Abstract
We recently showed that c-kit signal synergizes with glycoprotein (gp)130 signal mediated by a complex of interleukin (IL)-6 and soluble IL-6 receptor (IL-6/sIL-6R) to stimulate the expansion of human primitive hematopoietic progenitor cells and erythropoietin-independent erythropoiesis. In the present study, we examined the effect of a ligand for Flt3 (FL), whose receptor tyrosine kinase is closely related to c-kit, in combination with IL-6/sIL-6R on human hematopoiesis in vitro. In serum-containing methylcellulose clonal culture of cord blood CD34+ cells, whereas FL alone stimulated only granulocyte-macrophage (GM) colony formation, erythroid bursts and mixed colonies in addition to GM colonies were induced by FL with IL-6/sIL-6R, but not IL-6/sIL-6R alone. In suspension culture, CD34+ cells generated a small number of myeloid cells in the presence of FL or IL-6/sIL-6R alone. However, the addition of IL-6/sIL-6R to the culture with FL induced the generation of a significant number of erythroid cells and megakaryocytes in addition to myeloid cells. The combination of FL and IL-6/sIL-6R also induced a remarkable expansion of GM colony- and erythroid burst-forming cells and multipotential progenitors, although FL or IL-6/sIL-6R alone induced the generation of only a small number of progenitors for GM colonies. The synergistic effects of FL and IL-6/sIL-6R were confirmed in serum-free clonal and suspension cultures. In addition, the addition of anti-human gp130 monoclonal antibodies abrogated the synergistic action. These results indicate that Flt3 signal, as well as c-kit signal, synergizes with gp130 signal to stimulate human myelopoiesis, erythropoiesis and megakaryopoiesis, and the expansion of primitive multipotential hematopoietic progenitor cells.
HEMATOPOIESIS IS regulated by a variety of hematopoietic growth factors, all of which function by signaling through their specific receptors.1 Several cytokine receptors belonging to the subclass III receptor tyrosine kinase superfamily, including c-Fms and c-kit, which act as the receptors for macrophage colony-stimulating factor (M-CSF ) and stem cell factor (SCF ), respectively, have been shown to play key roles in hematopoiesis.2-7 Recently, the murine flt3 gene was identified and shown to encode a hematopoietic stem cell–associated tyrosine kinase receptor.4,8 A cDNA encoding the human homologue of the flt3 receptor was subsequently cloned from a CD34+ hematopoietic stem/progenitor cell–enriched library.6,7 Expression of human flt3 mRNA was observed in thymus, spleen, and bone marrow (BM). The flt3 receptor was used to clone Flt3 ligand (FL), which promotes the proliferation of murine and human hematopoietic stem and progenitor cells.9-14 Human BM and cord blood CD34+ cells form colonies in response to FL alone, or FL in combination with other cytokines including interleukin (IL)-3, granulocyte-macrophage colony-stimulating factor (GM-CSF ), and a GM-CSF–IL-3 fusion protein termed Pixy 321.9 12-16 However, information about the effects of FL on human hematopoiesis is still limited.
Glycoprotein (gp)130, a 130-kD transmembrane glycoprotein, was originally identified as a signal transducing component of the interleukin-6 receptor (IL-6R). It is also shared in common by receptors for IL-11, leukemia inhibitory factor, oncostatin M, ciliary neurotrophic factor (CNTF ), and cardiotrophin 1.17-21 We recently showed that gp130 is expressed on most cord blood CD34+ cells, and that gp130 signaling initiated by a complex of IL-6 and soluble IL-6 receptor (IL-6/sIL-6R) synergizes with SCF for the ex vivo expansion of hematopoietic progenitor cells and erythropoietin (Epo)-independent erythropoiesis.22-24 In the present study, we investigated the hematopoietic effects of FL in combination with IL-6/sIL-6R on human cord blood CD34+ cells. The results indicate that FL, like SCF, synergizes with gp130 signaling (initiated by IL-6/sIL-6R) to stimulate various types of human hematopoietic progenitor cells, including myelocytic, erythroid, megakaryocyte, and primitive multipotential progenitors.
MATERIALS AND METHODS
CD34+ cell purification. Human umbilical cord blood, collected according to institutional guidance, was obtained during normal full-term deliveries. Mononuclear cells (MNC) were separated by Ficoll-Hypaque density gradient centrifugation after depletion of phagocytes with Silica (Immuno Biological Laboratories, Fujioka, Japan). The MNC were resuspended at 3 to 5 × 107 cells/mL in phosphate-buffered saline (PBS) and mixed with Dynabeads M-450 CD34 (Dynal AS, Oslo, Norway), with a bead to cell ratio of 1:1. Cell-beads suspension was resuspended well and incubated at 4°C for 30 minutes with gentle rotation. After the incubation, the cell-beads volume was expanded and placed in a DYNAL MPC (Magnetic Particle Concentrator) to collect the Dynabeads M-450 CD34–rosetted cells. The rosetted cells were incubated with DETACHaBEAD CD34 (Dynal) at room temperature for 45 minutes for the detachment of Dynabeads M-450 CD34 from the positively selected cells. The released CD34+ cells were collected by placing the tube in the MPC and were evaluated by flow cytometric analysis and colony assay. Approximately 95% of the separated cells were CD34+ by flow cytometric analysis.
Cytokines, receptor, and antibodies. Recombinant human FL was prepared as described previously.9 IL-6 and sIL-6R were kindly provided by Tosoh Co (Kanagawa, Japan).25 Recombinant human SCF was kindly provided by Amgen Biologicals (Thousand Oaks, CA). Recombinant human IL-3 and Epo were generously provided by Kirin Brewery (Tokyo, Japan). Granulocyte colony-stimulating factor (G-CSF ) was kindly provided by Chugai Pharmaceutical Co (Tokyo, Japan). All the cytokines were pure recombinant molecules and were used at concentrations that induced optimal response in methylcellulose culture of human hematopoietic cells. These concentrations are 100 ng/mL of SCF and IL-6, 1,000 ng/mL of sIL-6R, 200 U/mL of IL-3, 2 U/mL of Epo, and 10 ng/mL of G-CSF. Anti-human gp130 monoclonal antibodies (MoAbs, GPX7, GPX22, and GPZ35)26 27 were gifts of Tosoh Co. The three anti-gp130 MoAbs recognize different epitopes on gp130 and have been shown to inhibit the IL-6–mediated biological response through inhibition of the IL-6–induced association of gp130 and IL-6R.
Clonal culture. The inoculated CD34+ cells and their progeny in the suspension culture were incubated in methylcellulose culture in triplicate at concentrations of 5 to 10 × 102 cells/mL for CD34+ cells and 2 to 10 × 103 cells/mL for cultured cells as previously reported.22,23 One milliliter of culture mixture containing cells, α-medium (Flow Laboratories, Rockville, MD), 0.9% methylcellulose (Shinetsu Chemical Co, Tokyo, Japan), 30% fetal bovine serum (FBS; Hyclone, Logan, UT), 1% deionized fraction V bovine serum albumin (BSA; Sigma, St Louis, MO), 5 × 10−5 mol/L mercaptoethanol, and various combinations of cytokines, was plated in each 35-mm standard nontissue culture dish (Nunc, Roskilde, Denmark) and incubated at 37°C in a humidified atmosphere flushed with 5% CO2 in air. Some studies were done with serum-free culture.22,23 It contained components identical to those in serum-containing culture, except 2% deionized crystallized BSA (Sigma), 200 μg/mL of human transferrin (Sigma), 160 μg/mL of soybean lecithin (Sigma), 96 μg/mL of cholesterol (Nakalai Tesque Inc, Kyoto, Japan), and 10 μg/mL of human recombinant insulin (Sigma) replaced fraction V BSA and FBS. One milliliter of culture mixture was plated in a 35-mm standard nontissue culture dish and incubated at 37°C in a humidified atmosphere flushed with 5% CO2 , 5% O2 , and 90% N2 in air. All cultures were done in triplicate and scored at day 14 of culture according to criteria reported previously.28-30 To assess the accuracy of the in situ identification, individual colonies were lifted with an Eppendorf micropipette under direct microscopic visualization, spread on glass slides using a cytocentrifuge (Cytospin 2; Shandon Southern Instruments, Sewickley, PA), and stained with May-Grunwald-Giemsa. For the blocking study, varying concentrations of anti-gp130 MoAbs were added at the beginning of the culture, and colony formation was observed at day 14. The abbreviations used for the colony types are as follows: GM, granulocyte-macrophage colonies; MK, megakaryocyte colonies; B, erythroid bursts; and Mix, mixed hematopoietic colonies.
Suspension culture. Purified CD34+ cells were incubated in suspension culture using a modification of the technique described recently.22,23 One milliliter of culture mixture containing 2 to 4 × 103 CD34+ cells, α-medium, 20% FBS, 1% deionized fraction V BSA, and different combinations of cytokines was incubated in 24-well tissue plates (Nunc) at 37°C in a humidified atmosphere flushed with 5% CO2 in air. Serum-free culture contained components identical to serum-containing culture, except 2% deionized crystallized BSA, 200 μg/mL of human transferrin, 160 μg/mL of soybean lecithin, 96 μg/mL of cholesterol, and 10 μg/mL of human recombinant insulin replaced fraction V BSA and FBS. One milliliter of culture mixture was plated in 24-well tissue plates and incubated at 37°C in a humidified atmosphere flushed with 5% CO2 , 5% O2 , and 90% N2 in air. At weekly intervals, cultures were demidepopulated by the removal of half the culture volume, which was then replaced by newly prepared medium with the same combinations of cytokines. Cells in the collected media were counted, spun with a cytocentrifuge, and stained with May-Grunwald-Giemsa and by the alkaline phosphatase anti-alkaline phosphatase technique using MoAbs against glycophorin A (Cosmo Bio, Tokyo, Japan) and gp IIb/IIIa (Nichirei Co, Tokyo, Japan).31 Total hematopoietic progenitor cells generated at each time point in the culture were evaluated by culturing a fraction of the expanded cells in methylcellulose containing a combination of SCF, IL-3, IL-6, Epo, and G-CSF.
Statistical analysis. For statistical comparison in scoring the number of colonies, Student's t-test was applied. The significance level was set at 0.01.
RESULTS
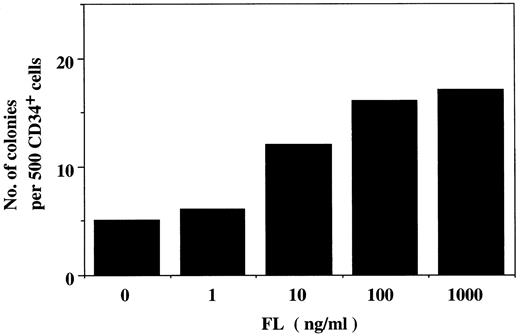
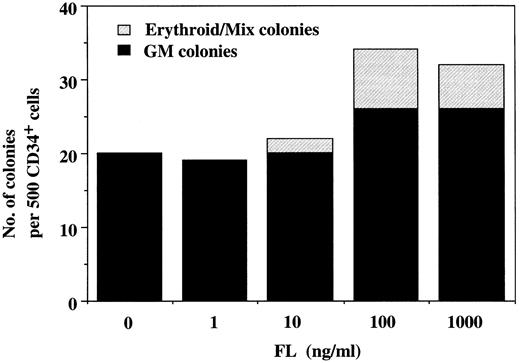
Synergistic action of FL with IL-6/sIL-6R on colony formation from cord blood CD34+ cells in serum-containing culture. To examine the effect of FL on human hematopoietic progenitor cells, we first performed methylcellulose clonal culture of 5 × 102 cord blood CD34+ cells in the presence of varying concentrations of FL. Although a small number of GM colonies were detected in the absence of cytokines, FL dose-dependently enhanced the GM colony formation as shown in Fig 1. The stimulatory effect of FL was obtained at a concentration of 1 ng/mL, and the maximal increase of colony number was observed at a concentration of 100 ng/mL. No other types of colonies were detectable at concentrations of up to 1,000 ng/mL of FL. To elucidate the interaction among FL, IL-6, and sIL-6R, we next examined the colony formation induced by different combinations of 100 ng/mL of FL, 100 ng/mL of IL-6, and/or 1,000 ng/mL of sIL-6R (Table 1). FL alone induced GM colonies as in the dose-response experiment. The addition of IL-6 to FL enhanced GM colony formation, whereas no synergistic interaction was observed between FL and sIL-6R. Although a combination of IL-6 and sIL-6R occasionally generated a few small erythroid bursts and Mix colonies in addition to GM colonies, the most pronounced effect was obtained with a combination of FL and IL-6/sIL-6R. The degree of the synergistic effect of FL and IL-6/sIL-6R varied among samples, but this combination induced the greatest number and largest erythroid bursts, GM, and Mix colonies; the most significant synergism was observed in the formation of Mix colonies. We then determined the effect of adding varying concentrations of FL to the culture containing 100 ng/mL of IL-6 plus 1,000 ng/mL of sIL-6R to confirm the synergistic action of FL with IL-6/sIL-6R on the formation of erythroid bursts and Mix colonies (Fig 2). The result showed that IL-6/sIL-6R supported only GM colony formation, and the addition of FL induced the increase in the number of GM colonies and the generation of erythroid bursts and Mix colonies in a dose-dependent manner. Maximal increase in the number of erythroid/Mix colonies, as well as GM colonies, was obtained at a concentration of 100 ng/mL of FL. These results indicate that FL in combination with IL-6/sIL-6R has a potent effect on hematopoietic progenitor cells, including myelocytic, erythroid, and multipotential progenitors (CFU-GM, BFU-E, and CFU-Mix, respectively).
Colony formation from cord blood CD34+ cells by varying concentrations of FL. 5 × 102 cord blood CD34+ cells were incubated in serum-containing culture, and the colonies were scored at day 14 of culture. FL alone induced only GM colony formation, and the number of the colonies increased in a dose-dependent manner, reaching a plateau at 100 ng/mL of FL. This concentration was assessed as an optimal dose.
Colony formation from cord blood CD34+ cells by varying concentrations of FL. 5 × 102 cord blood CD34+ cells were incubated in serum-containing culture, and the colonies were scored at day 14 of culture. FL alone induced only GM colony formation, and the number of the colonies increased in a dose-dependent manner, reaching a plateau at 100 ng/mL of FL. This concentration was assessed as an optimal dose.
Colony Formation From Cord Blood CD34+ Cells in the Presence of Various Combinations of FL, IL-6, and sIL-6R in Serum-Containing Culture
| Factor(s) . | No. of Colonies per 500 Cells . | ||||
|---|---|---|---|---|---|
| . | GM . | MK . | B . | Mix . | Total . |
| None | 4 ± 1 | 0† | 0 | 0 | 5 ± 1 |
| FL | 12 ± 2 | 0 | 0 | 0 | 12 ± 2 |
| IL-6 | 6 ± 2 | 1 ± 2 | 0 | 0 | 7 ± 0 |
| sIL-6R | 5 ± 1 | 0† | 0 | 0 | 5 ± 1 |
| IL-6 + sIL-6R | 18 ± 5 | 0 | 8 ± 1 | 7 ± 5 | 33 ± 5* |
| FL + IL-6 | 17 ± 3 | 0† | 0 | 0 | 17 ± 3 |
| FL + sIL-6R | 11 ± 1 | 0 | 0 | 0 | 11 ± 1 |
| FL + IL-6 + sIL-6R | 30 ± 3 | 0 | 11 ± 1 | 17 ± 3 | 58 ± 5* |
| Factor(s) . | No. of Colonies per 500 Cells . | ||||
|---|---|---|---|---|---|
| . | GM . | MK . | B . | Mix . | Total . |
| None | 4 ± 1 | 0† | 0 | 0 | 5 ± 1 |
| FL | 12 ± 2 | 0 | 0 | 0 | 12 ± 2 |
| IL-6 | 6 ± 2 | 1 ± 2 | 0 | 0 | 7 ± 0 |
| sIL-6R | 5 ± 1 | 0† | 0 | 0 | 5 ± 1 |
| IL-6 + sIL-6R | 18 ± 5 | 0 | 8 ± 1 | 7 ± 5 | 33 ± 5* |
| FL + IL-6 | 17 ± 3 | 0† | 0 | 0 | 17 ± 3 |
| FL + sIL-6R | 11 ± 1 | 0 | 0 | 0 | 11 ± 1 |
| FL + IL-6 + sIL-6R | 30 ± 3 | 0 | 11 ± 1 | 17 ± 3 | 58 ± 5* |
500 CD34+ cells were cultured in the presence of various combinations of 100 ng/mL of FL, 100 ng/mL of IL-6, and 1,000 ng/mL of sIL-6R, and the colonies were scored at day 14 of culture. The number of colonies indicates mean ± SD of triplicate cultures.
Colony formation by IL-6/sIL-6R was less than that by FL and IL-6/sIL-6R at P < .01%.
One colony was present in triplicate cultures.
Colony formation from cord blood CD34+ cells by varying concentrations of FL in the presence of 100 ng/mL of IL-6 and 1,000 ng/mL of sIL-6R. 5 × 102 cord blood CD34+ cells were incubated in serum-containing culture, and the colonies were scored at day 14 of culture. The number of colonies increased dose-dependently to FL. The formation of total and erythroid/Mix colonies was most pronounced at 100 ng/mL of FL.
Colony formation from cord blood CD34+ cells by varying concentrations of FL in the presence of 100 ng/mL of IL-6 and 1,000 ng/mL of sIL-6R. 5 × 102 cord blood CD34+ cells were incubated in serum-containing culture, and the colonies were scored at day 14 of culture. The number of colonies increased dose-dependently to FL. The formation of total and erythroid/Mix colonies was most pronounced at 100 ng/mL of FL.
Effect of anti-gp130 MoAbs on the colony formation by the combination of FL, IL-6, and sIL-6R. To verify the involvement of gp130 in the synergistic action of FL with IL-6/sIL-6R, we performed blocking studies using MoAbs against human gp130. The addition of anti-gp130 MoAbs to the methylcellulose culture (containing 100 ng/mL each of FL and IL-6 and 1,000 ng/mL of sIL-6R) inhibited the colony formation from cord blood CD34+ cells (Table 2). The colony number decreased to the level induced by FL alone at concentrations of 1 and 10 mg/mL of the MoAbs. In particular, the formation of erythroid bursts and Mix colonies was completely abrogated by the addition of 1 mg/mL of the MoAbs. These data clearly indicate that the observed effects mediated by IL-6/sIL-6R in the presence of FL are provided by the interaction of IL-6/sIL-6R with membrane-anchored gp130.
Effects of Varying the Concentration of Anti-gp130 MoAbs on Colony Formation by FL, IL-6, and sIL-6R
| Concentration of MoAbs . | No. of Colonies per 500 Cells . | |||
|---|---|---|---|---|
| (μg/mL) . | GM . | B . | Mix . | Total . |
| 0 | 20 ± 1 | 3 ± 1 | 5 ± 3 | 28 ± 5 |
| 1 | 11 ± 1 | 0 | 0 | 11 ± 1 |
| 10 | 12 ± 2 | 0 | 0 | 12 ± 2 |
| Concentration of MoAbs . | No. of Colonies per 500 Cells . | |||
|---|---|---|---|---|
| (μg/mL) . | GM . | B . | Mix . | Total . |
| 0 | 20 ± 1 | 3 ± 1 | 5 ± 3 | 28 ± 5 |
| 1 | 11 ± 1 | 0 | 0 | 11 ± 1 |
| 10 | 12 ± 2 | 0 | 0 | 12 ± 2 |
Varying concentrations of anti-gp130 MoAbs were added to the serum-containing culture of 500 CD34+ cells with 100 ng/mL of FL, 100 ng/mL of IL-6, and 1,000 ng/mL of sIL-6R, and the colonies were scored at day 14 of culture. The number of colonies indicates mean ± SD of triplicate cultures.
Generation of various blood cells and hematopoietic progenitor cells by the combination of FL and IL-6/sIL-6R. We have shown that SCF, in combination with IL-6/sIL-6R, potently stimulates the ex vivo expansion of human hematopoietic progenitor cells22 and the generation of erythroid cells in the absence of Epo in suspension culture of CD34+ cells.23 The results of our clonal culture assay suggest that FL in combination with IL-6/sIL-6R would have a similar effect. We then set up a suspension culture of 2 × 103 cord blood CD34+ cells with 100 ng/mL of FL alone or in combination with 100 ng/mL of IL-6 and 1,000 ng/mL of sIL-6R and incubated the culture for 3 weeks. When cultured with FL or IL-6/sIL-6R alone, cord blood CD34+ cells generated only a small number of myeloid cells (data not shown). By contrast, total cells cultured with FL and IL-6/sIL-6R significantly expanded (Table 3). The expanded cells contained megakaryocytes, erythroid, and blast cells in addition to myeloid cells. The nature of megakaryocytes and erythroid cells was confirmed by immunostaining with MoAbs against gp IIb/IIIa complex and glycophorin A, respectively. The gp IIb/IIIa–positive megakaryocytes were first detected at day 7 of culture and increased until day 14. The glycophorin A–positive erythroid cells constituted approximately 15% of the total culture cells at days 14 and 21. Some of the erythroid cells differentiated to normoblast and enucleated erythrocyte stage. Myeloid and blast cells also expanded, but myeloid cells kept increasing for 3 weeks whereas blast cells began to decrease at day 21. These results indicate that FL and IL-6/sIL-6R induce the generation from CD34+ cells of various types of hematopoietic cells, including erythroid cells, megakaryocytes, and immature blast cells in addition to myeloid cells.
Generation of Various Blood Cells From Cord Blood CD34+ Cells in Serum-Containing Suspension Culture With FL, IL-6, and sIL-6R
| Days in Culture . | Total Cell No. . | No. of Blood Cells . | ||||
|---|---|---|---|---|---|---|
| . | . | G . | M . | MK . | E . | Blast . |
| Day 7 | 2.0 × 104 | 800 | 1,580 | 600 | 0 | 17,020 |
| Day 14 | 10 × 104 | 10,900 | 47,500 | 1,000 | 12,900 | 27,700 |
| Day 21 | 12 × 104 | 28,080 | 66,240 | 840 | 19,920 | 4,920 |
| Days in Culture . | Total Cell No. . | No. of Blood Cells . | ||||
|---|---|---|---|---|---|---|
| . | . | G . | M . | MK . | E . | Blast . |
| Day 7 | 2.0 × 104 | 800 | 1,580 | 600 | 0 | 17,020 |
| Day 14 | 10 × 104 | 10,900 | 47,500 | 1,000 | 12,900 | 27,700 |
| Day 21 | 12 × 104 | 28,080 | 66,240 | 840 | 19,920 | 4,920 |
2,000 CD34+ cells were incubated in serum-containing suspension culture with 100 ng/mL of FL, 100 ng/mL of IL-6, and 1,000 ng/mL of sIL-6R. Every week, total cell numbers were counted, and cytospin preparations were stained with May-Grunwald-Giemsa. Morphology was examined under a light microscope. At least 200 cells were examined.
Abbreviations: G, granulocytes; M, macrophages; E, erythroid cells; Blast, blast cells.
We next analyzed hematopoietic progenitor cells in the suspension culture. In the culture with FL or IL-6/sIL-6R alone, there was little expansion of CFU-GM. However, the combination of FL and IL-6/sIL-6R induced the significant expansion of hematopoietic progenitor cells at days 14 and 21. When 2 × 103 cord blood CD34+ cells containing 1,372 hematopoietic progenitors were cultured, the number of total progenitors at days 7, 14, and 21 reached 853, 7,133, and 6,040, respectively. The expanded progenitors were of various types including CFU-GM, BFU-E, and CFU-Mix, but the most significant expansion was achieved with CFU-Mix at day 14. The 246 CFU-Mix in the inoculated 2 × 103 cord blood CD34+ cells had expanded to 1,700 by day 14. These results indicate that FL synergizes with IL-6/sIL-6R to stimulate the expansion of various types of hematopoietic progenitor cells, including CFU-GM, BFU-E, and CFU-Mix, supporting our observation in clonal culture.
Synergistic action of FL with IL-6/sIL-6R in serum-free culture of cord blood CD34+ cells. To exclude the effect of FBS on the results observed above, we performed clonal and suspension cultures under serum-free conditions. In the methylcellulose clonal culture of 1 × 103 cord blood CD34+ cells, IL-6/sIL-6R supported few colonies, and FL alone supported only small GM colonies (Table 4). In combination with IL-6/sIL-6R, however, FL induced a significant number of colonies, including GM colonies, erythroid bursts, and Mix colonies, confirming the results in the serum-containing culture. The serum-free suspension culture of 4 × 103 cord blood CD34+ cells also revealed a similar result to the serum-containing culture. Although a small number of myeloid cells were produced in the culture with FL or IL-6/sIL-6R alone at day 14 of culture, the combination of FL and IL-6/sIL-6R induced the generation of 1.85 × 105 cells, which consisted of 73.2% of myeloid cells, 0.5% of megakaryocytes, 20.5% of erythroid cells, and 5.9% of blast cells. These results indicate that the synergistic effect of FL and IL-6/sIL-6R on human hematopoietic cells requires no other cytokines.
Colony Formation From Cord Blood CD34+ Cells in the Presence of Various Combinations of FL, IL-6, and sIL-6R in Serum-Free Culture
| Factor(s) . | No. of Colonies per 1,000 Cells . | ||||
|---|---|---|---|---|---|
| . | GM . | MK . | B . | Mix . | Total . |
| FL | 17 ± 3 | 0 | 0 | 0 | 17 ± 3 |
| IL-6 + sIL-6R | 0 | 0 | 1 ± 1 | 0 | 1 ± 1 |
| FL + IL-6 + sIL-6R | 87 ± 7 | 0 | 4 ± 3 | 3 ± 1 | 95 ± 10 |
| Factor(s) . | No. of Colonies per 1,000 Cells . | ||||
|---|---|---|---|---|---|
| . | GM . | MK . | B . | Mix . | Total . |
| FL | 17 ± 3 | 0 | 0 | 0 | 17 ± 3 |
| IL-6 + sIL-6R | 0 | 0 | 1 ± 1 | 0 | 1 ± 1 |
| FL + IL-6 + sIL-6R | 87 ± 7 | 0 | 4 ± 3 | 3 ± 1 | 95 ± 10 |
1,000 CD34+ cells were cultured in the presence of various combinations of 100 ng/mL of FL, 100 ng/mL of IL-6, and 1,000 ng/mL of sIL-6R, and the colonies were scored at day 14 of culture. The number of colonies indicates mean ± SD of triplicate cultures.
Comparison between FL and SCF in synergistic effect with IL-6/sIL-6R. The above results suggested that the stimulatory pattern of FL is reminiscent of that of SCF. To compare the effect of SCF and FL in more detail, we performed methylcellulose culture of 5 × 102 CD34+ cells stimulated by FL or SCF in the presence of IL-6/sIL-6R (Table 5). A combination of FL, IL-6, and sIL-6R induced colonies of various lineages, but the number of colonies was less than those induced by a combination of SCF, IL-6, and sIL-6R. The addition of FL to the combination of SCF, IL-6, and sIL-6R failed to affect the colony formation. These results suggest that FL has a similar but weaker effect than SCF, and that the effect of FL overlaps that of SCF.
Comparison of Synergistic Effects Between FL and SCF With IL-6/sIL-6R
| Factor(s) . | No. of Colonies per 500 CD34+ Cells . | ||||
|---|---|---|---|---|---|
| . | GM . | MK . | B . | Mix . | Total . |
| FL + IL-6 + sIL-6R | 5 ± 0 | 8 ± 3 | 5 ± 1 | 28 ± 3 | 46 ± 3 |
| SCF + IL-6 + sIL-6R | 9 ± 1 | 5 ± 4 | 6 ± 3 | 85 ± 5 | 105 ± 6 |
| SCF + FL + IL-6 + sIL-6R | 10 ± 2 | 5 ± 2 | 4 ± 3 | 82 ± 8 | 101 ± 12 |
| Factor(s) . | No. of Colonies per 500 CD34+ Cells . | ||||
|---|---|---|---|---|---|
| . | GM . | MK . | B . | Mix . | Total . |
| FL + IL-6 + sIL-6R | 5 ± 0 | 8 ± 3 | 5 ± 1 | 28 ± 3 | 46 ± 3 |
| SCF + IL-6 + sIL-6R | 9 ± 1 | 5 ± 4 | 6 ± 3 | 85 ± 5 | 105 ± 6 |
| SCF + FL + IL-6 + sIL-6R | 10 ± 2 | 5 ± 2 | 4 ± 3 | 82 ± 8 | 101 ± 12 |
500 CD34+ cells were incubated in the presence of designated factor combinations in serum-containing culture, and the colonies were scored at day 14 of culture. Concentrations of cytokines were 100 ng/mL for FL, 100 ng/mL for SCF, 100 ng/mL for IL-6, and 1,000 ng/mL for sIL-6R. The number of colonies indicates mean ± SD of triplicate cultures.
DISCUSSION
The present study has shown that FL synergizes with IL-6/sIL-6R in human hematopoiesis. The combination of FL and IL-6/sIL-6R induced the formation of greater numbers of GM colonies in methylcellulose clonal culture and larger expansion of myeloid cells and their progenitors in suspension culture as compared with FL alone. Erythroid colony formation in clonal culture and terminal maturation and expansion of erythroid cells in suspension culture, which currently are considered to require Epo receptor signaling, were supported by the combination of FL and IL-6/sIL-6R in the absence of added Epo. FL alone did not affect erythropoiesis in accordance with previous reports.9,12,13,15 Megakaryopoiesis was also induced by the combination of FL and IL-6/sIL-6R in suspension culture. In clonal culture, the synergistic effect of these proteins on MK colony formation was unclear since serum-containing methylcellulose culture has been shown to be inappropriate for the growth of MK colonies.32 Moreover, the combination of proteins induced the generation of Mix colonies in clonal culture and the expansion of multipotential hematopoietic progenitors in suspension culture. Because similar results were also obtained in the serum-free culture, the synergism of FL with IL-6/sIL-6R did not require the effect of other cytokines. In addition, the synergistic effects were abrogated by the addition of anti-human gp130 MoAbs, suggesting that the effects mediated by IL-6/sIL-6R in the presence of FL were provided by the interaction of IL-6/sIL-6R with membrane-anchored gp130 on CD34+ cells. Taken together, the present results show that a Flt3 signal, together with a gp130 signal, stimulates human erythropoiesis and megakaryopoiesis as well as the expansion of primitive multipotential hematopoietic progenitors.
The Flt3 receptor was reported to be expressed at low levels on human BM CD34+ cells by flow cytometric analysis.12 A recent study of ours indicated that most cord blood CD34+ cells express gp130, whereas IL-6R is expressed on only a third of cord blood CD34+ cells. CD34+/IL-6R+ progenitors are already committed to the myeloid lineage, whereas CD34+/IL-6R− cells contain CFU-GM, BFU-E, megakaryocytic progenitors (CFU-MK), CFU-Mix, and long-term culture-initiating cells.24 These findings suggest that human CD34+ cells are composed of at least two subpopulations, Flt3+/gp130+/IL-6R+ and Flt3+/gp130+/IL-6R− cells, and that most of the former are CFU-GM whereas the latter consists of BFU-E, CFU-MK, and CFU-Mix in addition to CFU-GM. This explains the responsiveness of CD34+ cells to the combination of FL, IL-6, and sIL-6R observed in this study. FL and IL-6 might induce GM colony formation from Flt3+/gp130+/IL-6R+ CFU-GM and have no effect on Flt3+/gp130+/IL-6R− progenitors. By contrast, the addition of sIL-6R to the combination of FL and IL-6 might stimulate not only Flt3+/gp130+/IL-6R+ CFU-GM but also Flt3+/gp130+/IL-6R− CFU-GM and other types of progenitors expressing Flt3+/gp130+/IL-6R−, such as BFU-E, CFU-MK, and CFU-Mix.
We have recently indicated that signaling through c-kit synergizes with a gp130 signal mediated by IL-6/sIL-6R to induce the expansion of human primitive hematopoietic progenitor cells22 and the generation of erythroid cells from cord blood CD34+ cells.23 More recently, human megakaryopoiesis has been shown to be stimulated by the synergistic action of SCF and IL-6/sIL-6R (manuscript submitted). Thus, the signals generated via Flt3 and c-kit, both of which belong to the tyrosine kinase receptor superfamily and are expressed on human CD34+ cells,12,24 exhibit a similar degree of synergism in combination with gp130 signals in human hematopoiesis.22-24 However, the synergistic effects of the Flt3 signal appear to be weaker than those of the c-kit signal. There are several possible explanations for the difference in activity between Flt3 and c-kit signals in synergism with a gp130 signal. Firstly, the effect of transduction of the c-kit signal is essentially stronger than that of the Flt3 signal in the hematopoietic system. Secondly, the affinity of the gp130 signal to the c-kit signal is stronger than that to the Flt3 signal. Lastly, Flt3 expression diminishes earlier than c-kit during the maturation process of hematopoietic cells. We have also found that hepatocyte growth factor, a ligand for the Met tyrosine kinase receptor, synergized with IL-6/sIL-6R to some extent in erythroid colony formation in clonal culture of CD34+ cells, although other types of ligands for tyrosine kinase receptors, including epidermal growth factor, platelet derived growth factor, fibroblast growth factor (FGF ), and M-CSF, provided no synergistic stimulation with IL-6/sIL-6R on these cells (unpublished data). Recently, Ip et al have reported that FGF synergizes with CNTF, whose receptor contains gp130 as a signal transducing component, for the terminal differentiation of neuronal progenitor cells.19 Synergistic signals through gp130 and receptors with tyrosine kinase may have roles not only in hematopoiesis but also in other systems, such as the nervous system.
There has been great interest in the ex vivo expansion of human primitive hematopoietic progenitor cells for clinical application, including gene therapy. The combination of SCF, IL-6, and sIL-6R has been shown to be an attractive candidate for the expansion.22 However, SCF induces the generation of mast cells from human BM and cord blood CD34+ cells in vitro.33,34 Therefore, cultures containing SCF could possibly induce not only hematopoietic cells but also mast cells, and the administration of cells cultured with SCF may cause allergic reactions in the recipients. FL, in contrast to SCF, does not affect the proliferation or degranulation of mast cells.35 36 Accordingly, although the combination of FL with IL-6/sIL-6R has weaker activity than the mixture of SCF plus IL-6/sIL-6R, the combination may be more suitable to apply to clinical use in the ex vivo expansion of human hematopoietic progenitor cells.
Supported by grants from the Ministry of Education, Science, Sports, and Culture, Japan.
Address reprint requests to Tatsutoshi Nakahata, MD, Department of Clinical Oncology, The Institute of Medical Science, The University of Tokyo, 4-6-1 Shirokanedai, Minato-ku, Tokyo 108, Japan.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal