nucleotides between MARCO (a) and complementary nucleotide sequences of murine mitochondrial DNA fragment (b) are demonstrated with bold typing. The 3′ end of the serine tRNA region is indicated with an arrow. The PvuII site used to prepare the probe for northern blotting analysis is indicated with an arrowhead. The horizontal arrows indicate primers used for RT-PCR analysis (rightward direction for sense primer and leftward direction for antisense primer).
Nucleotide sequence of MARCO cDNA. The entire nucleotide sequence of the isolated cDNA clone, MARCO, is shown in (a). The nucleotide sequences of murine mitochondrial DNA fragment and rat mitochondrial cytochrome c oxidase/serine tRNA cDNA are shown in (b) and (c), respectively (from 3′ to 5′ direction) with the corresponding complementary sequence of MARCO. The nucleotide of MARCO is numbered from 5′ terminal. In each sequence profile, mismatched nucleotides between MARCO (a) and complementary nucleotide sequences of murine mitochondrial DNA fragment (b) are demonstrated with bold typing. The 3′ end of the serine tRNA region is indicated with an arrow. The PvuII site used to prepare the probe for northern blotting analysis is indicated with an arrowhead. The horizontal arrows indicate primers used for RT-PCR analysis (rightward direction for sense primer and leftward direction for antisense primer).
In vitro effects of cytochrome c oxidase in the TNF-α system.Sense cytochrome c oxidase-expressing transformants were obtained by electroporation with pBK-CMV-sense cytochrome c oxidase plasmid linearized with Mlu I, and cultured with G418. The obtained transformants and wild-type cells were cultured with or without recombinant human TNF-α (rhTNF-α) (20 ng/mL; Genzyme, Cambridge, MA) for the indicated periods and morphologic changes in HL-60 cells, viable cell count for U937 cells, and DNA fragmentation for both types of cells were determined as described above.
RESULTS
Isolation of cDNA clone.Complementary DNA was synthesized using mRNA extracted from HL-60 cells treated with ATRA, fractionated and ligated into the expression vector pBK-CMV. After electroporation into E coli, a library was constructed with 3 × 105 independent colonies, and further divided into subpools. Plasmids were isolated from each subpool and electroporated into HL-60 cells, smear slides of which were stained after culture for 2 or 3 days. The subpools were screened by light microscopy with reference to reduction in cell size, decreased basophilic staining of the cytoplasm, chromatin condensation and segmented nucleus, and increased cell death between 48- and 72-hour incubation. Most of the transfected cells on the prepared slides were intact blastic cells or dead cells because of electroporation, and 10 to 50 cells of a total of 1 × 104 HL-60 cells showed morphologic changes in positive subpools. The six positive subpools among the initial 600 were retransfected and precise morphologic comparisons were made between 48- and 72-hour incubation. Of these, three positive subpools were analyzed further. Extrachromosomal DNA was recovered and electroporated into E coli, then plasmids were prepared and screened to isolate single clones. Simultaneously, the positive subpools were recovered from E coli glycerol stocks and further screened. Three clones were identical, and their entire nucleotide sequence is shown in Fig 1. A database homology search revealed that this cDNA clone showed significant similarity to antisense RNA for rat mitochondrial cytochrome c oxidase/serine tRNA34 and also a murine mitochondrial gene fragment35 with 85.0% and 99.6% similarity at the nucleotide level, respectively. It was concluded that this cDNA clone was derived from antisense RNA for human mitochondrial cytochrome c oxidase/serine tRNA.*
Characterization of the isolated cDNA clone.To characterize this cDNA clone, named MARCO, transformants were obtained with the lac switch method in which a 3′SS eukaryotic lac-repressor protein expression plasmid (Hygromycin resistant) was cotransfected into HL-60 cells with pOPRSVI-MARCO expression plasmid (G418-resistant), in which the expression of this cDNA clone was controlled by Rous sarcoma virus-long terminal repeat (RSV-LTR) promoter with modified operator sequences from the lac operon to induce expression of the cDNA clone with IPTG stimulation.36 The yield of transformants was very low, which seemed to reflect leaky expression of this cDNA clone without IPTG stimulation (reported by the manufacturer). Three transformants (NF-1, 2, 3) of a total of 1 × 107 cells were obtained (Fig 2).
Nomenclature, origin, and transfected plasmid of the transformant cells used in this manuscript. CytoC means human mitochondrial cytochrome c oxidase in this figure.
Nomenclature, origin, and transfected plasmid of the transformant cells used in this manuscript. CytoC means human mitochondrial cytochrome c oxidase in this figure.
The transcript of MARCO was detected by northern blotting analysis (Fig 3). In this experiment, the cDNA fragment obtained after PvuII digestion (from the PvuII site indicated by the arrowhead in Fig 1, to the 3′ end of the cDNA) was used as a probe to eliminate serine tRNA region (Fig 1, indicated with the arrow at the 3′ end). Also, because MARCO encoded an antisense RNA for mitochondrial cytochrome c oxidase, a single-stranded probe was synthesized. In wild-type HL-60 cells, strong signals for cytochrome c oxidase were observed with or without IPTG when the sense-stranded probe, which could hybridize with antisense MARCO and thus detect cytochrome c oxidase, was used for northern blotting. In transformant NF-1 cells, the signal was detected without IPTG, and diminished in a time-dependent manner (Fig 3A), with almost no signal being detected after 48-hour stimulation with IPTG. These results indicated that in the transformant cells cytochrome c oxidase seemed to be downregulated by the induction of MARCO transcript. When HL-60 cells were treated with ATRA, this transcript was not detected after 84-hour incubation. Also, in the membrane a higher molecular weight band as well as major band and vague 18S band was detected in each lane, which might indicate that transcript of mitochondrial cytochrome c oxidase was mainly stopped at the near serine tRNA 3′ terminal, and also some of them extended beyond this region to serine tRNA.
Northern blot analysis using (A) sense probe, and (B) antisense probe. (A) Lanes 1 and 2, HL-60 transformed with 3′SS plasmid and pOPRSVI plasmid without insert (mock-transfection); 3, 4, 5, 6, and 7, HL-60 transformed with 3′SS plasmid and pOPRSVI-MARCO plasmid (NF-1); 8, and 9, wild-type HL-60 cells; 1 and 3, without IPTG; 4, with IPTG for 6 hours; 5, IPTG for 24 hours; 6, IPTG for 36 hours; 2 and 7, IPTG for 48 hours; 8, RA for 48 hours; 9, RA for 84 hours. (B) Lanes 1 and 2, mock-transfected HL-60 cells; 3 and 4, HL-60 transformant NF-1 cells; 1 and 3, without, and 2 and 4, with IPTG for 6 hours. In both (A) and (B), bottom panels show a rehybridization of the same blot to a human β-actin probe.
Northern blot analysis using (A) sense probe, and (B) antisense probe. (A) Lanes 1 and 2, HL-60 transformed with 3′SS plasmid and pOPRSVI plasmid without insert (mock-transfection); 3, 4, 5, 6, and 7, HL-60 transformed with 3′SS plasmid and pOPRSVI-MARCO plasmid (NF-1); 8, and 9, wild-type HL-60 cells; 1 and 3, without IPTG; 4, with IPTG for 6 hours; 5, IPTG for 24 hours; 6, IPTG for 36 hours; 2 and 7, IPTG for 48 hours; 8, RA for 48 hours; 9, RA for 84 hours. (B) Lanes 1 and 2, mock-transfected HL-60 cells; 3 and 4, HL-60 transformant NF-1 cells; 1 and 3, without, and 2 and 4, with IPTG for 6 hours. In both (A) and (B), bottom panels show a rehybridization of the same blot to a human β-actin probe.
When the antisense probe was used, which could hybridize with MARCO transcript, no signals were detected in wild-type HL-60 cells with or without IPTG nor in transformant NF-1 cells without IPTG. When NF-1 cells were treated with IPTG for 6 hours, however, the transcript was observed (Fig 3B).
This cDNA clone was characterized using NF-1 cells, which showed stable transformation and grew well in the absence of IPTG. The time dependency of morphologic changes on IPTG stimulation is shown in Fig 4. HL-60 cells underwent morphologic changes from blastic to mature neutrophilic appearance with chromatin condensation and nuclear segmentation after 48-hour incubation with IPTG. After 48-hour culture some of the cells also showed cell death. Obvious cell death was observed after 60 hours in culture, and after 96 hours almost no cells survived, although wild-type HL-60 cells and control transformants with no insert (mock transfection) showed no morphologic changes and grew well. Enzymatic maturation was also estimated with neutrophil alkaline phosphatase staining. Wild-type HL-60 cells showed no staining with neutrophil alkaline phosphatase, and mature neutrophilic granulocytes were stained. NF-1 cells showed no staining after incubation for 48 or 60 hours with IPTG (data not shown). Also, CD11β expression was analyzed with FACS. It was reported previously that the expression level of CD11β was very low in wild-type HL-60 cells, and when maturation was induced, the expression of this cell surface molecule was increased.2 However, the expression level of CD11β in NF-1 cells after 48-hour treatment with IPTG was similar level to that of wild-type HL-60 cells (data not shown). From these data no maturation-associated enzymes and molecules were induced when NF-1 cells were treated with IPTG, though morphologic changes were observed after IPTG stimulation.
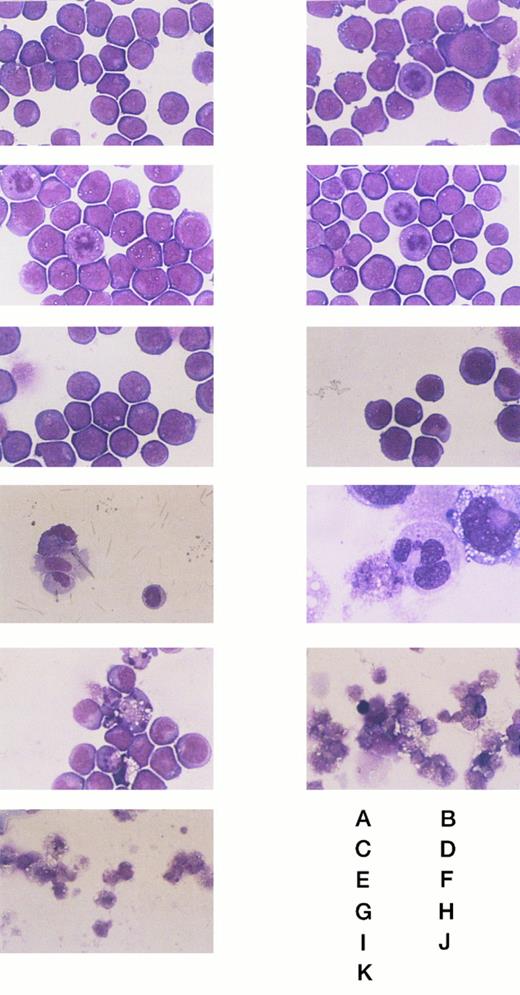
The morphologic changes of the wild-type and transformant HL-60 cells on IPTG stimulation. (A) and (B) Wild-type HL-60 cells. (C) and (D) Mock-transfected HL-60 cells. (E), (F), (G), (H), (I), (J), and (K) NF-1 cells. (A), (C), and (E) Without IPTG. (F) With IPTG for 24 hours. (G) and (H) With IPTG for 48 hours. (I) With IPTG for 60 hours. (J) With IPTG for 84 hours. (B), (D), and (K) With IPTG for 96 hours. Original magnification: (H) × 1000, and others × 400.
The morphologic changes of the wild-type and transformant HL-60 cells on IPTG stimulation. (A) and (B) Wild-type HL-60 cells. (C) and (D) Mock-transfected HL-60 cells. (E), (F), (G), (H), (I), (J), and (K) NF-1 cells. (A), (C), and (E) Without IPTG. (F) With IPTG for 24 hours. (G) and (H) With IPTG for 48 hours. (I) With IPTG for 60 hours. (J) With IPTG for 84 hours. (B), (D), and (K) With IPTG for 96 hours. Original magnification: (H) × 1000, and others × 400.
To determine the effects on growth of the transformants when the MARCO transcript was induced with IPTG, cells were assayed at the indicated periods after adding IPTG with a cell counting kit. This assay system is based on (3-(4,5-dimethylthiazol-2-yl)) 2,5-diphenyl tetrazolium bromide (MTT) assay, and monitors soluble formazan which is produced from tetrazolium salt. The tertazolium ring is reported to be cleaved only in active mitochondria, and formazam is not produced when mitochondrial activity is lost (Fig 5A).37-39 After 24-hour culture with 5 mmol/L IPTG, cell growth was decreased to half that of wild-type HL-60 cells, and after 60-hour incubation no growth was seen. Viable cell number was determined by Trypan blue dye exclusion assay, in which NF-1 cells started to die after 36 hours in culture with IPTG with no cells surviving after 72 hours (Fig 5B). Also, microscopically cell counting was proceeded when IPTG was added in the cultures. The result was shown in Fig 5C, in which with a little time delay cell number was decreased with the similar fashion to that from cell counting kit assay and dye exclusion one.
Time-dependency of cell death on the induction of MARCO transcript with the lac switch system. (A) The wild-type HL-60 cells or the transformant cells were treated with IPTG for the indicated periods and assayed with a cell counting kit. On the vertical axis, the O.D. from wild-type HL-60 cells is shown as 100%, while 0% indicates the O.D. from cell-free cultures. (•) Wild-type HL-60 cells; (▴) mock-transfected HL-60 cells; (○) NF-1 cells. (B) Cells were cultured for the indicated periods with IPTG, and viable cell number was counted by Trypan blue dye exclusion assay. (C) Cells were cultured as in (B), and cell counting was proceeded microscopically. (•) Wild-type HL-60 cells; (▴) mock-transfected HL-60 cells; (○) NF-1 cells. In each panel, values represent the mean of triplicate assays with standard error of means (SEM). This result is representative of five independent experiments.
Time-dependency of cell death on the induction of MARCO transcript with the lac switch system. (A) The wild-type HL-60 cells or the transformant cells were treated with IPTG for the indicated periods and assayed with a cell counting kit. On the vertical axis, the O.D. from wild-type HL-60 cells is shown as 100%, while 0% indicates the O.D. from cell-free cultures. (•) Wild-type HL-60 cells; (▴) mock-transfected HL-60 cells; (○) NF-1 cells. (B) Cells were cultured for the indicated periods with IPTG, and viable cell number was counted by Trypan blue dye exclusion assay. (C) Cells were cultured as in (B), and cell counting was proceeded microscopically. (•) Wild-type HL-60 cells; (▴) mock-transfected HL-60 cells; (○) NF-1 cells. In each panel, values represent the mean of triplicate assays with standard error of means (SEM). This result is representative of five independent experiments.
Changes in high molecular weight DNA were also examined to determine the effects of this cDNA transcript on the genomic DNA. As shown in Fig 6A, transformants NF-1, 2, and 3 showed genomic DNA fragmentation and smearing after incubation for 36 hours with 5 mmol/L IPTG. In contrast, wild-type HL-60 cells and transformants without IPTG stimulation showed little DNA fragmentation or smearing. The time-dependency of the genomic change in transformants after IPTG stimulation is shown in Fig 6B. High molecular weight DNA was extracted from NF-1 cells before and after stimulation with IPTG. After 36 hours in culture with IPTG, genomic DNA fragmentation was observed. After 72- and 84-hour incubation, the yield of the extracted DNA was very low and when DNA was extracted from 1 × 108 cells, DNA fragmentation and smearing were also observed. These observations indicated that after IPTG stimulation nonspecific DNA fragmentation followed by nucleosome formation occurred in transformant cells, followed by morphologic changes and cell death.
Estimation of DNA fragmentation after induction of MARCO with IPTG. (A) Cells were cultured with IPTG for 36 hours, then high molecular weight DNA was prepared and electrophoresed. Lane 1, MW marker (φX174/HaeIII); 2, wild-type HL-60 cells; 3, 4, and 5, HL-60 transformants NF-1, 2, and 3. (B) High molecular weight DNA was extracted at the indicated periods after IPTG treatment and electrophoresed. Lanes 1 and 14, MW marker; 2, 3, and 4, wild-type HL-60 cells; 5 and 6, mock-transfected HL-60 cells; 7, 8, 9, 10, 11, 12, and 13, NF-1 cells; 2, 5, and 7, no IPTG stimulation; 8, with IPTG for 24 hours; 9, with IPTG for 36 hours; 10, with IPTG for 48 hours; 11, with IPTG for 60 hours; 12, with IPTG for 72 hours; 13, with IPTG for 84 hours; 3 and 6, with IPTG for 96 hours; 4, with RA for 24 hours.
Estimation of DNA fragmentation after induction of MARCO with IPTG. (A) Cells were cultured with IPTG for 36 hours, then high molecular weight DNA was prepared and electrophoresed. Lane 1, MW marker (φX174/HaeIII); 2, wild-type HL-60 cells; 3, 4, and 5, HL-60 transformants NF-1, 2, and 3. (B) High molecular weight DNA was extracted at the indicated periods after IPTG treatment and electrophoresed. Lanes 1 and 14, MW marker; 2, 3, and 4, wild-type HL-60 cells; 5 and 6, mock-transfected HL-60 cells; 7, 8, 9, 10, 11, 12, and 13, NF-1 cells; 2, 5, and 7, no IPTG stimulation; 8, with IPTG for 24 hours; 9, with IPTG for 36 hours; 10, with IPTG for 48 hours; 11, with IPTG for 60 hours; 12, with IPTG for 72 hours; 13, with IPTG for 84 hours; 3 and 6, with IPTG for 96 hours; 4, with RA for 24 hours.
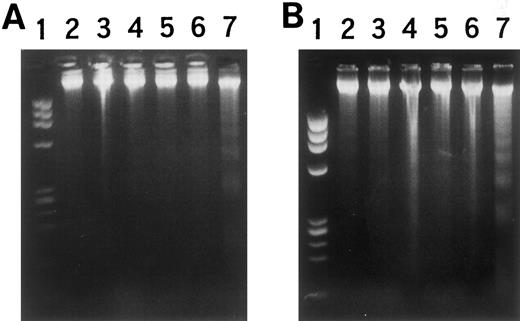
Effects on other hematopoietic cell lines.To elucidate the significance of MARCO, the effects of its expression were examined in other hematopoietic cell lines, erythroid progenitor K562 cells, and megakaryoblastic CMK cells, using transformants obtained with the lac switch method (Fig 2). The obtained transformants, NG-1 for K562 cells and NH-1 for CMK cells, showed morphologic changes with decreased basophilic staining of the cytoplasm, chromatin condensation, and nuclear changes after 2 days in culture with IPTG (data not shown). High molecular weight DNA fragmentation was also observed in these transformants when cultured with IPTG for 36 hours to induce the MARCO transcript, as was observed in HL-60 transformant NF-1 cells (Fig 7). Viable cell number was decreased in a time-dependent manner with IPTG, and no cells survived after 4 days in culture (data not shown). Thus, other kinds of hematopoietic cell lines also underwent cell death when MARCO transcription was induced.
DNA fragmentation in (A) NG-1 cells and (B) NH-1 cells with IPTG stimulation. (A) Lane 1, MW marker; 2 and 3, wild-type K562 cells; 4 and 5, mock-transfected K562 cells; 6 and 7, NG-1 cells; 2, 4, and 6, without, and 3, 5, and 7, with IPTG for 36 hours. (B) Lane 1, MW marker; 2 and 3, wild-type CMK cells; 4 and 5, mock-transfected CMK cells; 6 and 7, NH-1 cells; 2, 4, and 6, without, and 3, 5, and 7, with IPTG for 36 hours.
DNA fragmentation in (A) NG-1 cells and (B) NH-1 cells with IPTG stimulation. (A) Lane 1, MW marker; 2 and 3, wild-type K562 cells; 4 and 5, mock-transfected K562 cells; 6 and 7, NG-1 cells; 2, 4, and 6, without, and 3, 5, and 7, with IPTG for 36 hours. (B) Lane 1, MW marker; 2 and 3, wild-type CMK cells; 4 and 5, mock-transfected CMK cells; 6 and 7, NH-1 cells; 2, 4, and 6, without, and 3, 5, and 7, with IPTG for 36 hours.
RT-PCR.RT-PCR was performed to identify antisense RNA transcript product because using northern hybridization the obtained signal was too weak to be distinguished from the background (Fig 8). In this experiment, DNase treatment was applied to prevent mitochondrial DNA contamination. Also, to detect only antisense RNA for cytochrome c oxidase, first strand cDNA was synthesized with the antisense primer as used in 5′ RACE (Rapid Amplification of cDNA End), as described in Materials and Methods.33 The transcript was not detected in wild-type HL-60 cells nor control cells transformed with vector alone (Fig 8A). No MARCO transcript was observed in NF-1 cells without IPTG stimulation, but the transcript was detected following 6-hour incubation with IPTG (Fig 8A). Other features of cell death were also examined. As reported previously, factor-dependent cells show cell death after 6- to 12-hour incubation in the absence of the growth factors.40 Murine myeloblastic NFS-60 cells were reported to show growth dependency on rhG-CSF23; no RT-PCR product of the MARCO transcript was detected after 6-hour incubation in the absence of rhG-CSF (Fig 8B). Similar results were obtained when using human myeloblastic TF-1 cells, which showed growth dependency on rhGM-CSF (Fig 8B).24 When K562 cells, which showed enzymatic maturation to express globin when cultured with hemin,41 were examined with RT-PCR after treatment with hemin for 96 hours, the product of the MARCO transcript was not detected (Fig 8B). However, MARCO transcript was observed in HL-60 cells cultured with RA for 48 to 84 hours, concomitantly with the changes to mature neutrophilic granulocytes and induced cell death (Fig 8A). The transcript was not detected in human bone marrow nonadherent mononuclear cells or in blood lymphocytes. However, when RT-PCR product from blood neutrophils was electrophoresed, the 385 bp band was detected although no signal was seen when RT-PCR was performed in the absence of reverse transcriptase (Fig 8C). When the prepared blood neutrophilic granulocytes were treated with rhG-CSF for 3 or 6 hours in vitro, there were also detected RT-PCR products (Fig 8D). These observations demonstrated that MARCO was transcribed in neutrophilic granulocytes, and that MARCO was also transcribed even when G-CSF was added in the cultures, which is reported to delay apoptosis of mature neutrophilic granulocytes in culture and in vivo.
RT-PCR analysis of MARCO transcript. (A) Lane 1, MW marker; 2, 3, 4, 5, 14, 15, 16, and 17, wild-type HL-60 cells; 6, 7, 8, and 9, mock-transfected HL-60 cells; 10, 11, 12, and 13, NF-1 cells; 2, 4, 6, 8, 10, 12, 14, and 16, without, and 3, 5, 7, 9, 11, 13, 15, and 17, with reverse transcriptase; 2, 3, 6, 7, 10, and 11, without, and 4, 5, 8, 9, 12, and 13, with IPTG for 6 hours; 14 and 15, with RA for 48 hours; 16 and 17, with RA for 84 hours. (B) Lane 1, MW marker; 2, NF-1 cells; 3, 4, 5, and 6, NFS-60 cells; 7, 8, 9, and 10, TF-1 cells; 11, 12, 13, and 14, K562 cells; 3, 5, 7, 9, 11, and 13, without, and 2, 4, 6, 8, 10, 12, and 14, with reverse transcriptase; 3 and 4, with, and 5 and 6, without rhG-CSF for 6 hours; 7 and 8, with, and 9 and 10, without rhGM-CSF for 6 hours; 11 and 12, without, and 13 and 14, with hemin for 96 hours. (C) Lane 1, MW marker; 2 and 3, human bone marrow nonadherent mononuclear cells; 4 and 5, human blood lymphocytes; 6 and 7, human blood neutrophilic granulocytes; 8, NF-1 cells; 2, 4, and 6, without, and 3, 5, 7, and 8, with reverse transcriptase; 8, with IPTG for 6 hours. (D) Lane 1, MW marker; 2, 3, 4, 5, 6, 7, 8, and 9, human blood neutrophilic granulocytes; 2, 4, 6, and 8, without, and 3, 5, 7, and 9, with reverse transcriptase; 2, 3, 6, and 7, incubated for 3 hours, and 4, 5, 8, and 9, incubated for 6 hours; 2, 3, 4, and 5, without, and 6, 7, 8, and 9, with rhG-CSF.
RT-PCR analysis of MARCO transcript. (A) Lane 1, MW marker; 2, 3, 4, 5, 14, 15, 16, and 17, wild-type HL-60 cells; 6, 7, 8, and 9, mock-transfected HL-60 cells; 10, 11, 12, and 13, NF-1 cells; 2, 4, 6, 8, 10, 12, 14, and 16, without, and 3, 5, 7, 9, 11, 13, 15, and 17, with reverse transcriptase; 2, 3, 6, 7, 10, and 11, without, and 4, 5, 8, 9, 12, and 13, with IPTG for 6 hours; 14 and 15, with RA for 48 hours; 16 and 17, with RA for 84 hours. (B) Lane 1, MW marker; 2, NF-1 cells; 3, 4, 5, and 6, NFS-60 cells; 7, 8, 9, and 10, TF-1 cells; 11, 12, 13, and 14, K562 cells; 3, 5, 7, 9, 11, and 13, without, and 2, 4, 6, 8, 10, 12, and 14, with reverse transcriptase; 3 and 4, with, and 5 and 6, without rhG-CSF for 6 hours; 7 and 8, with, and 9 and 10, without rhGM-CSF for 6 hours; 11 and 12, without, and 13 and 14, with hemin for 96 hours. (C) Lane 1, MW marker; 2 and 3, human bone marrow nonadherent mononuclear cells; 4 and 5, human blood lymphocytes; 6 and 7, human blood neutrophilic granulocytes; 8, NF-1 cells; 2, 4, and 6, without, and 3, 5, 7, and 8, with reverse transcriptase; 8, with IPTG for 6 hours. (D) Lane 1, MW marker; 2, 3, 4, 5, 6, 7, 8, and 9, human blood neutrophilic granulocytes; 2, 4, 6, and 8, without, and 3, 5, 7, and 9, with reverse transcriptase; 2, 3, 6, and 7, incubated for 3 hours, and 4, 5, 8, and 9, incubated for 6 hours; 2, 3, 4, and 5, without, and 6, 7, 8, and 9, with rhG-CSF.
Effects of cytochrome c oxidase on the TNF-α system.As MARCO is an antisense RNA corresponding to the cytochrome c oxidase transcript, and it was reported that mitochondrial respiratory enzymes, especially cytochrome c oxidase, showed decreased activity in TNF-α–treated cells,22 it was possible that TNF-α would induce MARCO transcript in responsive cells. Morphologic changes of HL-60 cells treated with TNF-α were similar to those reported previously (data not shown)2,3 and to those induced by MARCO (Fig 4). RNA was extracted from TNF-α–treated HL-60 cells and RT-PCR was performed. As shown in Fig 9A, the MARCO transcript was detected after incubation for 6 hours with TNF-α. Also, the MARCO transcript was detected in U937 cells treated with TNF-α, which induces cell death as well as DNA fragmentation2 6 (Fig 9B).
RT-PCR analysis of MARCO in TNF-α–treated (A) HL-60 and (B) U937 cells. (A) Lane 1, MW marker; 2, 3, 4, and 5, HL-60 cells; 2 and 4, without, and 3 and 5, with reverse transcriptase; 2 and 3, without, and 4 and 5, with rhTNF-α. (B) Lane 1, MW marker; 2, 3, 4, and 5, U937 cells; 2 and 4, without, and 3 and 5, with reverse transcriptase; 2 and 3, without, and 4 and 5, with rhTNF-α.
RT-PCR analysis of MARCO in TNF-α–treated (A) HL-60 and (B) U937 cells. (A) Lane 1, MW marker; 2, 3, 4, and 5, HL-60 cells; 2 and 4, without, and 3 and 5, with reverse transcriptase; 2 and 3, without, and 4 and 5, with rhTNF-α. (B) Lane 1, MW marker; 2, 3, 4, and 5, U937 cells; 2 and 4, without, and 3 and 5, with reverse transcriptase; 2 and 3, without, and 4 and 5, with rhTNF-α.
Finally, to determine whether MARCO was essential for the TNF-α system, morphologic changes and cell death effects were estimated when TNF-α was added to cultures of HL-60 transformant NI-1 cells and U937 transformant NJ-1 cells in which mitochondrial cytochrome c oxidase was constitutively expressed at high levels under the control of the CMV immediate early promoter (determined by northern blotting analysis; data not shown) (Fig 2). Morphologic changes were not observed in NI-1 cells, although typical morphologic changes were demonstrated in wild-type HL-60 cells when treated with TNF-α (data not shown). After administration of TNF-α, cell death was observed in wild-type U937 cells, but this effect was suppressed in NJ-1 transformants (data not shown). Also, no high molecular weight DNA fragmentation was observed in NI-1 and NJ-1 transformants when treated with TNF-α, although DNA fragmentation was detected in wild-type HL-60 and U937 cells treated with TNF-α for 6 hours (Fig 10). These results indicated that in the TNF-α system MARCO might be inducibly transcribed, and also overexpression of cytochrome c oxidase suppressed the ability of TNF-α to induce high molecular weight DNA fragmentation in U937 cells and HL-60 cells.
Inhibition of high molecular weight DNA fragmentation induced by TNF-α. (A) Lane 1, MW marker; 2 and 3, mock-transfected HL-60 cells; 4 and 5, HL-60 transformant NI-1 cells; 2 and 4, without, and 3 and 5, with rhTNF-α for 6 hours. (B) Lane 1, MW marker; 2 and 3, mock-transfected U937 cells; 4 and 5, U937 transformant NJ-1 cells; 2 and 4, without, and 3 and 5, with rhTNF-α for 6 hours.
Inhibition of high molecular weight DNA fragmentation induced by TNF-α. (A) Lane 1, MW marker; 2 and 3, mock-transfected HL-60 cells; 4 and 5, HL-60 transformant NI-1 cells; 2 and 4, without, and 3 and 5, with rhTNF-α for 6 hours. (B) Lane 1, MW marker; 2 and 3, mock-transfected U937 cells; 4 and 5, U937 transformant NJ-1 cells; 2 and 4, without, and 3 and 5, with rhTNF-α for 6 hours.
DISCUSSION
During the maturation process, myeloid progenitor cells undergo typical morphologic changes such as chromatin condensation and nuclear segmentation for granulocytes, generation of apoptotic bodies for thrombocytes, and denucleation for erythrocytes.1 These changes represent programmed cell death,42 43 ie, the morphologic maturation steps of hematopoietic immature progenitor cells are a kind of slow cell death process. The results of the present study indicate that when antisense RNA for mitochondrial cytochrome c oxidase was transcribed in HL-60 cells, blastic morphologic features were changed to those of neutrophils followed by cell death. Also, K562 transformants and CMK transformants showed morphologic changes with chromatin condensation and nuclear segmentation as well as a decrease in basophilic staining in the cytoplasm when MARCO transcript was induced with IPTG for 2 days, and after 4 days cell death was observed in these transformant cells.
Blastic progenitor cells have been reported to show high levels of mitochondrial activity, and terminal morphologic maturation is accompanied by a decrease in mitochondrial activity.1 Neutrophilic granulocytes were reported to show decreases in number as well as activity of mitochondria,1,44 and erythrocytes contain no mitochondria.45 Cytochrome c oxidase has been reported to be essential for mitochondrial respiration in electron transfer and proton translocation, which seems to be linked to cellular proliferation.46 47 The inactivation of this transcript may block energy generation, which can consequently induce cell death.
Because the construct of MARCO represented sense serine tRNA portion and following antisense cytochrome c oxidase portion, there are two possible mechanisms to induce cell death when MARCO is introduced. The one is the effect of antisense cytochrome c oxidase, and the other is the effect of the fusion transcript of serine tRNA with other nucleotides. When our isolated cDNA clone was digested with PvuII to prepare a cDNA fragment without serine tRNA region and expressed with the lac switch expression vector system, morphologic changes, high molecular weight DNA fragmentation, and cell death were observed, which were similar to those observed when entire MARCO was expressed (data not shown). However, when sense serine tRNA portion was ligated with sense cytochrome c oxidase, or with sense or antisense β-galactosidase cDNA, and expressed with the lac switch expression system, cell death-inducing effects were not observed (data not shown). That is, the cell death effect reported here may be possibly induced by the antisense transcript of cytochrome c oxidase. It was reported that treatment with an antisense oligonucleotide corresponding to the nuclear-encoded cytochrome c oxidase subunit isoform decreased cytochrome c oxidase activity.48 Our results showed that RNA products encoded by mitochondrial genes may also be inactivated by antisense RNA when introduced into the cells by electroporation. Thus, antisense RNA treatment targeting mitochondrial products may be effective to change the characteristics and morphology of cells.
Our results also showed that MARCO is transcribed physiologically in neutrophilic granulocytes. Physiologic antisense RNA transcripts have been reported in various systems. In plasmid R1, duplex formation of CopT and CopA was reported.49 In Dictyosterlium, EB-4 PSV prespor gene stability is controlled by a normally transcribed antisense RNA.33 Oncogene products in mammalian cells, myb50 and myc,51 have been reported to be controlled at the transcriptional level by the production of sense and antisense RNA transcripts. In rat mitochondria, antisense RNA is naturally transcribed from the Ori L region.52 Also, rat mitochondrial NADH-dehydrogenase subunit changes sense mRNA stability by the transcription of antisense RNA.53 MARCO was demonstrated to be transcribed from the L strand compared with sense cytochrome c oxidase, which was encoded on the H strand.54 Thus, it may be possible that MARCO regulates cytochrome c oxidase mRNA stability to influence its activity.
Also, MARCO is inducibly transcribed in the TNF-α system as well as the ATRA one. Although the mechanism of signal transduction has not been determined, some reports have demonstrated signal transduction to the mitochondria.55 56 It may be possible that after TNF-α treatment, some molecules, including Ca2+, polypeptides, and even nucleic acids, can transduce signals from the cytoplasm or nucleus to the mitochondria, followed by the inducible transcription of MARCO.
These observations suggest that antisense RNA corresponding to mitochondrial transcripts may be effectively used for the treatment of leukemia, and this newly defined MARCO expression plasmid vector may be effective against myeloid leukemic blasts. We are currently examining the feasibility of treatment of myeloid leukemias using the MARCO delivery system both in vitro and in vivo.
ACKNOWLEDGMENT
We thank Dr S. Nagata and N. Yoshida for technical advice and encouragement; N. Sato and N. Yusa for DNA sequencing; A. Moriguchi, K. Ishikawa, and N. Miyajima for the continuous encouragement; and K. Nakano and A. Hirata for secretarial assistance.
DNA data bank accession no.: AB004246
Address reprint requests to Naoki Shirafuji, MD, the Department of Hematology/Oncology, The University of Tokyo, 6-1, 4-Shirokanedai, Minato-ku, Tokyo 108 Japan.









This feature is available to Subscribers Only
Sign In or Create an Account Close Modal