Abstract
The cDNAs encoding wild type (WT) human receptor tyrosine kinase c-Kit and a constitutively activated mutant, V816Kit, were introduced into granulocyte-macrophage colony-stimulating factor (GM-CSF )-dependent early murine hemopoietic cells, which had been transformed with activated Myb. WTKit cells were able to grow in the presence of the human ligand for Kit, stem cell factor (SCF ), but displayed reduced growth and clonogenic potential in either SCF or GM-CSF compared with the parental cells in GM-CSF. In contrast, V816Kit cells grew without factor at a higher rate than the parental cells in GM-CSF and displayed increased clonogenicity. Dissection of the growth characteristics in liquid culture showed that in the presence of appropriate factors, the different populations had similar proliferation rates, but that V816Kit profoundly increased cell survival compared with WTKit or parental cells. This suggests that the signals transduced by WTKit activated with SCF, and by V816Kit, were not identical. Also, WTKit and V816Kit-expressing cells both varied from the early myeloid progenitor phenotype of the parental cells and gave rise to a small number of large to giant adherent cells that expressed macrophage (α-naphthyl acetate) esterase and neutrophil (naphtol-AS-D-chloroacetate) esterase, were highly phagocytic and phenotypically resembled histiocytes. Thus, WTKit activated by SCF and V816Kit were able to induce differentiation in a proportion of Myb-transformed myeloid cells. The factor independent V816Kit cells, unlike the parental and WTKit expressing cells, were shown to produce tumors of highly mitotic, invasive cells at various stages of differentiation in syngeneic mice. These results imply that constitutively activated Kit can promote the development of differentiated myeloid tumors and that its oncogenic effects are not restricted to lineages (mast cell and B-cell acute lymphoblastic leukemia), which have been reported previously. Furthermore, the mixed populations of cells in culture and in the tumors phenotypically resembled the leukemic cells from patients with monocytic leukemia with histiocytic differentiation (acute myeloid leukemia-M5c), a newly proposed subtype of myeloid leukemia.
THE C-KIT RECEPTOR tyrosine kinase1 is activated on binding of its ligand stem cell factor (SCF ) and subsequent dimerization of receptor monomers.2 This leads to transphosphorylation of the tyrosine residues in the cytoplasmic domain and results in intracellular signalling.3 C-Kit is expressed by the majority of hematopoietic stem and progenitor cells in normal human bone marrow4,5 and plays a critical role in hematopoiesis in mice (reviewed in Galli et al6 ). Its role in human leukemia is equivocal.7
Several activating mutations in Kit have been described. An aspartic acid to valine substitution at residue 816 in the phosphotransferase domain of human c-Kit is known to cause phosphorylation and activation of the receptor in the absence of ligand.8 This mutation was originally identified in HMC-1, a human mast cell line derived from a patient with mast cell leukemia.9 The corresponding mutations, which replace Asp814 with Tyr in a murine mastocytoma cell line P815 and Asp 817 with Tyr in a rat mast cell line RBL-2H3, also exhibit constitutive phosphorylation.10,11 This mutation in human, murine, or rat c-Kit was also shown to result in constitutive activation of the receptor when expressed in human embryonic kidney cell line, 293T.8,10,11 Introduction of this Kit mutant into the factor-dependent cell line, FDC-P1, resulted in factor independent growth and tumorigenicity in nude mice12 and caused factor-independence, tumorigenicity, and differentiation of the IC-2, a murine mast cell line.13 The same point mutation in c-kit was also identified in the peripheral blood mononuclear cells of patients with mastocytosis with an associated hematologic disorder of predominantly myelodysplastic features.14 Additionally, c-Kit was found to be phosphorylated in the absence of exogenous SCF in a number of patients with acute myeloblastic leukemia, but the presence of any mutations was not determined.15 A recent study introducing normal and activated forms of c-Kit into the bone marrow of mice showed that expression of activated murine V814Kit could result in immature acute lymphoblastic leukemias. However, in vitro colony assays showed that introduction of V814Kit into bone marrow cells gave rise to factor independent granulocyte/macrophage, mast, and mixed colony-forming cells, and colony numbers were increased by the addition of SCF.16 Furthermore, the frequency and monoclonality of leukemias arising in these mice indicated that V814Kit alone did not transform the cells. The nature of the malignancy observed in these animals may reflect the predisposition of different lineages to subsequent activation of other oncogenes.
Our interest was in determining the effects of c-Kit and the constitutively activated mutant form of Kit in myeloid lineages, especially in synergy with the myb oncogene, which promotes immortalization of early myeloid cells. The activation of c-Myb, a nuclear transcription factor, is known to occur by carboxyl truncation or by disruption of the leucine zipper-like motif in the negative regulatory domain.17,18 Overexpression of activated Myb19 or wild type (WT) Myb20 is known to transform murine primary hematopoietic cells, resulting in long-term proliferation of immature myeloid cells blocked in an early granulocyte/macrophage progenitor state.19,20 These cells require exogenous granulocyte-macrophage colony-stimulating factor (GM-CSF ) and an autocrine factor21 for survival and proliferation. The responses of these Myb transformed hematopoietic cells (MTHC) to various growth factors with proliferative and differentiative effects has been previously studied.22 SCF alone was unable to sustain clonogenicity of MTHC, but displayed synergistic effects on growth in combination with GM-CSF and interleukin-3 (IL-3),22 similar in effect to that observed with hematopoietic progenitor cells (reviewed Broxmeyer et al23 ). Thus, these cells provide an excellent model for investigating the effects of WTKit and activated Kit on the survival, proliferation, differentiation, and tumorigenicity of early myeloid cells.
Our initial experiments showed that introduction of either activated Myb alone19,21 or activated Kit alone in murine primary hematopoietic cells failed to bring about growth of factor-independent cell lines or colonies, although V816Kit did promote differentiation in short term cultures (P. Ferrao, T.J. Gonda, and L.K. Ashman, unpublished data, 1996). However, the two oncogenes in synergy were able to give rise to factor-independent cell lines in culture and factor-independent colonies in methycellulose and were potently tumorigenic in syngeneic mice, as detailed in this report. These cells derived by introducing V816Kit into long-term MTHC cells, showed morphologic features of myeloid cells, and some displayed mature characteristics suggesting a differentiative effect of activated Kit, previously noted only in a mast cell line.13
MATERIALS AND METHODS
Construction of kit expression constructs. The WT human c-kit cDNA (encoding the isoform containing the GNNK amino acids24 ) in pBluescript was obtained from Dr D. Williams, Immunex, Seattle, WA. The c-kit cDNA as an Asp718 - Not1 fragment was excised from the vector by restriction enzyme digestion, subsequently blunt ended using Klenow polymerase and subcloned into the Hpa1 site in the polylinker of pRUFneo25 to give pRUFneo(WTkit). To confirm the orientation of Kit, the plasmid DNA was digested with Eco R1.
The V816 kit mutation was constructed by reverse transcriptase-polymerase chain reaction (RT-PCR) of RNA extracted from the HMC-1 cell line using primers 5′-GATAGTACTAATGAGTACATGG-3′ (sense) and 5′-GAATGGTCACCACGGGC-3′ (antisense), with denaturation for 1 minute at 94°C, annealing for 1 minute at 56°C, and extension for 1 minute at 72°C for 30 cycles, to obtain a 715-bp product (nucleic acid residues 2167-2882). This fragment was digested to 360 bp (2330-2690) with restriction endonuclease Cel II (Esp I) and directionally cloned into the Esp I sites of pRUFneo(WTkit). The mutation of A to T at 2468 bp, which causes an Asp to Val substitution at amino acid residue 816 results in the loss of a BsmAI site. Hence, DNA from bacterial clones was extracted and screened for the mutation by PCR (2400-2660) and the loss of the BsmAI site. The construct, pRUFneo(V816kit) containing the cloned PCR product, was sequenced to confirm the mutation. All plasmids used for transfection were purified using a Qiagen plasmid kit.
Generation of virus producing cells and analysis of viral titer. Ψ2 cells were transfected with either the pRUFneo(WTkit) or pRUFneo(V816kit) expression constructs as described previously.26 The transfectants were selected with 400 μg/mL of G418 until all untransfected cells were killed. The transfected pools were labeled with monoclonal antibody (MoAb) IDC3 (antihuman c-Kit monoclonal antibody27 ) and sorted using a FACStarPLUS cell sorter (Becton Dickinson, Mountain View, CA) for cells positive for human c-Kit. Of the total population, 1.8% of V816kit and 10.5% of WTkit transfected cells were positive for surface expression when compared with nontransfected cells labeled with the same antibodies. The top 5% and 10% of cells, respectively, were sorted and expanded.
The viral titer of the sorted ψ2 cells was determined by infection of NIH-3T3 cells using viral supernatant as described previously26 and was calculated to be 2 × 105 colony-forming units (cfu)/mL for the V816kit transfectants and 1.5 × 106 cfu/mL for the WTkit transfectants.
Generation of infectants. All cell lines were derived from parent MTHC cells, which were generated from primary fetal liver cells by infection with pRUF(CT3Myb) and were maintained in culture supplemented with GM-CSF for 2 months as previously described.20 Stocks of these cells were infected with V816kit and WTkit viruses by cocultivation with the ψ2 transfectants for 2 days in Dulbecco's modified Eagle's medium (DMEM) containing 20% fetal calf serum (FCS) and 400 U/mL GM-CSF. The infectants were selected with 1 mg/mL G418 until uninfected cells were killed.
Colony assay. Parental and infected MTHC were washed three times in Hanks' Balanced Salt Solution (HBSS) and added at the indicated cell density to a methylcellulose mix containing 1.4% methylcellulose, 20% FCS, 1% bovine serum albumin in Iscove's modified Dulbecco's medium (IMDM), containing either 400 U/mL murine GM-CSF or 100 ng/mL human SCF. Triplicate 1-mL aliquots were plated and incubated in a humidified atmosphere of 5% CO2 at 37°C. After either 1 or 2 weeks, colonies consisting of more than 50 cells were counted.
Cell survival and proliferation assay. The assay and analysis was performed essentially as described previously.28 Cells were washed in HBBS and labeled with a lipophilic dye PKH26 (Sigma, St Louis, MO) according to manufacturer's instructions and seeded at 2 × 104 cells per well in duplicate into three 24-well trays. To each well, medium containing either 400 U/mL murine GM-CSF, 100 ng/mL human SCF, or no added factor was added to a final volume of 1 mL. On the same day (day 0) and at day 2 and day 4, the total cells in each well was harvested, centrifuged, and resuspended 300 μL phosphate-buffered saline (PBS). To each tube, 50 μL of Standard-Brite calibration beads (Coulter, Hialeah, FL) at a fixed concentration (6 × 104/mL) was added. The cell count, bead count, and the mean fluorescence intensity (MFI) for each sample was analyzed on a Profile II Flow cytometer (Coulter). The total cell number was calculated from the bead count and the cell count in the sample analyzed. The average number of divisions was calculated based on the MFI at each time point relative to the MFI at day 0, as each division results in halving of the fluorescence of individual cells. The survival or maintenance was calculated as the percentage retention of fluorescence units (MFI multiplied by the number of viable cells) relative to day 0.
Immunofluorescence and immunohistochemistry. Nonadherent cells were harvested, washed in PBS/0.1% BSA/0.1% sodium azide (PBA) and resuspended in antibody diluted in PBA + 10% normal rabbit serum to block Fc receptors. Either IDC3 (anti-human c-kit), ACK2 (anti-murine c-kit), 30H12 (anti-Thy1.2), MI/70 (anti-Mac1 biotin conjugated), 8C5 (anti-Gr1 fluorescein isothiocyanate [FITC]-conjugated), F4/80 (anti-macrophage marker), or no antibody was added for 1 hour on ice. Cells were washed to remove unbound antibody and then incubated with either FITC-conjugated sheep antimouse immunoglobulin, FITC-conjugated sheep anti-rat Ig (Silenus, Hawthorn, Vic., Australia) or FITC-conjugated Strepavidin (Amersham, Buckinghamshire, UK), as appropriate, for 1 hour on ice in the dark. Unbound antibody was again washed off and the cells fixed in 1% paraformaldehyde in PBS and analyzed on the Profile II Flow cytometer (Coulter) to detect fluorescence of the cells.
Cells resuspended in FCS were cytocentrifuged onto slides and fixed in 5% formaldehyde/47.5% acetone/47.5% methanol for 30 seconds, then washed once in distilled water and twice in Tris-buffered saline (TBS), pH 7.6. IDC3 antibody, diluted in PBS with 10% normal rabbit serum, was added to the slide and incubated overnight at 4°C in a humidified container. Slides were washed three times in TBS and secondary antibody (rabbit antimouse Ig; Dako, Carpinteria, CA) was added for 30 minutes. The slides were washed again three times and incubated with tertiary antibody (alkaline phosphatase antialkaline phosphatase mouse monoclonal, APAAP) for 30 minutes at room temperature. The additions of secondary and tertiary antibodies were repeated twice more with each incubation lasting for 10 minutes. The slides were washed, placed in substrate (mix of napthol AS-MX phosphate as the substrate and Fast Red as the indicator) at room temperature for 30 minutess, washed, and counterstained with hematoxylin.29 Cells were mounted in a glycerol/glycine solution and photographed.
Phagocytosis assay. Cells were cultured in tissue culture chambers on chamber slides (Nunc, Naperville, IL) for 4 days. Nonadherent cells were discarded and adherent cells washed in DMEM. A total of 200 μL of DMEM supplemented with 20% nonheat inactivated FCS and 2 μL of latex beads (Fluoresbrite plain YG20 microspheres, 2.5% solids-latex, diameter 1.8 μm; Polyscience Inc, Warrington, PA) was added to each well and incubated for 2 hours. The chambers were removed and the slides washed thoroughly in PBS before mounting. Cells were observed using a confocal microscope (Biorad, Microscopy Division, Hertfordshire, UK).
Enzyme staining. Nonspecific (α-naphthyl acetate) and chloro-acetate esterase were detected esentially as described previously.30 Cells suspended in FCS were cytocentrifuged onto slides and fixed in esterase fixative (2.86 mmol/L Na2HPO4 , 14.7 mmol/L KH2PO4 , 45% vol/vol acetone, 25% vol/vol formalin in distilled water) for 30 seconds at 4°C and washed three times in distilled water. In a Coplin jar, slides were allowed to stain for 45 minutes at room temperature in an α-naphthyl acetate solution of pH 6.2 containing Pararosanalin. The slides were washed three times in distilled water and stained for 1 hour at room temperature in a napthol-AS-D-chloroacetate solution of pH 7.4 containing Fast Blue. The slides were again washed three times in distilled water and counterstained in 2% methylgreen. Cells expressing nonspecific esterase were detected by a red-brown coloring and chloro-acetate esterase by a blue coloring as detailed previously.
Tumorigenicity assay. Cells were harvested, washed in HBBS, and aliquots of 2 × 106/mouse injected subcutaneously into the right flank of groups of four 8-week-old syngeneic (CBA strain) female mice. The mice were observed for visible tumors each week and killed for autopsy when tumors were 5 to 10 mm in diameter. The tumors were placed in Tissue TeK OCT compound (Miles Inc, Elkhart, IN) and frozen by immersion in isopentane precooled in liquid nitrogen and stored at −70°C. Sections of 5 μm from the fresh frozen samples were stained for enzyme markers as described above. Other tumor specimens were formalin fixed, paraffin embedded, sectioned at 5 μm, and stained with hematoxylin/eosin to examine morphology.
RESULTS
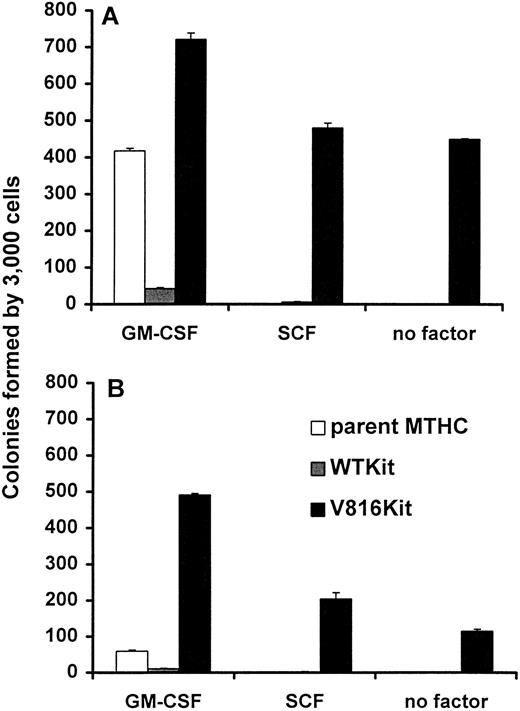
Generation of Kit-expressing MTHC. MTHC infected with retroviruses encoding wild type human c-Kit (WTKit) and the mutant form containing an Asp to Val substitution at residue 816 (V816Kit) were selected in medium containing G418 and then plated in methylcellulose with GM-CSF or without factor. The numbers of colonies obtained after 1 week are shown in Fig 1. As expected, parental MTHC and WTKit cells were unable to grow in the absence of exogenous growth factor, however, the V816Kit cells were able to form colonies, albeit at a 50-fold lower frequency than parental cells in GM-CSF, indicating that some of these cells were factor-independent.
Colony formation by MTHC infected with retroviruses carrying human Kit encoding constructs. The parent MTHC infected with retroviruses containing either RUFneo, c-kitRUFneo, or mutant kitRUFneo are represented as Vector, WTKit, and V816Kit, respectively. After 1 week of G418 selection in the presence of GM-CSF, cells were plated in triplicate (20,000 per dish) in methylcellulose containing either murine GM-CSF (▪) or no factor (□). Colonies containing more than 50 cells were scored 1 week after plating and the mean colony numbers ± the SEM are shown with the asterisk (*) indicating an absence of colonies.
Colony formation by MTHC infected with retroviruses carrying human Kit encoding constructs. The parent MTHC infected with retroviruses containing either RUFneo, c-kitRUFneo, or mutant kitRUFneo are represented as Vector, WTKit, and V816Kit, respectively. After 1 week of G418 selection in the presence of GM-CSF, cells were plated in triplicate (20,000 per dish) in methylcellulose containing either murine GM-CSF (▪) or no factor (□). Colonies containing more than 50 cells were scored 1 week after plating and the mean colony numbers ± the SEM are shown with the asterisk (*) indicating an absence of colonies.
Immunofluorescence data (not shown) indicated that not all of the G418 resistant cells expressed surface Kit protein; therefore, the cells were selected to obtain populations that were all expressing Kit. The V816Kit cells were selected for growth in liquid culture in the absence of growth factor. The WTKit cells were isolated by fluorescence-activated cell sorting based on surface expression of human c-Kit and then selected for growth in human SCF (which is not able to activate murine c-Kit31 ). All further experiments involved parental MTHC maintained in murine GM-CSF, WTKit cells maintained in human SCF, and V816Kit cells maintained in the absence of added factor.
Proliferative capacity of Kit-expressing MTHC. The growth rates, the average number of cell divisions, and the survival of the selected cell populations in liquid culture are shown in Fig 2A-C, D-F, and G-I, respectively. Like the parental MTHC, the Kit-expressing cells grew in the presence of murine GM-CSF, however, the V816Kit cell population increased at a faster rate than the parental MTHC, while WTKit cells grew at a relatively slower rate (Fig 2A). A possible reason for this is the change in growth factor, as the cells were previously maintained in SCF; although it is probably more likely to be a reflection of prior differentiation of this cell group in SCF (see below and Fig 6). The average number of divisions of the three groups over the course of the assay, were very similar (Fig 2D), so the variation in cell number was primarily due to the great differences in the survival of these cells (Fig 2G). No death of V816Kit cells was seen over the 4-day culture period, while considerable turnover of WTKit and the parental cells was observed. The latter could reflect the requirement for other autocrine factors21 in the culture medium, which were removed by washing the cells before assay. On the other hand, a high rate of death, as well as proliferation, has been observed in cells transformed by another oncogenic transcription factor, c-Myc.32 In medium containing human SCF, the parental MTHC did not survive (Fig 2H), while the WTKit cells proliferated (Fig 2B), but showed rapid turnover, as in GM-CSF (Fig 2H). Figure 2C, F, and I show that in the absence of growth factors, only the V816Kit cells were able to survive and proliferate. The growth rate of these cells was higher in the presence of GM-CSF than without added factors, and GM-CSF could to some extent enhance their proliferation and survival. However, it is important to note that the V816Kit cell populations always doubled at a higher rate than the other cell populations, including the parental cells.
Growth of Kit expressing MTHC in liquid culture. Parental MTHC maintained in murine GM-CSF, WTKit expressing MTHC maintained in human SCF, and V816Kit expressing MTHC maintained without factor were washed and seeded in duplicate wells at 2,000 cells per 200 μL. The upper three panels show the number of viable cells recovered (mean ± standard deviation [SD]) at day 0, 2, and 4 after culture in medium supplemented with (A) murine GM-CSF, (B) human SCF, and (C) no added factor. The middle three panels show the average number of cell divisions of the viable populations in the presence of (D) murine GM-CSF, (E) human SCF, and (F ) no added factor. The bottom three panels show the survival of cells relative to the total viable cells at day 0 in culture containing (G) murine GM-CSF, (H) human SCF, and (I) no added factor.
Growth of Kit expressing MTHC in liquid culture. Parental MTHC maintained in murine GM-CSF, WTKit expressing MTHC maintained in human SCF, and V816Kit expressing MTHC maintained without factor were washed and seeded in duplicate wells at 2,000 cells per 200 μL. The upper three panels show the number of viable cells recovered (mean ± standard deviation [SD]) at day 0, 2, and 4 after culture in medium supplemented with (A) murine GM-CSF, (B) human SCF, and (C) no added factor. The middle three panels show the average number of cell divisions of the viable populations in the presence of (D) murine GM-CSF, (E) human SCF, and (F ) no added factor. The bottom three panels show the survival of cells relative to the total viable cells at day 0 in culture containing (G) murine GM-CSF, (H) human SCF, and (I) no added factor.
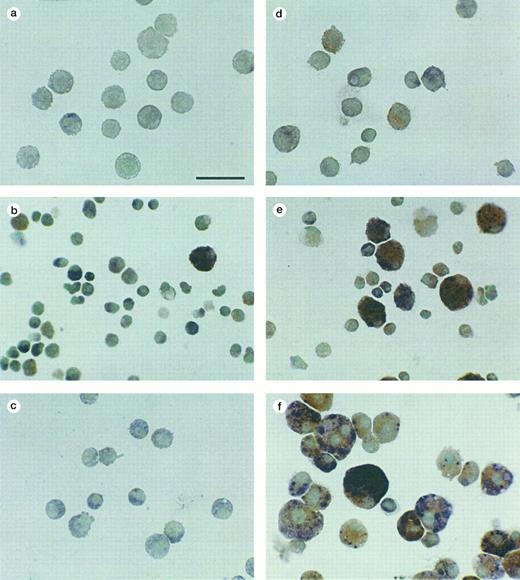
Esterase expression by the different MTHC populations. The cell groups are the same as those shown in Fig 5. Red staining is indicative of α-naphthyl acetate esterase and blue is indicative of naphtol-AS-D-chloroacetate esterase, with methyl green as the counterstain. (a and d) Nonadherent and adherent parental MTHC cultured in murine GM-CSF, (b and e) nonadherent and adherent WTKit-expressing MTHC maintained in human SCF, and (c and f ) nonadherent and adherent V816Kit-expressing MTHC maintained without factor. The bar in (a) is equivalent to 38 μm and all photographs are at the same magnification.
Esterase expression by the different MTHC populations. The cell groups are the same as those shown in Fig 5. Red staining is indicative of α-naphthyl acetate esterase and blue is indicative of naphtol-AS-D-chloroacetate esterase, with methyl green as the counterstain. (a and d) Nonadherent and adherent parental MTHC cultured in murine GM-CSF, (b and e) nonadherent and adherent WTKit-expressing MTHC maintained in human SCF, and (c and f ) nonadherent and adherent V816Kit-expressing MTHC maintained without factor. The bar in (a) is equivalent to 38 μm and all photographs are at the same magnification.
Clonogenic capacity of Kit-expressing MTHC. The Kit-expressing cell populations in liquid cultures were heterogeneous and contained more adherent cells than the parental population; hence, the adherent and nonadherent cells were analyzed separately. Figure 3 shows that the parental MTHC were not able grow in semisolid medium with SCF or without factor, and the WTKit cells were unable to grow without factor, as was the case in liquid culture (see Fig 2). In GM-CSF, the V816Kit cells formed more colonies and the WTKit cells less colonies than the parental cells. The number of colonies formed in all instances by WTKit cells was very low despite the fact that the cells could be maintained indefinitely in liquid culture. Additionally in a separate colony assay, it was observed that unwashed WTKit cells (in the medium used during culture) formed higher numbers of colonies than the same number of cells that were washed before plating (data not shown). This suggested that the WTKit cells required additional factors that could enhance proliferation, possibly produced in an autocrine or paracrine manner. The relative number of colonies formed by the adherent and nonadherent fractions was similar for each of the three populations, but in all cases, the adherent cells formed fewer colonies than the nonadherent cells; this will be discussed later.
Clonogenic growth of Kit expressing MTHC. Cells from the same cultures of parental, WTKit, and V816Kit MTHC as for Fig 2, were plated at 3,000 per dish in methylcellulose containing murine GM-CSF, human SCF, or no factor. The graphs show the average number of colonies ± standard error of mean (SEM) at 2 weeks after plating. (A) Shows the results for nonadherent cells collected from suspension and (B) the results for adherent cells from the same culture, harvested by trypsinization.
Clonogenic growth of Kit expressing MTHC. Cells from the same cultures of parental, WTKit, and V816Kit MTHC as for Fig 2, were plated at 3,000 per dish in methylcellulose containing murine GM-CSF, human SCF, or no factor. The graphs show the average number of colonies ± standard error of mean (SEM) at 2 weeks after plating. (A) Shows the results for nonadherent cells collected from suspension and (B) the results for adherent cells from the same culture, harvested by trypsinization.
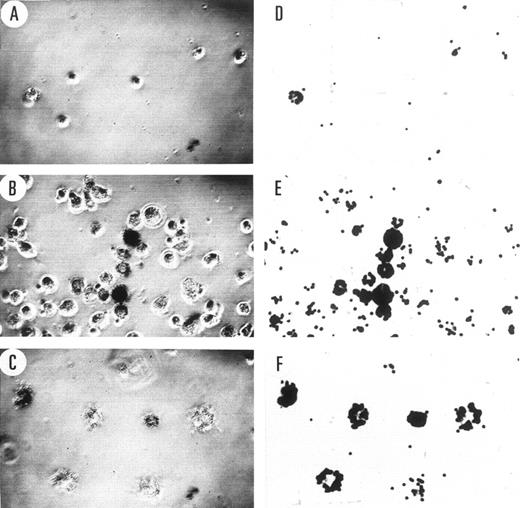
Colony morphology. Visual inspection showed a marked variation in the morphology of the colonies formed by the different cell lines. The relative percentages of the different types of colonies formed by the cell lines are shown in Table 1 and representative examples are shown in Fig 4. Using the criteria previously applied to the analysis of differentiation of myeloid leukemias,33 the compact colonies are suggestive of immature highly proliferative cells, the diffuse colonies of more differentiated cells types such as mature macrophages, and the mixed colonies, a mixture of both mature and immature cells. The nonadherent parental cells formed mainly compact colonies in comparison to the adherent cells, indicating the adherent cells were more differentiated or more readily underwent differentiation. WTKit adherent cells formed very few total colonies (Fig 3) and the majority were of a mixed phenotype (Table 1), together indicating either that most of these cells were differentiated or that they required other factors to proliferate. The majority of nonadherent V816Kit cells formed compact colonies suggesting that the clonogenic cells in the population were immature and highly proliferative, and they were not influenced by the presence of GM-CSF or SCF. The adherent V816Kit cells also formed mostly compact colonies with some variations with the addition of GM-CSF or SCF.
Relative Percentages of the Various Colony Types Formed by the MTHC Cells
| . | % Compact . | % Diffuse . | % Mixed . |
|---|---|---|---|
| Nonadherent cells | |||
| MTHC in GM | 79 | 13 | 9 |
| WTKit in GM | 29 | 24 | 46 |
| WTKit in SCF | 45 | 38 | 17 |
| V816Kit in GM | 96 | 1 | 3 |
| V816Kit in SCF | 87 | 6 | 7 |
| V816Kit without factor | 88 | 3 | 9 |
| Adherent cells | |||
| MTHC in GM | 23 | 27 | 50 |
| WTKit in GM | 8 | 17 | 75 |
| WTKit in SCF | 0 | 0 | 100 |
| V816Kit in GM | 81 | 5 | 14 |
| V816Kit in SCF | 65 | 9 | 26 |
| V816Kit without factor | 68 | 5 | 27 |
| . | % Compact . | % Diffuse . | % Mixed . |
|---|---|---|---|
| Nonadherent cells | |||
| MTHC in GM | 79 | 13 | 9 |
| WTKit in GM | 29 | 24 | 46 |
| WTKit in SCF | 45 | 38 | 17 |
| V816Kit in GM | 96 | 1 | 3 |
| V816Kit in SCF | 87 | 6 | 7 |
| V816Kit without factor | 88 | 3 | 9 |
| Adherent cells | |||
| MTHC in GM | 23 | 27 | 50 |
| WTKit in GM | 8 | 17 | 75 |
| WTKit in SCF | 0 | 0 | 100 |
| V816Kit in GM | 81 | 5 | 14 |
| V816Kit in SCF | 65 | 9 | 26 |
| V816Kit without factor | 68 | 5 | 27 |
The colonies formed in methylcellulose (Fig 3) were scored 3 weeks after plating as compact (shown in Fig 4a), diffuse (shown in Fig 4b), and mixed (shown in Fig 4c and d). The number of halo colonies varied depending on the time after plating and were therefore counted as mixed colonies. The numbers above show the percentage of the different colony types of the total number counted.
Morphology of the MTHC colonies. (a through d) Examples of the various types of colonies observed in methylcellulose. Examples shown are indicative of (a) a compact colony, (b) a diffuse colony, (c) a mixed colony with a compact center, and diffuse cells surrounding and (d) a mixed halo colony with a compact center, diffuse cells radiating outward and a compact ring of cells surrounding it. (e and f ) The presence of giant cells in colonies and, for comparison, in liquid culture of Kit-expressing MTHC. (e) Shows the size comparison of a giant cell colony and a normal-sized compact MTHC colony. (f ) The same comparison in liquid culture with the arrows indicating a nonadherent giant cell in the middle of the photograph and an adherent giant cell to the extreme right.
Morphology of the MTHC colonies. (a through d) Examples of the various types of colonies observed in methylcellulose. Examples shown are indicative of (a) a compact colony, (b) a diffuse colony, (c) a mixed colony with a compact center, and diffuse cells surrounding and (d) a mixed halo colony with a compact center, diffuse cells radiating outward and a compact ring of cells surrounding it. (e and f ) The presence of giant cells in colonies and, for comparison, in liquid culture of Kit-expressing MTHC. (e) Shows the size comparison of a giant cell colony and a normal-sized compact MTHC colony. (f ) The same comparison in liquid culture with the arrows indicating a nonadherent giant cell in the middle of the photograph and an adherent giant cell to the extreme right.
Morphological and phenotypic characteristics of Kit-expressing MTHC. As shown in Fig 4e and f, giant cells were observed when V816Kit cells formed colonies in methylcellulose and when maintained in liquid culture, respectively. However, it should be noted that the examples in Fig 4e illustrate the most extreme difference that was observed between normal and large-sized cells. There was a wide range in cell sizes observed in the Kit-expressing MTHC cell populations. The relative number of the larger cells compared with the smaller progenitor-type cells in the total population was low in liquid culture and in the colonies formed in methylcellulose. The majority of the large to giant cells in liquid culture were adherent and an increase in the proportion of adherent cells was observed as the cultures became more dense. With prolonged culture of the adherent population, the larger cells were found to increase in size and granularity, with many displaying large vacuoles, often encompassing smaller cells or cell debris.
Cytocentrifuge smears of the different cell populations were stained with Giemsa to show the cellular morphology. The photographs shown in Fig 5 are representative of the proportions of the various phenotypes of cells in the total population. The parental cells were mainly nonadherent (on average 88%) and were of an immature myeloid phenotype (Fig 5a), while the small adherent fraction consisted of differentiated macrophages and neutrophils (Fig 5d). This was also the case with parental MTHC infected with the vector alone. The nonadherent WTKit cells (Fig 5b) were extremely heterogeneous with the majority of cells being similar to the parent type; most of the others were smaller in size and resembled mature neutrophils at various stages of differentiation. A few large cells displayed features of mature macrophages showing lighter stained areas in the cytoplasm. The adherent population (10% to 33%) of WTKit cells contained a higher proportion of these large cells, as well as some small neutrophilic cells (Fig 5e). The nonadherent factor-independent V816Kit cells were a largely homogeneous population of immature myeloid progenitor cells closely resembling the parental MTHC (Fig 5c), but contained the occasional large cell with very obvious internal vacuoles, which sometimes contained other cells, as seen in Fig 4f. There was an increase in the number of large to giant cells with other cells bound to them in the adherent fraction of V816Kit cells (Fig 5f ). The proportions of nonadherent and adherent V816Kit cells in culture varied dramatically with changes in the cell density, with a marked increase in adherent cells at high density (data not shown).
Morphology of nonadherent (a through c) and adherent (d through f ) populations derived from liquid culture. Cytocentrifuge preparations were stained with Wright-Giemsa. The fields of view shown are representative of the total cell populations. (a and d) Nonadherent and adherent parent MTHC cultured in murine GM-CSF; (b and e) nonadherent and adherent WTKit-expressing MTHC maintained in human SCF; (c and f ) nonadherent and adherent V816Kit-expressing MTHC maintained without factor. The bar in (a) is equivalent to 38 μm and all photographs are at the same magnification.
Morphology of nonadherent (a through c) and adherent (d through f ) populations derived from liquid culture. Cytocentrifuge preparations were stained with Wright-Giemsa. The fields of view shown are representative of the total cell populations. (a and d) Nonadherent and adherent parent MTHC cultured in murine GM-CSF; (b and e) nonadherent and adherent WTKit-expressing MTHC maintained in human SCF; (c and f ) nonadherent and adherent V816Kit-expressing MTHC maintained without factor. The bar in (a) is equivalent to 38 μm and all photographs are at the same magnification.
Lineage characterization by esterase staining. The expression of lineage-specific esterases by the cells is shown in Fig 6; in which the fields of view were chosen to illustrate most of the different cell types that were observed on enzyme staining. The α-naphthyl acetate esterase is normally expressed by mature macrophages and the naphthol-AS-D-chloroacetate esterase by mature neutrophils.30 The nonadherent parent MTHC did not express either esterase, consistent with their immature state. The strong esterase staining by a proportion of the Kit-expressing cells indicated that compared with the parental MTHC, they were more mature and the cell populations were more heterogeneous. The large to giant cells seen in the adherent fractions were macrophage-like cells, but contained various levels of naphthol-AS-D-chloroacetate esterase. Enzyme staining separately for the two esterases showed that some large cells expressed high levels of both esterases and this double staining is probably the cause of the black coloring observed (Fig 6f ) when the stains were done consecutively. It was not clear if these cells were bilineage cells or if they were macrophages that had acquired the neutrophil esterase by phagocytosing other (neutrophilic) cells. The presence of these large cells in the V816Kit population, as well as in the WTKit population maintained in SCF, indicated that signalling through Kit resulted in maturation of proliferating myeloid cells.
Phagocytic capacity. The ability of the adherent cells to phagocytose fluorescent beads is shown in Fig 7. The parental MTHC showed minimal phagocytic ability except for a few spontaneously differentiated mature macrophages. The WTKit cells again were extremely heterogeneous and ranged from no phagocytic ability to very high, with no obvious correlation between cell size and phagocytic capacity. Only the giant cells in the V816Kit population remained adherent after washing and all showed phagocytic ability. Some of these cells already contained other cells and debris within them, and it is possible that this may have compromised the extent of phagocytosis of the beads.
Phagocytosis of fluorescent beads by adherent MTHC. The images in the two columns are of the same field of view as observed by phase-contrast microscopy shown on the left (A through C) and fluorescence microscopy shown on the right (D through F ). The cells can be seen in the images on the left and the phagocytosed fluorescent beads on the right. The number of the cells in the field of view is indicative of the proportion of cells that were strongly adherent to glass in cultures of similar cell density. (A and D) Show parental MTHC, (B and E) show WTKit-expressing MTHC, and (C and F ) show V816Kit-expressing MTHC. The cells were observed at an initial magnification of 40×.
Phagocytosis of fluorescent beads by adherent MTHC. The images in the two columns are of the same field of view as observed by phase-contrast microscopy shown on the left (A through C) and fluorescence microscopy shown on the right (D through F ). The cells can be seen in the images on the left and the phagocytosed fluorescent beads on the right. The number of the cells in the field of view is indicative of the proportion of cells that were strongly adherent to glass in cultures of similar cell density. (A and D) Show parental MTHC, (B and E) show WTKit-expressing MTHC, and (C and F ) show V816Kit-expressing MTHC. The cells were observed at an initial magnification of 40×.
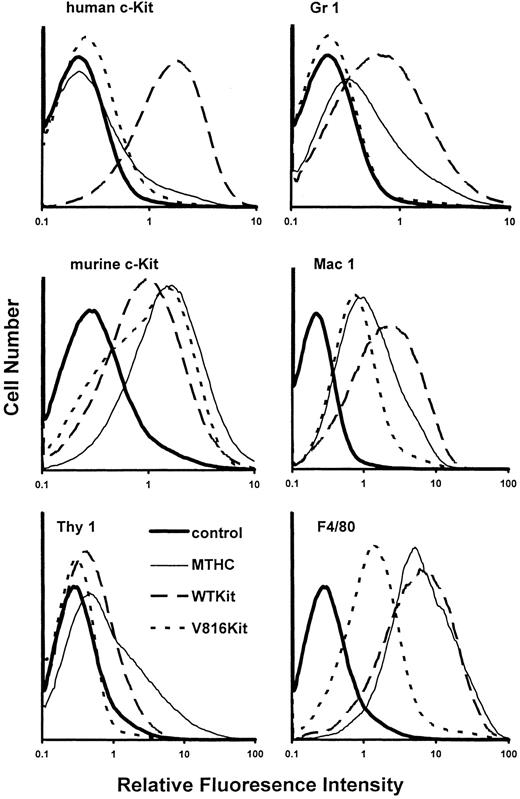
Cell surface marker analysis. Further characterization of the MTHC by immunofluorescence is shown in Fig 8. Cell surface expression of human Kit by the different cell populations was determined using MoAb 1DC3. Human Kit was expressed by all WTKit cells, but was not detectable on the surface of V816Kit cells. Low surface expression of this mutant form of murine Kit was also observed in other cell types12 and was thought to be due to its downregulation caused by continuous degradation.34 A low level of surface expression of constitutively phosphorylated mutant c-Fms receptors, again due to receptor degradation, has also been previously observed.35 Therefore, it is likely that our failure to detect V816Kit surface expression was due to downregulation caused by degradation of V816Kit in MTHC. This was investigated further by immunocytochemistry (see below and Fig 9).
Cell surface marker expression. The histograms show the relative surface expression profiles of the indicated cell surface antigens by nonadherent parental MTHC cultured in murine GM-CSF, WTKit-expressing MTHC maintained in human SCF, and V816Kit-expressing MTHC maintained without factor. The negative control shown in each panel corresponds to parental MTHC labeled with the secondary antibody used in each case and is representative of background.
Cell surface marker expression. The histograms show the relative surface expression profiles of the indicated cell surface antigens by nonadherent parental MTHC cultured in murine GM-CSF, WTKit-expressing MTHC maintained in human SCF, and V816Kit-expressing MTHC maintained without factor. The negative control shown in each panel corresponds to parental MTHC labeled with the secondary antibody used in each case and is representative of background.
Expression of human Kit as detected by APAAP (A through C) and tumor histopathology (D through H). The red color shown in the photographs in (A through C) indicates the presence of human Kit protein detected with IDC3 antibody on parental MTHC (A), WTKit-expressing MTHC (B), and V816Kit-expressing MTHC (C). The parental cells do not express human c-Kit and, therefore, (A) represents a negative control. The bar in (C) represents 15 μm and provides the scale for (A, B, and C). The right panel shows photographs of tumor sections. Photographs (D, E, and F ) show hematoxylin and eosin staining of a paraffin-embedded tumor section and (G and H) show esterase expression in frozen sections. (D) Shows a region through the center of the vascularized tumor, (E and F ) show invasion of the tumor cells into surrounding fat tissue and a blood vessel, respectively. (G and H) Show regions of staining for predominantly macrophage or neutrophil esterase, respectively. The arrows in photograph (G) indicate the larger cells showing phagocytosis of other cells. The bar in (D) represents 38 μm and provides the scale for (D) through (H).
Expression of human Kit as detected by APAAP (A through C) and tumor histopathology (D through H). The red color shown in the photographs in (A through C) indicates the presence of human Kit protein detected with IDC3 antibody on parental MTHC (A), WTKit-expressing MTHC (B), and V816Kit-expressing MTHC (C). The parental cells do not express human c-Kit and, therefore, (A) represents a negative control. The bar in (C) represents 15 μm and provides the scale for (A, B, and C). The right panel shows photographs of tumor sections. Photographs (D, E, and F ) show hematoxylin and eosin staining of a paraffin-embedded tumor section and (G and H) show esterase expression in frozen sections. (D) Shows a region through the center of the vascularized tumor, (E and F ) show invasion of the tumor cells into surrounding fat tissue and a blood vessel, respectively. (G and H) Show regions of staining for predominantly macrophage or neutrophil esterase, respectively. The arrows in photograph (G) indicate the larger cells showing phagocytosis of other cells. The bar in (D) represents 38 μm and provides the scale for (D) through (H).
The early markers, Thy1 and murine c-Kit, found on progenitor cells were analyzed, as it was shown previously that the level of surface expression of Thy1 and murine c-Kit varied on MTHC with time in culture.20 The parental MTHC were maintained for 2 months in GM-CSF before the introduction of Kit and were shown to be heterogeneous in their expression of both markers. The results in Fig 8 show that introduction of human Kit resulted in a decrease in expression of Thy1 and murine c-Kit compared with the parental MTHC, suggesting that the Kit-expressing populations were more mature. The WTKit cells also showed increased levels of mature markers of the granulocyte lineage (Gr1) and the monocytic/marophage lineage (Mac1 and F4/80 antigen), compared with the other cells, again indicating that they were more differentiated. The levels of these markers on V816Kit cells were low; however, it should be noted that the results shown in Fig 8 illustrate expression only by the nonadherent cells. The low numbers, variation in size of the adherent cells, and fragility of the giant cells made it difficult to analyze them using flow cytometry. The results that were obtained indicated that in most cases, the profiles were similar to the nonadherent cells, except for subpopulations of adherent V816Kit cells, which showed high levels of Gr1 and F4/80 antigen expression. This indicated that there was a greater difference in the nonadherent and adherent populations of the V816Kit cells than the other MTHC, in agreement with the results shown in Figs 5 and 6.
Despite the fact that the V816Kit cells did not express any detectable cell surface human Kit, they were able to survive without factor, unlike parental MTHC and WTKit MTHC. To confirm the expression of human Kit in these cells, immunocytochemical staining was used to detect both cell surface and intracellular Kit protein. The results of immunostaining with MoAb 1DC3 using the APAAP method of detection for total human Kit protein expression are shown in Fig 9A through C. The parental cells did not express human c-Kit (the light staining is background). The WTKit cells have high levels of total Kit protein, but the V816Kit cells have low and, in some cases, undetectable levels, which is again consistent with rapid turnover of activated Kit protein.
Tumorigenic potential. Aliquots of 2 × 106 nonadherent cells per mouse of either parental MTHC, WTKit MTHC, or V816Kit MTHC were injected subcutaneously into groups of four syngeneic (CBA) mice. The mice were monitored for the development of tumors for up to 3 months. Neither the parental MTHC nor the WTKit cells gave rise to tumors in any of the mice. In contrast, the V816Kit produced visible tumors after 2 weeks in three mice, with the fourth mouse in this group displaying a tumor at 6 weeks after injection. Analysis of this late-arising tumor showed that it was partly intradermal and partly subcutaneous. All tumors were excised and fresh frozen or formalin-fixed for phenotypic analysis or dispersed to obtain tumor cells that were cultured in vitro. The tumor-derived cells were able to proliferate in the absence of factor in vitro and were able to generate heterogeneous populations containing large adherent cells similar to those shown in Fig 5f. Sections of the tumor obtained from paraffin-embedded tumor masses were stained with hematoxylin and eosin to show the morphology of the cells and are shown in Fig 9D. The cells show nuclear characteristics similar to malignant histiocytes, with many cells undergoing mitosis. The cells were found to invade surrounding muscle (not shown) and fat tissue (Fig 9E), as well as blood vessels (Fig 9F ) of the mouse. Sections of fresh frozen tumor were stained for esterases. Variable staining was observed with areas positive for expression of α-naphyl–esterase containing a few strong staining larger cells (Fig 9G) and other areas positive staining for naphthol-AS-D-chloroacetate esterase expression (Fig 9H). This indicated that the tumors formed by V816Kit MTHC consisted of granulocyte and macrophage cells at various stages of differentiation, thus resembling the cell populations growing in vitro.
DISCUSSION
Expression of constitutively activated Kit was shown previously to result in factor independence and tumorigenicity of myelomonocytic FDC-P1 cells12 and to induce a differentiated phenotype in the IC-2 mast cell line.13 Myb-transformed hematopoietic cells (MTHC) represent a good model for investigating the effects of activated Kit in myeloid progenitor cells. Like FDC-P1, MTHC lines are factor-dependent, show characteristics of early myeloid progenitor cells, are highly proliferative and clonogenic (reviewed in Gonda19 ), but in contrast, have a known immortalizing event and are also capable of differentiating under some conditions.22 Additionally, in MTHC (that usually express low levels of endogenous c-Kit) SCF was shown to be able to act synergistically with other growth factors22, as in normal progenitor cells, implying that they could respond to Kit signals. Our results show that continuous overexpression of human c-Kit (WT) or an activated Kit mutant (V816) in MTHC allowed growth in the presence of human SCF alone or in the absence of factor, respectively. This allowed us to observe the effects of WTKit activated by SCF and mutant Kit in early myeloid cells. There were potentially two important comparisons: (1) the difference between the effects of Kit activity compared with GM-CSF activity in MTHC and (2) the difference between WTKit activated by SCF and the constitutively activated V816Kit mutant.
Both WTKit plus SCF and V816Kit were able to provide a proliferative signal in MTHC in the absence of GM-CSF. Apart from proliferation of MTHC, Kit activation was also able to induce phenotypic changes. Introduction of either WTKit (plus SCF ) or V816Kit resulted in more heterogeneous populations of cells with an increase in the number of large to giant adherent cells. These cells were shown by several criteria to be mature differentiated cells of the macrophage lineage with phagocytic activity. They were not detected in a parental MTHC population grown in GM-CSF, indicating that Kit activity in MTHC produced some different signals to GM-CSF. This differentiation and maturation phenomenon was only observed in a proportion of the cells, however, clonal cell populations obtained from the different types of colonies in methylcellulose (see Table 1) all gave rise to heterogeneous cell populations in culture containing mature adherent cells (data not shown). Therefore, the phenotypic heterogeneity in the cell populations in liquid culture and in colony assays was probably due to intrinsic properties that induced activation of differentiation pathways and not a result of heterogeneity in the initial cell population. While the mechanism underlying differentiation of MTHC is not yet understood, these cells may provide a useful model for investigating differentiative signals induced by Kit activity in myeloid cells.
The other important observation was the difference between WTKit (plus SCF ) and V816Kit activity in MTHC. WTKit (plus SCF ) induced more differentiated cells with a decreased growth rate and lower clonogenic potential than parental MTHC, whereas V816Kit induced mixed populations of immature and mature cells with increased survival and growth rates and clonogenic potential. This indicated that the mutant Kit was able to provide a maturation effect in a proportion of MTHC, but this was not linked with decrease in proliferation of the majority of the population. A possible reason for this could lie in differences between the activities of WTKit (plus SCF ) and V816Kit in MTHC. It was recently shown that expression of the corresponding murine mutant, D814YKit, in the mast cell line, IC-2, resulted in degradation of the phosphatase PTP1C,36 also known as HCP, SHP1, and SHPTP1, which is the product of the motheaten (me) gene. This phosphatase is a negative regulator of Kit37 and IL-3 receptor38 signaling and is also known to interact with Grb2, mSos1, and Vav.39 Degradation of this phosphatase is also likely to occur in V816Kit-expressing MTHC and would result in loss of negative regulation of many of PTP1C substrates. As many of the substrates, including Kit itself, are hematopoietic growth factor receptors, this would result in enhanced activity of all these proteins and may be a contributing factor for the high growth capacity observed in phenotypically mature V816Kit-expressing MTHC populations. The murine form of this mutant was also found to exhibit altered sites of autophosphorylation and altered substrate specificity,36 which may also contribute to the differences observed between WTKit + SCF and V816Kit.
The expression of V816Kit in MTHC resulted in the generation of heterogeneous cell populations in vitro in the absence of factor, as well as in the production of tumors. In both cases, the cells were at various stages of differentiation with some cells of giant size displaying phagocytosis of other smaller cells. The giant cells grown in vitro phenotypically resembled histiocytes due to their vacuolated cytoplasm and binucleation. Additionally, the tumor cells showed histopathologic features similar to the malignant cells in murine malignant histiocytosis induced by a transforming murine retrovirus carrying the Ha-ras oncogene.40 Taken together, our results show that in the presence of an immortalizing agent, here provided by activated Myb, V816Kit provides strong survival, proliferative, and differentiative stimuli.
Unlike other mature cells of hematopoietic origin, human mast cells express high levels of Kit41 and in mice, functional Kit is critical for mast cell development.6 Correspondingly, activating mutations in Kit have been identified in human8,14 and rodent10,11 malignant mast cells. The Kit ligand SCF is also an important growth and differentiation factor for early hematopoietic cells,6 however, activating mutations of Kit have not been reported in corresponding malignant cells, such as AML. Additionally, only one patient sample (AML-M2) of 33 cases examined in our laboratory was positive for the presence of the V816 mutation in Kit (S.R. Cole and L.K. Ashman, unpublished data). The results reported here suggest an explanation for this finding in that V816Kit provided a strong differentiative, as well as proliferative and survival stimulus for early myeloid cells. Thus, similar mutant forms of Kit may be associated with myeloid malignancies of other differentiated types, as well as those of mast cells.
In this light, it is interesting to note that there have been two reports of patients showing evidence of histiocyte-like cells linked with leukemia. This has lead to a proposal for the classification of a new type of acute histiocytic leukemia, AML-M5c,42 and as acute monocytic leukemia with histiocytic differentiation.43 These histiocyte-like cells contain large, often kidney-shaped nuclei, with broad irregular membranes, granular chromatin, and prominent nucleoli, features similar to the nuclei of the tumor cells shown in Fig 9D. Also, it has been proposed that these diseases are caused by the proliferation of activated macrophages/histiocytes,42 a phenomenon we have observed in clonogenic assays of V816Kit MTHC (Fig 4e). Therefore, the histiocyte-like giant cells we have observed in vitro and in vivo that were derived from cell populations expressing oncogenic Myb and oncogenic Kit may be of particular relevance in investigating these types of leukemia. It will be important to test such malignant cells for mutations in c-kit and other synergistically acting oncogenes such as myb.
ACKNOWLEDGMENT
We thank Paul Sincock for assistance with confocal microscopy, Kathy Janz, Division of Histopathology, Institute of Medical and Veterinary Science, Adelaide, Australia, for tissue sectioning, Gaby Aylett for assistance with FACS analysis, and Tony Cambareri for assistance with FACS analysis, presentation of figures and graphs. Growth factors GM-CSF and SCF were kindly provided by Dr Tracy Wilson (Walter and Eliza Hall Institute, Melbourne, Australia) and Dr Ian McNiece (Amgen, Thousand Oaks, CA) and monoclonal antobodies by Dr Andreas Strasser and Dr Ivan Bertoncello.
Supported by grants from the National Health and Medical Research Council (NHMRC), Canberra, Australia. P.F. is an Australian Postgraduate Research Award Scholar and L.K.A. and T.J.G. are NHMRC Senior Research Fellows.
Address reprint requests to Leonie K. Ashman, PhD, Leukaemia Research Unit, Hanson Centre for Cancer Research, Institute of Medical and Veterinary Science, Box 14 Rundle Mall PO, Adelaide, SA 5000, Australia.


![Fig. 2. Growth of Kit expressing MTHC in liquid culture. Parental MTHC maintained in murine GM-CSF, WTKit expressing MTHC maintained in human SCF, and V816Kit expressing MTHC maintained without factor were washed and seeded in duplicate wells at 2,000 cells per 200 μL. The upper three panels show the number of viable cells recovered (mean ± standard deviation [SD]) at day 0, 2, and 4 after culture in medium supplemented with (A) murine GM-CSF, (B) human SCF, and (C) no added factor. The middle three panels show the average number of cell divisions of the viable populations in the presence of (D) murine GM-CSF, (E) human SCF, and (F ) no added factor. The bottom three panels show the survival of cells relative to the total viable cells at day 0 in culture containing (G) murine GM-CSF, (H) human SCF, and (I) no added factor.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/11/10.1182_blood.v90.11.4539/3/m_bl_0028f2.jpeg?Expires=1765897187&Signature=ebAEEcXz3-eTOv5MECFNnZpb8dJxUR-ISJ6tupOIzlXJ3jh-CQ2R9-7IKndEd~nTGISO3pc3ZGcXEJ66Ly1iW1U6sJNKCqm8LCzWGIKtwzOo54JbcoWtT8-GFQj7JlziLKEbfTOccDCHubZuPoRUXsplfnGfTXfyUkvGIwE3XyUKcDTKfcHviKkxCuGCOcT4MLJRihqSbPgEHnfD14vZi2XBYk8-1i729266H~p1uOKjsqeMNgnMzx3FtZPACgXZ5vKAWN4UQQcVwJl9JX2nxwN7pS29tCY00sjLKQR8udiMI579s7nbQpkPgHZ1FlpPlabtRTZ8Tv7MBms8QHEsew__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal