Abstract
Epstein-Barr virus (EBV) latent infection in B cells persists over years or decades despite a sustained cytotoxic immune response to viral antigens. We present data that methylated EBV DNA can be detected in the normal lymphocytes of healthy volunteers. Whereas methylation of foreign DNA has been recognized as a potential cellular defense mechanism, methylation of EBV DNA may be an essential part of the virus life cycle in vivo, explaining the persistence of virus-infected B cells in the face of immune surveillance. Methylation of the C promoter helps to prevent expression of the immunodominant antigens expressed from this promoter. First recognized in tumors, methylation-associated evasion of immune surveillance is not an aberration restricted to tumor tissue but is detected in normal EBV-infected lymphocytes. Methylation of the viral genome in latency also provides an explanation for the CpG suppression associated with EBV but not other large DNA viruses.
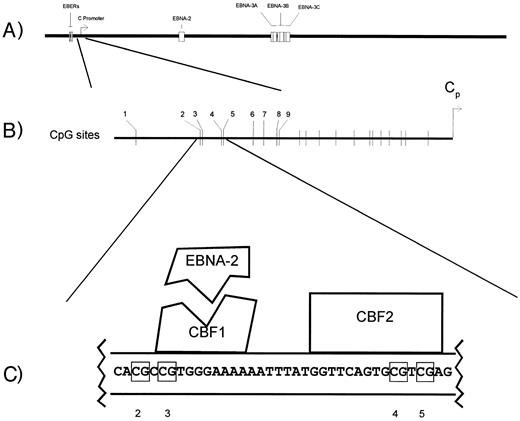
IN VITRO INFECTION of peripheral blood mononuclear cells (PBMCs) under appropriate conditions generates latently infected B-lymphoblastoid cell lines.1 These Epstein-Barr virus (EBV)-immortalized latently infected lymphoblastoid cell lines express 11 EBV latency genes. Among the proteins expressed by these lymphoblastoid cell lines are the immunodominant EBV nuclear antigens (EBNA-2, -3A, -3B, and -3C) recognized by CD8(+) cytotoxic T cells on a range of HLA backgrounds.2,3 The transcripts for these antigens originate from the major latency promoter, the C promoter (Cp) (Fig 1A). This promoter is silent in EBV(+) Hodgkin's and Burkitt's lymphomas and these immunodominant antigens are not expressed.4-6 The restricted pattern of viral antigen expression in Hodgkin's and in Burkitt's lymphoma is thought to be important for tumor survival insofar as the cytotoxic T-cell response to these proteins is maintained for years and might be expected to eliminate cells expressing these proteins in vivo. C promoter silence is at least in part a function of CpG methylation of a region upstream of the C promoter in the EBNA-2 response region.7,8 Two sequence-specific cellular DNA binding activities footprint in this region.9,10 The first corresponds to CBF1, which interacts with the viral protein EBNA-2 to facilitate activation of the C promoter (Fig 1C). The second corresponds to CBF2. The in vitro binding activity of CBF2 but not CBF1 is inhibited by CpG methylation.7 Sensitivity to methylation-mediated transcriptional repression may be explained in part by inhibition of CBF2 binding.
Aspects of the EBV genome and C promoter regulation. (A) The linear EBV genome. (B) CpG sites upstream of the C promoter whose methylation status was monitored by genomic sequencing (nos. 1 through 9). (C) The EBNA-2 responsive region of the C promoter. The schematic illustrates the nucleotides protected by CBF1 and CBF2 in DNAse protection assays and the interaction of EBNA-2 with CBF1.
Aspects of the EBV genome and C promoter regulation. (A) The linear EBV genome. (B) CpG sites upstream of the C promoter whose methylation status was monitored by genomic sequencing (nos. 1 through 9). (C) The EBNA-2 responsive region of the C promoter. The schematic illustrates the nucleotides protected by CBF1 and CBF2 in DNAse protection assays and the interaction of EBNA-2 with CBF1.
Aberrant methylation of cellular genes and their transcriptional regulatory regions is a well-recognized phenomenon in tumors and tumor cell lines.11 Evidence has recently emerged suggesting that methylation of transcriptional regulatory regions of tumor suppressor genes may be a step in multistep tumorigenesis akin to deletion or mutation of tumor-suppressor genes.12 Methylation of viral promoters suppressing transcription of immunodominant antigens may be another way in which methylation in tumors contributes to tumorigenesis.4 Because primary infection of B lymphocytes in vitro leads to the establishment of EBV-infected B-cell lines in which the viral genome is not methylated, there has been a presumption that methylation of the viral genome was a phenomenon confined to tumors.13,14 However, in light of evidence suggesting that some features of Burkitt's lymphoma, including silence of the C promoter, might reflect the phenotype of latently infected normal lymphocytes in vivo,15-17 we wished to determine whether methylation of the C promoter might also play a role in protecting EBV-infected peripheral blood lymphocytes from immune surveillance.
We wished to determine whether EBV DNA in PBMCs in vivo is methylated. The frequency of EBV-infected cells in PBMCs from healthy seropositives is less than 1/50,000 by virtually all estimates.15 17 EBV genomes in PBMCs are therefore too rare to have their CpG methylation status determined by conventional Southern blot hybridization techniques using methylation-sensitive and -insensitive isoschizomers. The bisulfite genomic sequencing technique yields information about CpG methylation and involves a polymerase chain reaction (PCR) amplification step. We have previously applied this technique to the analysis of the EBV Cp in tumors. By virtue of the PCR amplification step, we thought this technique might allow the analysis of EBV methylation in PBMCs.
MATERIALS AND METHODS
PBMCs and cell lines. Peripheral blood was collected from healthy volunteers at Johns Hopkins according to a protocol approved by the institutional human investigations review boards. Mononuclear cells were isolated by density gradient centrifugation in Ficoll-Hypaque. Rael and Akata are EBV-associated Burkitt's-derived cell lines. B95-8 is an EBV-immortalized lymphoblastoid B-cell line. These lines are maintained in RPMI1640 with 10% fetal calf serum. DNA was isolated by standard techniques involving sodium dodecyl sulfate, proteinase K, phenol, and phenol-chloroform extraction.18
Genomic sequencing. Genomic sequencing was performed on extracted DNA using the bisulfite treatment method, with minor modifications.7,19 Briefly, 1 to 10 μg of genomic DNA was digested with EcoRI, alkali-denatured, neutralized, precipitated, and treated with sodium bisulfite/hydroquinone. After desalting procedures (Wizard DNA Cleanup System; Promega, Madison, WI), PCR was performed and the amplification products were cloned and sequenced. The sequence of the region was also determined without bisulfite modification using the same procedure of PCR followed by cloning. DNA that had incompletely reacted with bisulfite could be recognized by the persistence of cytosines (C's) in the C lane, even in the absence of CpG methylation sites. Such incompletely reacted DNA was not further analyzed. The primers used have been previously described and include the CBF1 and CBF2 binding sites within the EBNA-2 response element of the C promoter.7 As is standard in this technique, the primers bind to regions without CpG sequences and therefore there is no bias during PCR amplification.
Southern blot hybridization. Genomic DNA samples (5 to 10 μg) prepared after drug treatment were digested with a 10-fold excess of BamHI and Hpa II or BamHI and Msp I (GIBCO-BRL, Gaithersburg, MD) according to the manufacturer's instructions. After digestion, samples were electrophoresed in a 1.5% agarose gel, transferred to nylon membrane (Biotrans; ICN, Irvine, CA), and probed with nick-translated EBV BamHI-C probe (pSL93).20 All quantitative determinations were performed using a Molecular Dynamics Phosphorimager (Molecular Dynamics, Sunnyvale, CA).
RESULTS
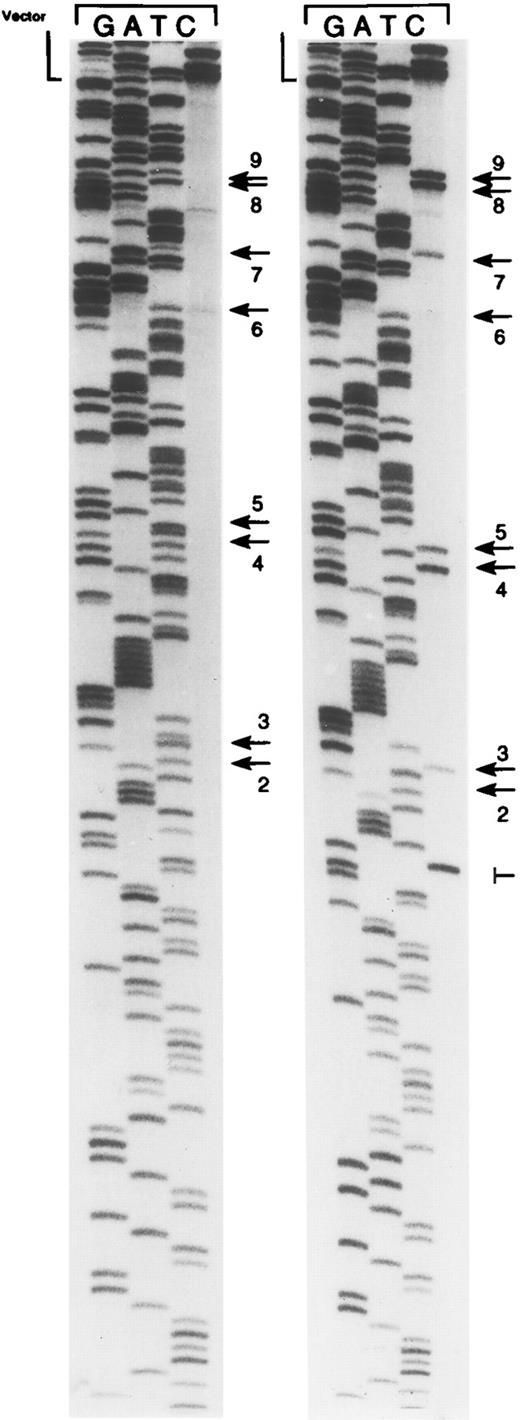
Bisulfite genomic sequencing involves treatment of genomic DNA with sodium bisulfite leading to the conversion of cytosine to uracil. 5-Methylcytosine does not react under these conditions. Bisulfite treatment results in two strands of DNA that are no longer complementary and that can be differentially amplified by PCR. The ability to amplify viral DNA from DNA extracted from PBMCs is a prerequisite for the analysis of methylation patterns. We studied DNA extracted from PBMCs from seven healthy volunteers. Six volunteers were EBV-seropositive and one was seronegative. Using primers to the Cp region of the EBV genome, amplification was successful in four of the seropositive volunteers. Amplification was not detected from the seronegative volunteer or from two of the seropositive donors. Five to six cloned plasmid inserts were sequenced by the dideoxy technique from each donor where amplification yielded an appropriate product (Fig 2 and Table 1). A total of 22 cloned inserts were sequenced and the methylation status at each of 9 CpG sites was analyzed. Of a total of 198 sites examined, 96 (48%) were methylated. In previous investigations, we have suggested that methylation of a particular CpG site (site no. 4) might play a crucial role in the regulation of the C promoter by blocking the binding of CBF2, a cellular DNA binding activity associated with transcriptional activation. This site was methylated in 11 of 22 (50%) cloned inserts. Site no. 2 was usually not methylated (6 of 22). Almost half of the cloned inserts (10 of 22 [45%]) showed a complete absence of methylation.
Representative bisulfite sequencing autoradiogram showing cloned inserts of PCR-amplified bisulfite-treated DNA from PBMCs from donor 2. Examples of cloned inserts with and without methylation of CpG sites in the EBNA-2 responsive region of the C promoter are shown. Arrows indicate CpG dinucleotides. ‖─, a Taq polymerase error. Unmethylated CpG sites are displayed as TpG as a result of the bisulfite treatment, whereas methylated CpG sites are displayed as CpG. CpG sites are numbered as in Table 1. Site no. 1 is not shown. The vector/insert boundary is denoted as vector.
Representative bisulfite sequencing autoradiogram showing cloned inserts of PCR-amplified bisulfite-treated DNA from PBMCs from donor 2. Examples of cloned inserts with and without methylation of CpG sites in the EBNA-2 responsive region of the C promoter are shown. Arrows indicate CpG dinucleotides. ‖─, a Taq polymerase error. Unmethylated CpG sites are displayed as TpG as a result of the bisulfite treatment, whereas methylated CpG sites are displayed as CpG. CpG sites are numbered as in Table 1. Site no. 1 is not shown. The vector/insert boundary is denoted as vector.
Summary of Genomic Sequencing
| . | Cloned Insert . | CpG Site No. . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . |
| Donor 1 | 1 | − | − | − | − | − | − | − | − | − |
| 2 | − | − | − | − | − | − | − | − | − | |
| 3 | − | − | − | − | − | − | − | − | − | |
| 4 | + | + | − | + | + | + | + | + | + | |
| 5 | − | − | − | − | − | − | − | − | − | |
| 6 | + | + | − | + | + | + | + | + | + | |
| Donor 2 | 1 | + | − | + | + | + | + | + | + | + |
| 2 | − | − | − | − | − | − | − | − | − | |
| 3 | − | − | − | − | − | − | − | − | − | |
| 4 | + | − | + | + | + | − | + | + | + | |
| 5 | − | − | − | − | − | − | − | − | − | |
| Donor 3 | 1 | + | + | + | + | + | + | + | + | + |
| 2 | − | − | − | − | − | − | − | − | − | |
| 3 | − | − | − | − | − | − | − | − | − | |
| 4 | + | + | + | + | − | + | + | + | + | |
| 5 | + | + | + | + | + | + | + | + | + | |
| Donor 4 | 1 | + | − | + | + | + | + | + | + | + |
| 2 | + | − | + | + | + | + | + | + | + | |
| 3 | + | − | + | + | + | + | + | + | + | |
| 4 | − | − | − | − | − | − | − | − | − | |
| 5 | − | + | + | − | + | + | + | + | + | |
| 6 | + | − | + | + | + | + | + | + | + | |
| . | Cloned Insert . | CpG Site No. . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . |
| Donor 1 | 1 | − | − | − | − | − | − | − | − | − |
| 2 | − | − | − | − | − | − | − | − | − | |
| 3 | − | − | − | − | − | − | − | − | − | |
| 4 | + | + | − | + | + | + | + | + | + | |
| 5 | − | − | − | − | − | − | − | − | − | |
| 6 | + | + | − | + | + | + | + | + | + | |
| Donor 2 | 1 | + | − | + | + | + | + | + | + | + |
| 2 | − | − | − | − | − | − | − | − | − | |
| 3 | − | − | − | − | − | − | − | − | − | |
| 4 | + | − | + | + | + | − | + | + | + | |
| 5 | − | − | − | − | − | − | − | − | − | |
| Donor 3 | 1 | + | + | + | + | + | + | + | + | + |
| 2 | − | − | − | − | − | − | − | − | − | |
| 3 | − | − | − | − | − | − | − | − | − | |
| 4 | + | + | + | + | − | + | + | + | + | |
| 5 | + | + | + | + | + | + | + | + | + | |
| Donor 4 | 1 | + | − | + | + | + | + | + | + | + |
| 2 | + | − | + | + | + | + | + | + | + | |
| 3 | + | − | + | + | + | + | + | + | + | |
| 4 | − | − | − | − | − | − | − | − | − | |
| 5 | − | + | + | − | + | + | + | + | + | |
| 6 | + | − | + | + | + | + | + | + | + | |
Abbreviations: +, methylated CpG site; −, unmethylated CpG site.
We also investigated the methylation status of another locus within the BamHI-C fragment of the viral genome, the EBER region. Two genes, EBER-1 and -2, are transcribed by RNA polymerase III and expressed in abundance in latently infected Burkitt's cell lines. Genomic sequencing in a Burkitt's cell line (Rael) showed that, in contrast to the C promoter region that is consistently methylated, there was no methylation in the EBER region (data not shown). This confirms studies using methylation-sensitive and -insensitive restriction enzymes, suggesting that this region of the genome is not methylated.21
We have compared the results of the bisulfite PCR technique as applied to analysis of the EBV Cp with restriction enzyme-based Southern blot techniques and found a close correspondence in EBV cell lines. Thus, using restriction enzyme-Southern blot techniques, the Cp region of Rael (a Burkitt's cell line that is tightly latent) is entirely methylated, that of B95-8 (a lymphoblastoid cell line) is entirely unmethylated, and that of Akata (a Burkitt's cell line with active lytic infection) is a mixture of methylated and unmethylated DNA (data not shown). Using the bisulfite PCR technique, 7 of 7 Rael Cp region clones were completely methylated, 7 of 7 B95-8 region clones were completely unmethylated, and 4 of 8 Akata Cp region clones were completely methylated, whereas 3 were completely unmethylated and 1 was partially methylated. Furthermore, after treatment with inhibitors of DNA methyltransferase such as 5-azacytidine, changes in methylation pattern as determined by genomic sequencing parallel changes in methylation pattern as determined by Southern blot hybridization (K.D.R. and R.F.A., manuscript submitted).
DISCUSSION
We have shown that methylation of the EBV genome is detectable in PBMCs from healthy individuals. Thus methylation of this region, previously identified in Burkitt's lymphoma, Hodgkin's disease, and nasopharyngeal carcinoma, does not necessarily reflect aberrant methylation associated with malignancy but may reflect a more fundamental interaction of virus and cell. Detection of methylated viral genomes in the peripheral blood is consistent with a growing body of evidence that EBV latency in B cells in the normal host does not resemble EBV latency in immortalized lymphoblastoid cell lines. Other laboratories have shown that the patterns of viral gene expression in PBMCs are different from EBV-immortalized lymphoblastoid cell lines, with a consistent finding being that C promoter transcripts for the EBNAs are not detected in PBMCs.15,22 23 We now demonstrate that, whereas lymphoblastoid cell lines in general do not show methylation of the viral genome, there is methylation of the viral genome in PBMC.
One of the functions that has been ascribed to cytosine methylation is that of a genome-wide defense system that inactivates foreign parasitic sequences such as transposable elements and proviral DNA.24-26 It would appear that EBV has subverted this function. Rather than protecting against viral infection, methylation of the viral genome may ensure that a proportion of infected cells survive cytotoxic T-cell immune surveillance. Evidence to support this hypothesis comes from analysis of the interaction between methylation and CpG content. In vertebrate DNA, the frequency of the CpG dinucleotide is much lower than would be predicted by chance. This CpG suppression is believed to be a consequence of enhanced spontaneous deamination of methylcytosine versus cytosine over evolutionary time.26 A corollary is that CpG methylation should be associated with CpG suppression. In this light, it is interesting to note that there is no significant CpG suppression in large DNA viruses. The exception to the rule is EBV with a CpG relative abundance of 0.60 and its lymphotropic simian cousin, herpesvirus saimiri, with a relative abundance of 0.33.13 The presence of CpG suppression in these lymphotropic herpesviruses is consistent with the hypotheses that methylated EBV genomes detected in PBMCs are (1) susceptible to mutagenesis by deamination of methylcytosines and (2) important in perpetuating the virus over evolutionary time. In contrast, there is no evidence that herpes simplex, varicella zoster, or cytomegalovirus is CpG methylated even in latency.
Is there evidence that the viral genome can reactivate from a methylated latent state? Certainly in Burkitt's tumor cell lines, methylation of viral episomes does not represent a viral dead end. Nearly all potential methylation sites in a region of the C promoter are methylated in the Rael Burkitt's cell line that is a producer cell line that releases infectious virions after lytic induction. Experiments in our laboratory show that lytic replication in methylated Burkitt's cell lines induced by phorbol esters yields unmethylated viral DNA as determined by Southern blot hybridization with methylation-sensitive and methylation-insensitive restriction enzymes (unpublished results). A possible explanation for this observation is that the cellular DNA methyltransferase does not associate with the viral DNA polymerase or is otherwise inactive during lytic viral replication. During latent replication of viral DNA, it is the host cell DNA polymerase rather than the viral DNA polymerase that is active in replicating the EBV episome.
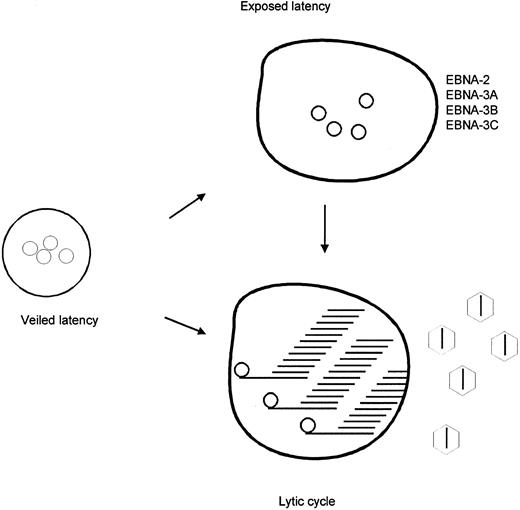
Model of EBV in peripheral blood lymphocytes. Lymphocytes infected by EBV are suggested to exist in three different states. In veiled latency, the immunodominant EBNA's are not expressed, the C promoter is methylated and its silence enforced. In exposed latency, the immunodominant EBNA's are expressed and the C promoter is not methylated. In lytic infection, it is proposed that the C promoter is not methylated.
Model of EBV in peripheral blood lymphocytes. Lymphocytes infected by EBV are suggested to exist in three different states. In veiled latency, the immunodominant EBNA's are not expressed, the C promoter is methylated and its silence enforced. In exposed latency, the immunodominant EBNA's are expressed and the C promoter is not methylated. In lytic infection, it is proposed that the C promoter is not methylated.
Evidence that methylation of the viral genome is regulated or limited so as to preserve select viral functions comes from study of the EBER region. The EBERs are two genes transcribed by RNA polymerase III. Their functions remain ill-defined, but they are expressed in high abundance in Burkitt's cell lines and tumors, in EBV(+) Hodgkin's tumors, and in PBMCs from healthy EBV-seropositive individuals.27 We investigated the methylation status of the EBER region of the viral genome by performing genomic sequencing in a Burkitt's cell line (Rael). Although also located within the BamHI-C fragment of the genome, in contrast to the C promoter region that is consistently methylated, the EBER region showed no methylation (data not shown). This confirms data presented by others using methylation sensitive and insensitive restriction enzymes suggesting that this region of the genome is not methylated.21 The nature of the mechanism that limits the spread of methylation and so protects the EBERs is not known, but it is clear that expression of genes encoding immunodominant proteins driven from the BamHI-C fragment of the genome is suppressed by methylation, whereas the EBER genes that may be important in viral persistence remain unmethylated and their products expressed.
In summary, we have shown the presence of methylated viral genomes in peripheral blood lymphocytes. We propose that these correspond to a reservoir of viral infection in which the immunodominant EBNAs are not expressed and the C promoter is silent (Fig 3). Thus, methylation of the viral episome may play a crucial role in perpetuating viral infection. Intermittently, and by as yet unknown mechanisms, viral episomes in these cells demethylate leading to C promoter activation with expression of latency genes or to lytic activation with production of new virions (lytic cycle). In support of this model, it should be noted that lytic viral gene products have recently been demonstrated in the PBMCs of normal seropositive individuals.28
R.F.A. is a Leukemia Society Scholar. This work was supported by Grants No. CA63532 and CA15396 (to R.F.A.).
Address reprint requests to Richard F. Ambinder, MD, PhD, 418 N Bond St, Johns Hopkins Oncology Center, Baltimore, MD 21231.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal