Abstract
The tendency for gastric mucosa-associated lymphoid tissue (MALT) lymphoma cells preferentially to localize around reactive B-cell follicles, both in the mucosa and regional lymph nodes, coupled with their immunophenotype, has led to the proposal that the normal cell counterpart of this lymphoma is the marginal zone B cell. In keeping with this proposition, lymphocytes expressing the lymphoma idiotype have been detected in the splenic marginal zone in a single case of gastric MALT lymphoma. To confirm that this truly represented preferential homing of MALT lymphoma to the splenic marginal zone, we have now re-examined this case, together with 17 other cases, using both immunohistochemical and molecular methods in an attempt to establish clonal identity between the gastric lymphoma and cells in the splenic marginal zone. In three cases, the spleen was characterized by marked expansion of marginal zones by cells showing the same pattern of Ig light chain restriction as the gastric lymphoma. None of the remaining 15 cases showed histologic evidence of lymphomatous infiltration. Analysis of the Ig genes by polymerase chain reaction (PCR), cloning, and sequencing confirmed clonal identity between the splenic marginal zone infiltrates and the gastric lymphoma in the histologically involved cases. Amplifiable DNA could be extracted from only 5 of the remaining 15 cases. In 3 of these cases, including the case previously studied using an anti-idiotype, involvement of the splenic marginal zone could be confirmed using microdissection and clone-specific PCR. No involvement could be detected in the remaining 2 cases. In addition, we have shown that mucosal addressin cell adhesion molecule-1 (MAdCAM-1), the primary homing receptor of gut-mucosa for lymphocytes, was strongly expressed by the sinus lining cells of the splenic marginal zone. These results provide strong evidence for preferential involvement of the marginal zone when gastric MALT lymphomas disseminate to the spleen, which is in keeping with the notion that the marginal zone B cells are the normal counterparts of MALT lymphoma cells.
GASTRIC B-CELL LYMPHOMAS of mucosa-associated lymphoid tissue (MALT), together with MALT lymphomas in general, tend to localize around reactive B-cell follicles. This property has led to the suggestion that the normal cell counterpart of this group of lymphomas may be the marginal zone B cell.1 Both the phenotypic and genotypic features of MALT lymphoma are in keeping with this. Marginal zone B cells and MALT lymphoma cells are CD20+, CD5−, CD23−, CD21+, CD35+ and possess rearranged Ig genes that show frequent somatic mutations.2-5
The most prominent physiologic marginal zone is found in the spleen and it would be expected, therefore, that, when that organ is involved by MALT lymphoma, the tumor cells should localize in the marginal zone. Gastric MALT lymphomas infrequently disseminate to the spleen but the observation, in a single case, that a subpopulation of cells in the marginal zone of an otherwise normal spleen expressed the tumor Ig idiotype was taken as evidence of preferential homing to that region.6 This would support the suggestion that the marginal zone B cell is the normal cell counterpart of MALT lymphoma. However, the relevance of this observation has been contested, because idiotypes may not necessarily be tumor-specific.7,8 Moreover, a polymerase chain reaction (PCR) study of 11 spleens removed from patients with gastric MALT lymphoma failed to show any evidence of cryptic splenic involvement despite the prominence of the splenic marginal zone in this group of patients.9
A recent review of our cases of low-grade gastric MALT lymphoma, in which a splenectomy had been performed, showed 3 cases in which the histologic appearance was compatible with splenic involvement. This has provided us with an opportunity to re-examine this question. We have investigated these 3 cases together with the original case referred to above and a further 14 cases in which splenectomy tissue was available. We have used PCR in an attempt to identify the clonal rearranged Ig gene in both the tumor and spleen tissues of each case followed by cloning and sequencing of the PCR products and application of clone-specific PCR to microdissected compartments of the spleen where indicated. To examine the role of adhesion molecules in the tumor dissemination, we have also immunostained mucosal addressin cell adhesion molecule-1 (MAdCAM-1), the primary homing receptor of the gut-mucosa for lymphocytes,10 in the involved spleens.
PATIENTS AND METHODS
A total of 18 cases of gastric MALT lymphoma, of which 15 were retrieved from the files of the Department of Pathology, Queen Elizabeth Hospital (Hong Kong) and 3 from the files of the Department of Histopathology, University College London Medical School (London, UK), were included in the study. A partial gastrectomy and splenectomy had been performed in each case. Paraffin-embedded formalin-fixed tissues were available in each case and fresh frozen tissues were available in 2 cases (nos. 3 and 4). The clinical features and histology of hematoxylin and eosin-stained sections were reviewed (Table 1). Case no. 4 has been the subject of a previous publication.6
Clinical Features of MALT Lymphomas Successfully Examined by Molecular Methods
| Patient . | Age/Sex . | Presentation . | Treatment . | Pathology . | Follow-Up . |
|---|---|---|---|---|---|
| 1 | 39/M | Gastrointestinal bleeding, barium meal showed “carcinoma” | Partial gastrectomy and splenectomy | Extensive low-grade MALT lymphoma of the stomach, gastric lymph nodes. Spleen 260 g, histologically involved. | Staging negative, radiotherapy, well for 14 yrs. Relapsed with lymphoma in the lung, cervical nodes and bone marrow. Chemotherapy administered, alive and well after 1.5 yrs. |
| 2 | 72/M | Epigastric discomfort, biopsy showed MALT lymphoma. | Partial gastrectomy and splenectomy | Extensive infiltration by low-grade MALT lymphoma of the stomach, gastric lymph nodes involved. Spleen 200 g, histologically involved. | Staging showed liver, marrow involvement. Chemotherapy administered, alive and well at 1.5 yrs. |
| 3 | 59/F | Dyspepsia, biopsy showed chronic gastritis. Gastric perforation, emergency surgery. | Partial gastrectomy, partial jenunectomy and splenectomy | Extensive low-grade MALT lymphoma of the stomach, gastric lymph nodes, small intestinal deposits. Spleen 200 g, histologically involved. | Staging showed bone marrow involvement. Chemotherapy administered, alive and well. |
| 4 | 63/M | Epigastric pain 4 yrs, biopsy showed MALT lymphoma | Partial gastrectomy, partial jenunectomy and splenectomy | Multifocal mucosal low-grade MALT lymphoma of the stomach, gastric lymph nodes negative, small intestinal deposit. Spleen 120 g, histologically uninvolved. | Chemotherapy administered, well for 2 yrs, then multiple lymphomatous infiltrates in ribs and vertebrae. Lost to follow-up. |
| 5 | 55/F | Epigastric discomfort 10 years, biopsy showed MALT lymphoma | Partial gastrectomy and splenectomy | Extensive low-grade MALT lymphoma of the stomach, gastric lymph nodes. Spleen 115 g, histologically uninvolved. | Chemotherapy administered, well for 18 mos. Dead of heart failure (?) due to doxorubicin cardiotoxicity. No evidence of recurrent lymphoma; no autopsy. |
| 6 | 64/M | NA | Partial gastrectomy and splenectomy | Low-grade MALT lymphoma, invading up to submucosa, gastric lymph node involved. Coexistence of gastric adenocarcinoma. Spleen normal size, histologically uninvolved. | NA |
| 7 | 74/F | NA | Partial gastrectomy and splenectomy | Predominantly high-grade lymphoma with focal low-grade MALT components, full thickness invasion, gastric lymph node involved. Spleen normal size, histologically uninvolved. | NA |
| 8 | 79/M | Epigastric pain. Endoscopy showed a large gastric ulcer and biopsy showed high-grade NHL. | Partial gastrectomy, partial jenunectomy and splenectomy | Predominantly high-grade lymphoma of the stomach with focal low-grade MALT components, gastric lymph node involved, small intestine deposit. Spleen 145 g, histologically uninvolved. | Staging showed widespread mediastinal lymphadenopathy and patient developed a vocal cord paralysis, chemotherapy administered, pulmonary shadowing (drug-induced alveolar damage?). Died 9 mos after surgery. |
| Patient . | Age/Sex . | Presentation . | Treatment . | Pathology . | Follow-Up . |
|---|---|---|---|---|---|
| 1 | 39/M | Gastrointestinal bleeding, barium meal showed “carcinoma” | Partial gastrectomy and splenectomy | Extensive low-grade MALT lymphoma of the stomach, gastric lymph nodes. Spleen 260 g, histologically involved. | Staging negative, radiotherapy, well for 14 yrs. Relapsed with lymphoma in the lung, cervical nodes and bone marrow. Chemotherapy administered, alive and well after 1.5 yrs. |
| 2 | 72/M | Epigastric discomfort, biopsy showed MALT lymphoma. | Partial gastrectomy and splenectomy | Extensive infiltration by low-grade MALT lymphoma of the stomach, gastric lymph nodes involved. Spleen 200 g, histologically involved. | Staging showed liver, marrow involvement. Chemotherapy administered, alive and well at 1.5 yrs. |
| 3 | 59/F | Dyspepsia, biopsy showed chronic gastritis. Gastric perforation, emergency surgery. | Partial gastrectomy, partial jenunectomy and splenectomy | Extensive low-grade MALT lymphoma of the stomach, gastric lymph nodes, small intestinal deposits. Spleen 200 g, histologically involved. | Staging showed bone marrow involvement. Chemotherapy administered, alive and well. |
| 4 | 63/M | Epigastric pain 4 yrs, biopsy showed MALT lymphoma | Partial gastrectomy, partial jenunectomy and splenectomy | Multifocal mucosal low-grade MALT lymphoma of the stomach, gastric lymph nodes negative, small intestinal deposit. Spleen 120 g, histologically uninvolved. | Chemotherapy administered, well for 2 yrs, then multiple lymphomatous infiltrates in ribs and vertebrae. Lost to follow-up. |
| 5 | 55/F | Epigastric discomfort 10 years, biopsy showed MALT lymphoma | Partial gastrectomy and splenectomy | Extensive low-grade MALT lymphoma of the stomach, gastric lymph nodes. Spleen 115 g, histologically uninvolved. | Chemotherapy administered, well for 18 mos. Dead of heart failure (?) due to doxorubicin cardiotoxicity. No evidence of recurrent lymphoma; no autopsy. |
| 6 | 64/M | NA | Partial gastrectomy and splenectomy | Low-grade MALT lymphoma, invading up to submucosa, gastric lymph node involved. Coexistence of gastric adenocarcinoma. Spleen normal size, histologically uninvolved. | NA |
| 7 | 74/F | NA | Partial gastrectomy and splenectomy | Predominantly high-grade lymphoma with focal low-grade MALT components, full thickness invasion, gastric lymph node involved. Spleen normal size, histologically uninvolved. | NA |
| 8 | 79/M | Epigastric pain. Endoscopy showed a large gastric ulcer and biopsy showed high-grade NHL. | Partial gastrectomy, partial jenunectomy and splenectomy | Predominantly high-grade lymphoma of the stomach with focal low-grade MALT components, gastric lymph node involved, small intestine deposit. Spleen 145 g, histologically uninvolved. | Staging showed widespread mediastinal lymphadenopathy and patient developed a vocal cord paralysis, chemotherapy administered, pulmonary shadowing (drug-induced alveolar damage?). Died 9 mos after surgery. |
Abbreviation: NA, not available.
Immunohistochemistry. Paraffin sections from the gastric MALT lymphoma, gastric lymph nodes, and spleen were immunostained with CD20, CD3, anti-IgM, anti-IgD, anti-κ Ig light chain, and anti-λ Ig light chain using the streptavidin-biotin method preceded by heat retrieval of antigen using a domestic pressure cooker.11 A cervical lymph node from case no. 1 was similarly studied.
In cases no. 3 and 4, frozen sections of spleen were immunostained with a monoclonal antibody against human MAdCAM-1.12 In case no. 4, to show the relationship of neoplastic B cells with MAdCAM-1 expression, frozen sections of the spleen were double immunostained with the anti-idiotype (7G3) recognizing the tumor Ig6 and antihuman MAdCAM-1 monoclonal antibody. The double immunostaining method has been described elsewhere.13
DNA preparation and microdissection. In each case, paraffin sections of tumor and spleen were used for DNA extraction.14 DNA samples were also prepared from an enlarged cervical lymph node in case no. 1 and an enlarged gastric lymph node in case no. 2. In cases no. 4 through 8, cell populations from the marginal zone, follicle center, and red pulp of the spleen were separately microdissected from hematoxylin and eosin-stained frozen or paraffin sections and DNA was prepared from these microdissected cells.15
PCR amplification of the rearranged Ig heavy chain genes. The Ig gene was PCR-amplified from framework (Fr) 2 or Fr3 to the joining (JH) region using a seminested PCR protocol.14 All PCR reactions were performed using a hot start procedure,16 and appropriate positive (a follicular lymphoma) and negative controls (without template DNA) were included in each experiment. All samples were analyzed in duplicate.
Cloning and sequencing of Ig PCR products. This was performed on 8 successfully amplified cases (Table 1). PCR products were purified on Sephacryl S-400 MicroSpin columns (Pharmacia, St Albans, UK), ligated to TA cloning vector pCR II and transfected into One Shot competent cells (INVαF′) according to the manufacturer's protocol (Invitrogen, Leek, The Netherlands). The transfected cells were plated onto LB-ampicillin agar plates containing X-gal. White colonies were transferred to a fresh LB-ampicillin agar plate containing X-gal and grown overnight for secondary selection. Confirmed white colonies were then transferred into 150 μL of LB medium containing 50 μg/mL ampicillin and cultured at 37°C for 4 hours. The cultures (100 μL) were centrifuged, resuspended in 20 μL of water, and heated at 98°C for 10 minutes. After centrifugation, the resulting supernatants were used for PCR with Sp6 and T7 primers.
The PCR products showing the expected insert size were sequenced using an ABI sequencer with dye terminators (Perkin Elmer, Warrington, UK). Six PCR clones from each sample were sequenced.
Sequence analysis. Analysis of sequences was performed using Wisconsin GCG software (provided by the Human Genome Mapping Project, Cambridge, UK) and the GenBank Database. The diversity and joining regions were identified from published germline D17-23 and JH sequences.24
Clone-specific PCR. In cases no. 4 through 8, primers specific to individual tumor clones were designed from the unique VDJ junction sequence of the tumor-derived Ig genes. The specificity of these primers were confirmed by searching the GenBank Database using FASTA program in the GCG (Wisconsin University, WI) software package. By combination of the clone-specific primer with appropriate consensus primer to the Fr2, Fr3, or JH region, clone-specific PCR was designed and applied for detection of tumor cell dissemination in various microcompartments of the spleen. The conditions for clone-specific PCR such as annealing temperature and magnesium concentration were optimized in each case using both positive (corresponding tumor DNA) and negative controls (10 to 15 nonrelated lymphoma samples). PCR was performed using an appropriate “touch down” program with a hot start procedure.14
RESULTS
Clinical features. Of the 18 cases examined, 8 cases were successfully analyzed by molecular methods and were the focus of the study. The clinical features of these cases are summarized in Table 1. Five of the patients were men and 3 were women. Their ages ranged from 39 to 79 years. All patients documented presented with epigastric pain or discomfort, and gastric biopsies were performed in 5. In patients no. 2, 4, 5, and 8, the biopsy showed low-grade MALT lymphoma, and in patient no. 3 the biopsy was interpreted as chronic gastritis associated with Helicobacter pylori infection (low-grade MALT lymphoma on review). Partial gastrectomy with splenectomy was performed in each case. In patients no. 3, 4, and 8, a small intestinal focus of lymphoma was resected. Postoperative staging showed marrow involvement in patients no. 2 and 3, with liver involvement in patient no. 2. Treatment comprised abdominal radiotherapy and/or chemotherapy. Patient no. 1 relapsed after 14 years with disease in the cervical lymph nodes, lung, and bone marrow and received chemotherapy. Patients no. 1 through 4 remain alive and well. Patient no. 5 died 3 years after gastrectomy but without evidence of recurrent lymphoma, and patient no. 8 died 9 months after gastrectomy.
Pathology. The histology of the gastric tumor in cases no. 1 through 6 was typical of low-grade MALT lymphoma, whereas the gastric tumor in cases no. 7 and 8 was predominantly high grade with focal low-grade MALT components. Reactive lymphoid follicles were present and these were surrounded by the neoplastic infiltrate, comprising centrocyte-like cells and their variants, which invaded the lamina propria, forming characteristic lympho-epithelial lesions. In cases no. 1, 2, 3, 5, 7, and 8, there was deep extension of the lymphoma into the muscularis, and in case no. 6, the tumor invaded the submucosa. All of the cases described above showed obvious lymph node involvement. In case no. 4, the lymphoma appeared confined to the mucosa. Lymph node involvement was confirmed in cases no. 1, 2, 3, 5, 7, and 8, and the lymphomatous nature of the small intestinal lesion was confirmed in cases no. 3, 4, and 8.
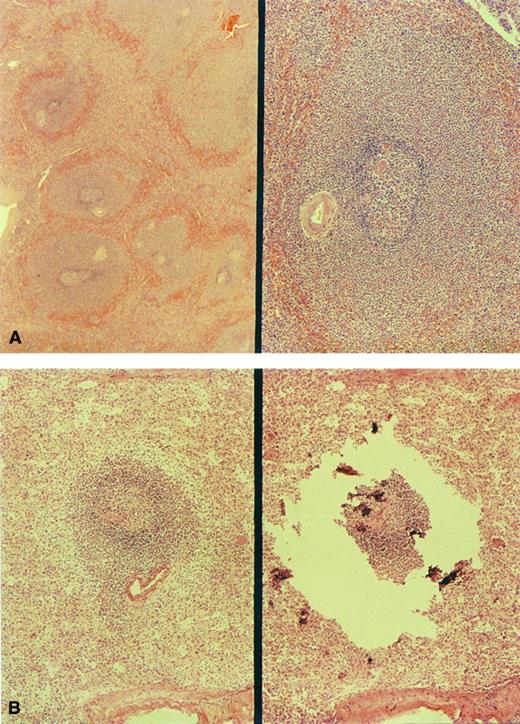
In cases no. 1 through 3, sections of spleen showed striking widening of marginal zones external to a preserved mantle (Fig 1A). The breadth of the marginal zones was up to three times the diameter of the follicle center. The cells within these areas resembled those of the gastric tumor. Small clusters of similar cells were present in the red pulp. Splenic histology in cases no. 4 through 8 was normal (Fig 1B).
Morphology of the spleen involved by MALT lymphoma. (A) The markedly enlarged marginal zones in case no. 1 (left, low-power view; right, high-power view). (B) marginal zone in case no. 4; right, microdissection of the same marginal zone cell population.
Morphology of the spleen involved by MALT lymphoma. (A) The markedly enlarged marginal zones in case no. 1 (left, low-power view; right, high-power view). (B) marginal zone in case no. 4; right, microdissection of the same marginal zone cell population.
Immunohistochemistry. In each case, cells comprising the gastric lymphoma expressed CD20; were IgM+, IgD−; and showed Ig light chain restriction (λ in case no. 2 and κ in the remaining cases). Staining with anti-IgD served to highlight the preserved polytypic mantle zone around reactive (polytypic) follicle centers. In cases no. 1 through 3, a similar staining pattern was seen in the splenic marginal zones that comprized IgM+, IgD− monotypic lymphoma cells external to a preserved IgD+ mantle. Small clusters of CD20+ cells in the red pulp showed the same light chain restriction as the cells in the marginal zone. No B-cell populations showing Ig light chain restriction could be detected in the spleen in the remaining cases. Specifically the splenic marginal zones were polytypic.
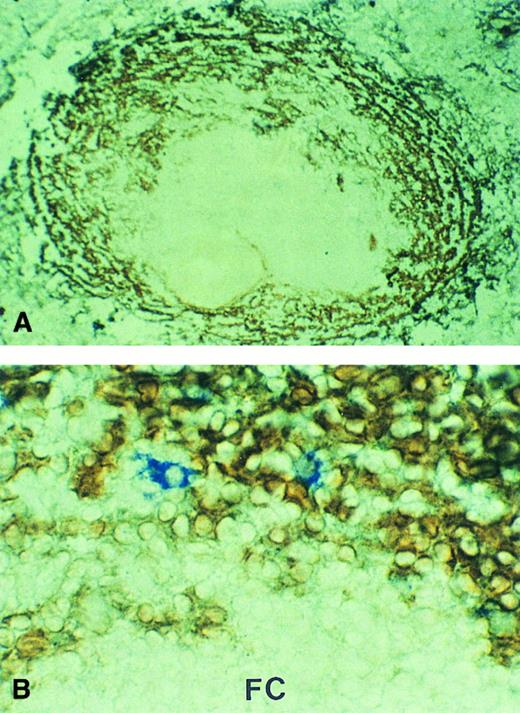
In cases no. 3 and 4, MAdCAM-1 was strongly expressed by marginal zone sinus lining cells surrounding the white pulp (Fig 2A). The pattern of expression was identical to that observed in histologically normal spleens removed for non-neoplastic diseases (data not shown). In case no. 4, double immunostaining showed that cells recognized by the anti-idiotype were almost exclusively located in the MAdCAM-1–expressing marginal zone sinuses (Fig 2B).
MAdCAM-1 expression in the spleen involved by MALT lymphoma. Marginal zone sinuses surrounding the white pulp showed strong MAdCAM-1 expression (brown). (A) Case no. 3, low-power view; (B) case no. 4, in which the tumor cells were immunostained by the anti-idiotype (blue; high-power view). FC, follicle center.
MAdCAM-1 expression in the spleen involved by MALT lymphoma. Marginal zone sinuses surrounding the white pulp showed strong MAdCAM-1 expression (brown). (A) Case no. 3, low-power view; (B) case no. 4, in which the tumor cells were immunostained by the anti-idiotype (blue; high-power view). FC, follicle center.
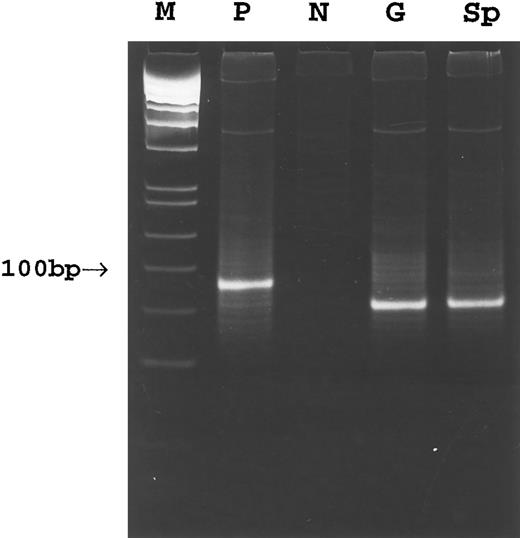
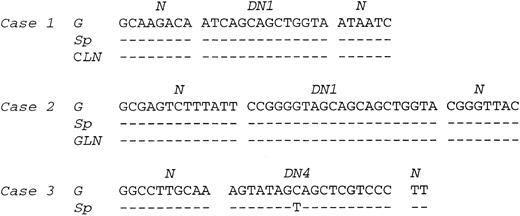
PCR. Amplification of the rearranged IgH genes using consensus primers was attempted in all 18 cases but successful only in cases no. 1 through 8. In the remaining 10 cases, amplification was unsuccessful due to poor DNA quality. PCR analysis of DNA extracted from gastric lymphoma tissue in cases no. 1 through 8 showed a monoclonal pattern. A monoclonal band identical to that of the gastric lymphoma was detected in DNA from the cervical lymph node in case no. 1, the gastric lymph node in case no. 2, and the spleen in cases no. 1 through 3 (Fig 3). A polyclonal pattern was found from DNA extracted from splenic tissue in cases no. 4 through 8. Further analysis of cell populations microdissected from the splenic marginal zone, follicle center, and red pulp in cases no. 4 through 8 also showed a polyclonal pattern (Fig 4A). Cloning and sequencing of the PCR products from different lesions of cases no. 1 through 3 showed an identical dominant clone in each case (Fig 5), indicating a common clonal lineage.
PCR amplification of the Fr3-JH region of the rearranged Ig gene in case 3. M, molecular weight marker; P, positive control; N, negative control; G, gastric tumor; Sp, spleen.
PCR amplification of the Fr3-JH region of the rearranged Ig gene in case 3. M, molecular weight marker; P, positive control; N, negative control; G, gastric tumor; Sp, spleen.
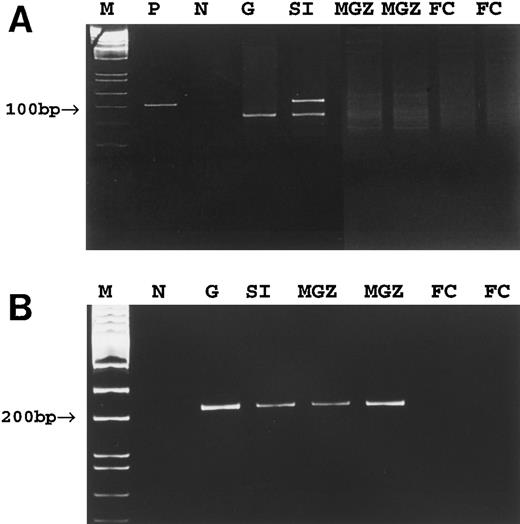
PCR detection of tumor clone in case no. 4. (A) Conventional PCR using consensus Fr3 and JH primers. (B) Tumor clone-specific PCR. M, molecular weight marker; P, positive control; N, negative control; G, gastric tumor; SI, small intestinal tumor; MGZ, microdissected cells from the marginal zone; FC, microdissected cells from the follicle center.
PCR detection of tumor clone in case no. 4. (A) Conventional PCR using consensus Fr3 and JH primers. (B) Tumor clone-specific PCR. M, molecular weight marker; P, positive control; N, negative control; G, gastric tumor; SI, small intestinal tumor; MGZ, microdissected cells from the marginal zone; FC, microdissected cells from the follicle center.
Comparison of VDJ sequences between gastric tumor, lymph node, and spleen lesions in cases no. 1 through 3. N and D regions are indicated. G, gastric tumor; Sp, spleen; CLN, cervical lymph node; GLN, gastric lymph node.
Comparison of VDJ sequences between gastric tumor, lymph node, and spleen lesions in cases no. 1 through 3. N and D regions are indicated. G, gastric tumor; Sp, spleen; CLN, cervical lymph node; GLN, gastric lymph node.
To determine whether tumor cells were present in the spleen of cases no. 4 through 8, despite being undetectable by conventional PCR, highly sensitive clone-specific PCR using primers specific to tumor clones (Fig 6) was used. Using this approach, an identical PCR band showing the expected size was detected in both the primary tumors and cell populations microdissected from the splenic marginal zone of cases no. 4 through 6 and red pulp of cases no. 4 and 5 (Fig 4B). However, no PCR products were detected in the cell populations microdissected from the follicle center of the spleen of these cases (Fig 4B). In cases no. 7 and 8, clone-specific PCR showed a band of the predicted size in the corresponding gastric tumor but not in the various microdissected cell populations of the spleen.
The VDJ junction sequence of cases no. 4 through 8. The region used as clone specific primer is indicated in bold and italic letters. In cases no. 4 through 7, the clone-specific primer was designed in reverse orientation and PCR was performed in conjunction with Fr2 (in cases no. 4 and 5) or Fr3 (cases no. 6 and 7) consensus primer, whereas in case no. 8, the clone-specific primer was designed in forward orientation and paired with LJH primer for PCR. G, gastric tumor; SI, small intestinal tumor. N and D region are indicated.
The VDJ junction sequence of cases no. 4 through 8. The region used as clone specific primer is indicated in bold and italic letters. In cases no. 4 through 7, the clone-specific primer was designed in reverse orientation and PCR was performed in conjunction with Fr2 (in cases no. 4 and 5) or Fr3 (cases no. 6 and 7) consensus primer, whereas in case no. 8, the clone-specific primer was designed in forward orientation and paired with LJH primer for PCR. G, gastric tumor; SI, small intestinal tumor. N and D region are indicated.
DISCUSSION
The identification of a marginal zone around the reactive B-cell follicles in Peyer's patches and the detection of marginal zone B cells within the dome epithelium, forming the so-called lympho-epithelium of MALT, first led to the suggestion that the marginal zone B cell could be the normal cell counterpart of this type of MALT lymphoma.1 The histologic features of MALT lymphoma in which lymphoma cells preferentially localize around reactive B-cell follicles both within the tumor and in involved lymph nodes are in keeping with this suggestion. Immunophenotypic studies have shown that MALT lymphoma cells share the immunophenotype of marginal zone B cells2 and genotypic investigations have shown that both marginal zone and MALT lymphoma B cells have mutated Ig genes.3-5 The demonstration in 6 of the cases reported here of specific homing of MALT lymphoma cells to the marginal zone of the spleen is further strong evidence that the marginal zone B cell is the normal cell counterpart of MALT lymphomas.
Identification of lymphoma cells in the spleen depends on the extent of tumor involvement and the sensitivity of detection methods used. In cases no. 1 through 3, in which striking enlargement of marginal zones was observed, both immunostaining of light chains and conventional PCR of rearranged Ig heavy chain genes indicated tumor involvement. This was further confirmed by sequence analysis of the PCR products. Whereas, in the remaining cases, in which no morphologic evidence of splenic dissemination was found, neither immunostaining for light chains nor conventional PCR showed splenic involvement by lymphoma. Of the 5 cases successfully analyzed by more sensitive clone-specific PCR, tumor cells were detected in the splenic marginal zone and red pulp in 3 cases. The limitation of conventional PCR in detection of scanty disseminated tumor cells may, therefore, account for the failure of detection of splenic dissemination in a previous study,9 in which no evidence of splenic involvement was found in 11 cases of gastric MALT lymphoma.
The prominent localization of disseminated MALT lymphoma cells to the splenic marginal zone but not other parts of the spleen suggests that there may be adhesive interactions between the tumor cells and the cells in this microcompartment. Mounting evidence has shown that the traffic of different lymphocyte subsets at various anatomical sites is controlled by the homing receptors expressed by the lymphocytes and the addressins expressed by the specialized vascular endothelia.25,26 The mucosal addressin MAdCAM-1 has been shown to be the main receptor for traffic of circulating lymphocytes to the mucosal tissues.10 In mice, it is expressed by the high endothelial venules of Peyer's patches and mesenteric lymph nodes and additionally by sinus lining cells of the splenic marginal zone.10,25,27 The ligand for MAdCAM-1 is the α4β7 integrin that is expressed by a subset of T and B lymphocytes28,29 and also, albeit inconstantly, by MALT lymphoma cells.30 The human homolog of murine MAdCAM-1 has been recently identified.31 The human MAdCAM-1 mRNA has been shown to be expressed by the mucosal tissues and spleen.31 Here, using immunohistochemistry, we confirm that MAdCAM-1 is strongly expressed by the sinus-lining cells of the human splenic marginal zone similar to that observed in the mouse spleen. This observation, together with the demonstration of tumor cells primarily in MAdCAM-1–positive areas of the spleen in case no. 4, suggests that the preferential localization of disseminated MALT lymphoma cells in the splenic marginal zone may be a reflection of their normal homing properties.
It should be emphasized that the histologic and immunohistochemical features of secondary splenic involvement by gastric MALT lymphoma are quite different from those of so-called splenic marginal zone lymphoma (SMZL). In that tumor, reactive follicle centers are surrounded or overrun by IgM+, IgD+ lymphoma cells that manifest a biphasic cytologic appearance.32 There is an inner zone of small cells with an outer zone of larger, sometimes nucleolated cells that superficially resemble marginal zone B cells. Importantly, the normal IgD+ polytypic mantle is not preserved and, thus, the lymphoma cells cannot be said to be in the marginal zone. Thus, despite its name, it is most unlikely that the normal cell counterpart of SMZL is the marginal zone B cell.
ACKNOWLEDGMENT
The authors thank Dr C.E. Keen for providing materials from case no. 8.
Supported by the Cancer Research Campaign.
Address reprint requests to Peter G. Isaacson, DM, FRCPath, Department of Histopathology, University College London Medical School, Rockefeller Bldg, University St, London WC1E 6JJ, UK.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal