To the Editor:
In a recent issue of Blood, Inoue et al1 reported aberrant overexpression of Wilms tumor gene (wt1) mRNA in leukemic blasts compared with immature hematopoietic progenitors. CD34+ bone marrow and cord blood cells derived from nine healthy volunteers were sorted into 10 subsets (CD34−, CD34+CD33+/−, CD34+CD38+/−, CD34+HLA-DR+/−, CD34+c-kithigh/low/−). Primary acute myeloid leukemia (AML) blast populations were sorted into CD34+/−CD33+/− subsets. The populations obtained from normal individuals expressed very low levels of wt1 mRNA. In contrast, the investigators described significantly higher (at least 10-fold) wt1 expression in AML blasts regardless of the surface marker pattern. They concluded that wt1 is aberrantly overexpressed in malignant blasts and that overexpression of wt1 may contribute to the pathogenesis of AML.
In contrast, ongoing studies in our laboratory addressing expression levels of wt1 in healthy progenitors and malignant blast cells do not show significant differences of wt1 expression in AML blasts in comparison to immature progenitors.
Bone marrow was aspirated from 7 healthy volunteers and 9 representative AML patients of 129 with no (2 samples), low (2 samples), moderate (2 samples), and high (3 samples) wt1 mRNA expression level, as described previously,2 with a blast proportion of greater than 90% at diagnosis, after informed consent was obtained. Mononuclear cells were isolated and CD34+ cells from healthy individuals were separated using Dynabeads (Baxter, Munich, Germany), obtaining about 5 × 104 cells with a purity of 90% to 95%.
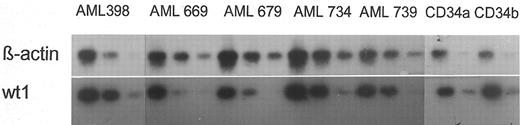
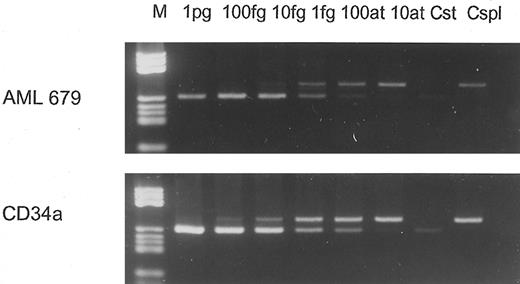
wt1- and β-actin–specific reverse transcription-polymerase chain reaction (RT-PCR) were performed with 30 and 20 cycles, respectively. To compare directly wt1 expression levels in AML blasts versus hematopoietic progenitors, the samples were amplified all at once using one master mix for each gene for RT and PCR, respectively. wt1 primers annealing in the wt1 3′-untranslated region (3′UTR) were used as described before,3,4 resulting in enhanced PCR sensitivity compared with our routine wt1-PCR.2 Different dilutions of cDNA were amplified. PCR products were Southern-blotted (Fig 1) and quantified using the PhosphorImager system and IPLab-Gel software (Molecular Dynamics, Krefeld, Germany). Because wt1-specific PCR was performed with 30 cycles and thus reached the range of saturation (nonexponential amplification), the results were confirmed by competitive PCR, as described previously,2 using a different mimic with binding sites for the 3′UTR primers. In Fig 2, two competitive PCRs are shown performed with a normal CD34+ population and an AML sample expressing wt1 at the moderate level (as described previously2 ). Both PCRs were performed with equal amounts of target cDNA verified by β-actin–specific PCR with 20 cycles.
Expression of β-actin and wt1 genes in 5 representative AML blast samples and 2 CD34+ normal progenitor populations. Amplification of the cDNA derived from AML blasts was performed with 100 (left), 10−1 (medium), and 10−2 (right) dilutions. cDNA derived from normal cells was diluted 100 (left) and 10−1 (right) due to the lower cell number obtaind for RNA extraction. Dilutions were performed to avoid PCR saturation due to initial excess of template.
Expression of β-actin and wt1 genes in 5 representative AML blast samples and 2 CD34+ normal progenitor populations. Amplification of the cDNA derived from AML blasts was performed with 100 (left), 10−1 (medium), and 10−2 (right) dilutions. cDNA derived from normal cells was diluted 100 (left) and 10−1 (right) due to the lower cell number obtaind for RNA extraction. Dilutions were performed to avoid PCR saturation due to initial excess of template.
Competitive PCR performed with 10−1 dilution of AML 679 (upper panel) and 100 dilution of CD34a (lower panel), containing equal amounts of cDNA as verified by β-actin PCR. In both PCRs, dilutions (10−1 per step) starting from 1 pg standard cDNA were performed and coamplified with identical amounts of sample cDNA as used for the experiment shown in Fig 1. The wt1 cDNA amount in AML 679 10−1 was 1.5 fg; in CD34a 100, 2 fg was determined. Cst, standard control; Cspl, sample control.
Competitive PCR performed with 10−1 dilution of AML 679 (upper panel) and 100 dilution of CD34a (lower panel), containing equal amounts of cDNA as verified by β-actin PCR. In both PCRs, dilutions (10−1 per step) starting from 1 pg standard cDNA were performed and coamplified with identical amounts of sample cDNA as used for the experiment shown in Fig 1. The wt1 cDNA amount in AML 679 10−1 was 1.5 fg; in CD34a 100, 2 fg was determined. Cst, standard control; Cspl, sample control.
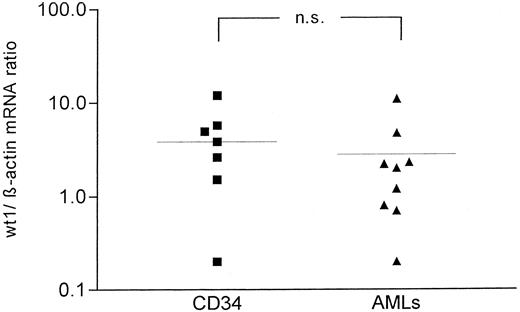
All samples of normal CD34+ cells expressed detectable levels of wt1 mRNA. All of the leukemic samples expressed wt1 mRNA, reflecting enhanced wt1 PCR sensitivity to the method described earlier.2 Additionally, CD34+ progenitors resembled the distribution of low, intermediate, and high expression of wt1 as found in malignant blasts of AML patients (Fig 3).2 5 As shown, we did not find differences regarding wt1 mRNA expression of normal CD34+ progenitors and malignant blasts. The average expression of wt1 in normal CD34+ cells was not significantly different from wt1 expression in AML blasts as evaluated by Wilcoxon-Mann-Whitney U-test (Fig 3).
wt1/β-actin ratio of CD34+ normal progenitors (left) versus AML blasts (right). The difference in wt1/β-actin ratio was not significant (P = .21).
wt1/β-actin ratio of CD34+ normal progenitors (left) versus AML blasts (right). The difference in wt1/β-actin ratio was not significant (P = .21).
Our data are according to Inoue et al1 in respect to the appearance of wt1 expression in CD34+ hematopoietic progenitor populations. The different strengths of wt1 expression in our CD34+ progenitor samples may be explained by the heterogeneity of the populations, consisting of different subpopulations. Recently, we showed that wt1 mRNA is expressed by a subpopulation of CD34+ progenitors in course of in vitro differentiation and subsequently downregulated during differentiation.3,4 However, our data are in striking contrast to that of Inoue et al1 regarding overexpression of wt1 in AML compared with normal precursors.
The discrepancy of our results and those of Inoue et al1 is hard to explain. It is possible that the nested PCR assay out of the amplification range (35 + 14 cycles) in company with a very low cell number used for RNA extraction (2 × 103 cells) does not reflect the initial mRNA amount in a linear manner. However, Fraizer et al6 reported equal expression of wt1 in fluorescence-activated cell sorted CD34+ bone marrow cells and in AML blasts as well, although only two bone marrow samples were tested; this is consistent with our data, despite the different methods used.
A possible difference of wt1 expression in normal and malignant cells has considerable impact for the value of wt1 as a marker for detection of (residual) leukemic blasts and for its relevance for the molecular pathophysiology of acute leukemias. Equal expression of wt1 in healthy progenitors and malignant blasts may complicate detection of minimal residual disease using wt1 PCR by background wt1 expression in the bone marrow. Because wt1 is expressed in more mature leukemias as well, our data suggest that wt1 expression is related to malignant transformation rather by the persistence of its expression (lack of downregulation) than by overexpression.
Response
We thank Maurer et al for their interest in our recent report.1-1 They reported controversial results of whether WT1 expression levels in normal CD34+ cells are comparable to those in acute myelocytic leukemia (AML) blast cells. We should like to discuss the cause of discrepancy between our results and their results from two points: sampling of CD34+ cells and methods of WT1 detection.
They separated CD34+ cells from bone marrow (BM) using Dynabeads, whereas we isolated CD34+ cells from BM or umbilical cord blood (CB) using fluorescence-activated cell sorting (FACS).1-1 The purity of CD34+ cells is usually lower in CD34+ cell samples separated by Dynabeads than in those isolated by FACS. Thus, contaminated cells (the majority are lymphocytes and monocytes) might let the WT1 expression levels elevate in the Dynabeads-separated samples. However, it seems unlikely, because we cannot detect WT1 expression in lymphocytes and monocytes. We also quantitated WT1 expression levels in seven CD34+ cell samples that were separated by leukapheresis from peripheral blood stem cells mobilized with chemotherapy and granulocyte colony-stimulating factor (G-CSF ) and then purified using the MACS system (Miltenyi, Cologne, Germany). The WT1 expression was at 2.5 × 10−2 level (the level in leukemic cell line K562 was defined as 1.0, as described previously1-2 ) in one sample and at less than 10−2 level in the other six samples (unpublished data). Menssen et al1-3 also could not detect WT1 mRNA in 91% pure CD34+ cells that were separated by the same procedure (MACS system) from the leukapheresis product of peripheral blood stem cells mobilized with chemotherapy and G-CSF. On the other hand, Fraizer et al1-4 reported that, in FACS-sorted CD34+ BM cells, WT1 expression levels were comparable with those in K562 and AML blast cells. Taken together, we determined low levels (10−2 or <10−2) of WT1 in both FACS- and magnetic beads-sorted CD34+ cells by reverse transcription-polymerase chain reaction (RT-PCR), whereas Maurer et al and Fraizer et al1-4 detected WT1 expression by RT-PCR at the levels comparable with those in K562 and AML blast cells in magnetic beads-separated or FACS-sorted CD34+ cells, respectively. Thus, it appears unreasonable that the cause of controversial results is ascribed to the difference in sampling of the CD34+ cells.
As for our PCR conditions, they supposed that the nested PCR assay out of the amplification range (35 + 14 cycles) in company with a very low cell number used for RNA extraction (2 × 103 cells) does not reflect the initial mRNA amount in a linear manner. However, under our PCR conditions, in all the samples in which the first-round PCR products were undetectable, the subsequent nested PCR could not also detect PCR products.1-1 Thus, WT1 expression levels of all the CD34+ samples were indeed quantitated only by the first-round PCR of 35 cycles, as we mentioned in our report1-1 that PCR was performed for 35 cycles with the outer primers to quantitate 100 to 10−2 levels of WT1 expression. Furthermore, serial 1:10 dilutions of standard cDNAs prepared from a small number of K562 cells (2 × 103) were always PCR-amplified simultaneously with the samples to determine the WT1 expression levels of the samples relative to those of K562 cells.1-1 As for cell number used for RNA extraction, 3 CD34+ BM and 2 CD34+ CB cell samples FACS-sorted by only single color for CD34 indeed contained greater than 104 cells,1-1 which are satisfactory for RNA extraction followed by cDNA synthesis and PCR, although it was not mentioned in detail in our report.1-1 WT1 expression levels in the 3 CD34+ BM cell samples were less than 10−2, 1.0 × 10−2, and 2.2 × 10−2, respectively. Likewise, the levels in the 2 CD34+ CB cell samples were 1.5 × 10−2 and 2.4 × 10−2, respectively.1-1 We determined WT1 expression at 1.0 to 2.2 × 10−2 and 2.0 to 8.0 × 10−4 levels in CD34+ BM cells1-1 and in BM mononuclear cells,1-2 respectively. These WT1 values are reasonable because normal BM contains 1% to 4% CD34+ cells. Thus, the issues pointed out by them are unreasonable.
The results that strongly encourage us are those of Menssen et al.1-3,1-5 They performed immunofluorescence assay using three different anti-WT1 monoclonal antibodies and one anti-WT1 polyclonal antibody (C-19; Santa Cruz Biotechnology, Santa Cruz, CA). K562 and HL60 leukemic cells and blast cells of AML patients showed strong nuclear immunofluorescence, whereas peripheral blood CD34+ cells and reactive BM mononuclear cells did not. Baird and Simmons1-6 detected WT1 protein in the nuclei of CD34+ BM cells in immunofluorescence assay using the same antibodies (C-19), but at a lower level compared with that seen in HL60 cells. This reduced level of WT1 protein expression was in accord with the lower level of mRNA expression seen in the CD34+ cells compared with HL60 cells.1-6 These results confirmed our claim.
In conclusion, at the present time, we should like to consider that normal CD34+ cells express WT1 at low levels, but not at levels comparable with those in AML blast cells, and that the discrepancy between our and their results could be ascribed to the differences in optimization of PCR conditions.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal