Abstract
Clinical studies have indicated that folate deficiency may enhance the development of various malignancies. In animal studies that examined the effect of folate deficiency on malignancies, conflicting results have been reported. In some studies, folate deficiency increased the development and growth of malignant tumors; in others, it decreased the development and growth of malignancies. We examined the effect of transient folate deficiency on the development of leukemia in mice infected with the anemia-inducing strain of Friend leukemia virus. Friend virus disease can be considered as a model for human acute leukemias that are preceded by a preleukemic period. These include leukemias that develop in patients who received previous chemotherapy and/or radiation therapy, as well as patients with chronic granulocytic leukemia or myelodysplasia. Folate deficiency around the time of Friend virus-infection delayed the onset but increased the incidence of leukemia. The rates of rearrangement of the Spi-1 (PU.1 ) oncogene by provirus integration and alteration of the p53 tumor-suppressor gene were the same in leukemia cell lines derived from folate-deficient mice as they were in cell lines from control mice. These results indicate that folate deficiency did not exert its enhancement of leukemogenesis through changes in either Spi-1 or p53, even though these two genes have been found to be the most frequently altered ones in Friend virus-induced leukemias. Our results suggest that folate deficiency may enhance the development of acute leukemia in patients who are at high risk for this disease.
FOLATE AND VITAMIN B12 (Cobalamin) are essential nutrients. Deficiency of either leads to megaloblastic anemia. Megaloblastic anemia is a disease in which the hematopoietic precursor cells of erythrocytes, granulocytes, and platelets are destroyed by programmed cell death (apoptosis).1 This apoptosis can result directly from decreased intracellular folate in folate deficiency or indirectly from functionally decreased intracellular folate in cobalamin deficiency. Megaloblastic anemia can be rapidly reversed by the administration of the deficient vitamin, but these patients appear to be at increased risk for development of leukemia. Epidemiological studies of patients with cobalamin-treated pernicious anemia have shown threefold2 and fourfold3 increases in the risk of developing leukemia. In the first year after diagnosis, one study3 showed an incidence of leukemia at 0.0019% that was increased more than 13-fold over the expected rate of 0.00015%. Although no studies have been performed with large numbers of patients treated for megaloblastic anemia due to folate deficiency, a family with pancytopenias due to defective cellular uptake of folate had a greatly increased incidence of acute leukemia.4 Folate deficiency also appears to predispose to other types of malignancy. Decreased incidences of premalignant and malignant changes in uterine, cervical,5 bronchial,6 and colonic7 epithelia were found in susceptible patients who had received oral folate supplementation as compared with similar patients who were not supplemented. In animal models, conflicting results about the effects of folate deficiency on the development of malignancies have been reported. These conflicting results appear to be due to variations in experimental designs including the tumor model used and the timing, dosage, and form of folate used in dietary manipulations.8 Folate administration decreased growth of virus-induced mammary carcinomas in mice9, and chemically induced colonic cancers developed more frequently in rats fed a folate-free diet, as compared with controls.10 Although these studies were consistent with the human studies described above, other animal experiments had opposite results. Dietary deficiency of folate retarded the growth of transplanted Rous sarcomas in chicks11,12 and Walker carcinosarcomas in rats.13 Transgenic mice that develop nerve sheath tumors had a delayed onset when fed a folate-free diet.14 In yet another study, the incidence of mammary tumors was similar for rats that were folate-deficient and controls that were normal at the time of exposure to a chemical carcinogen.15
All previous animal studies examining the effects of folate deficiency on malignancies required the use of a tumor model, a transplanted tumor, a chemically or virus-induced tumor, or genetically determined tumor.8 In all of these studies, folate deficiency by itself has never been shown to have any leukemogenic effects. In many experiments with transiently but severely folate-deficient mice followed various periods up to 1 year after the deficiency, we have never had any mouse develop signs of leukemia. Thus, to study the effects of transient dietary folate deficiency on the development of leukemia, we used a murine leukemia model. This model uses the “anemia-inducing” strain of Friend leukemia virus (FVA) to induce a two-stage hematopoietic disease.16-18 The two phases of the disease caused by this retrovirus complex are (1) a preleukemic phase that varies in length from 2 months to more than 6 months after infection; and (2) a malignant, leukemic phase that promptly results in the death of the mouse. Thus, Friend virus disease can be considered as a model for human acute leukemias that are preceded by a preleukemic period. The preleukemic phase of Friend disease is characterized by an acute erythroblastosis that results in transient, abrupt splenomegaly such that the spleen increases from the normal 100 mg to greater than 1,000 mg by 3 to 4 weeks after infection. Hemorrhage and occasionally necrosis accompany this acute splenomegaly and they are associated with death in about 3% to 10% of mice at 3 to 4 weeks after infection. Accompanying this acute splenomegaly are moderate increases in leukocytes (white blood cells) due to immature erythroblasts in the blood, decreased platelets due to the increased spleen size, and slightly decreased hematocrit levels due to an increased blood volume that accompanies the splenomegaly.19 This acute erythroblastosis begins to resolve by about 5 to 6 weeks after infection. The erythroblastosis regresses, the spleen shrinks, and the blood counts return to the normal range. During this resolution period, which lasts about 3 to 4 weeks, there are no deaths because the acute erythroblastosis phase is resolving and the malignant leukemia phase has not yet begun to kill the mice. Malignant erythroleukemias develop at 9 weeks or more after infection. These leukemias are easily transplanted to other mice and frequently form growth factor-independent cell lines in vitro.20,21 In a majority of these leukemia cell lines that have been examined, provirus integration activates the Spi-1 (PU.1 ) gene.22-24 The other frequent genetic change found in these leukemia cell lines is a deletion, disruption, or mutation of the p53 tumor-suppressor gene.25,26 Other oncogenes are infrequently activated by Friend provirus integration in leukemic cell lines.27
MATERIALS AND METHODS
Female weanling (4-week-old) CD2F1 mice were purchased from the National Cancer Institute (Frederick, MD). The experimental groups of mice were fed an amino acid-based, folate-free diet28 (Dyets, Inc, Bethlehem, PA). Control mice received the same diet containing 2 mg folic acid/kg of diet. Both the folate-free and control diets contained 100 mg/kg of succinylsulfathiazole.28 Two weeks after starting the diets, the mice were infected with 103 spleen focus-forming units29 of Friend virus complex via tail vein injection. The FVA virus complex was the cloned pseudotype SFFVA /FRE cl-3/MuLV 201.30 The virus was originally obtained from Dr W.D. Hankins (National Cancer Institute) and maintained by passage of infectious plasma in BALB/c mice. One group of experimental mice, designated folate-deficient 1, was switched from the folate-free diet to the control diet after 1 month, ie, 2 weeks after FVA infection. A second group of experimental mice, designated folate-deficient 2, was switched from the folate-free diet to the control diet after 2 months, ie, 6 weeks after FVA infection. The control groups of mice were fed the control diet throughout. Figure 1 shows the temporal relationships between diets and FVA infection for each group of mice. In multiple previous experiments, uninfected mice were made folate-deficient by being fed the folate-free diet and then, after switching to the control diet, were observed for various periods up to 1 year. No mouse ever developed any evidence of leukemia, splenomegaly, hepatomegaly, or unexplained death.
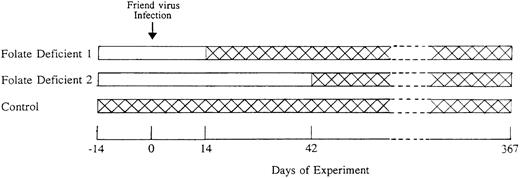
Scheme used to test the effects of folate deficiency in the early preleukemic phase of Friend virus disease. Two weeks before Friend virus infection (Day −14), mice were fed either folate-free diet (□) or control diet (). All mice were infected with Friend virus on Day 0. Folate deficient 1 group was switched to control diet after 4 weeks of folate-free diet (Day 14) and folate-deficient 2 group was switched to control diet after 8 weeks of folate-free diet (Day 42). The control group was fed the control diet throughout the entire course of the experiment. Mice were observed until they developed leukemia or they survived for 1 year.
Scheme used to test the effects of folate deficiency in the early preleukemic phase of Friend virus disease. Two weeks before Friend virus infection (Day −14), mice were fed either folate-free diet (□) or control diet (). All mice were infected with Friend virus on Day 0. Folate deficient 1 group was switched to control diet after 4 weeks of folate-free diet (Day 14) and folate-deficient 2 group was switched to control diet after 8 weeks of folate-free diet (Day 42). The control group was fed the control diet throughout the entire course of the experiment. Mice were observed until they developed leukemia or they survived for 1 year.
Two experiments were performed using these three groups of mice. In one experiment from 10 to 30 mice were killed immediately before virus infection and at 2, 6, 9, and 12 weeks after infection. Blood samples were obtained from the anesthetized mice just before cervical dislocation. The spleens and livers of the mice were weighed. Single-cell suspensions were made from the spleens by passing the contents through nylon mesh filters and counting with a hemacytometer. Nucleated spleen cell numbers rather than weights were used as a measure of spleen size, because mice occasionally had intrasplenic hemorrhage that increased spleen weights but did not affect nucleated cell numbers. The total folate content of the livers was determined using a Lactobacillus casei bioassay, as described previously.31 At 12 weeks, 3 of 30 mice from the control group and 2 of 30 mice from the folate-deficient 1 group were excluded because they had acute leukemia (see below for criteria of acute leukemias).
In the second experiment, the mice were monitored with periodic blood counts and examinations until they either died or they developed three or more of the following signs of leukemia: WBC greater than 50,000/μL, hematocrit levels less than 20%, ruffled fur, listlessness, palpable hepatosplenomegaly, or neurological deficits such as limb paralysis. Mice were killed when they developed three or more of these signs. Those mice that survived without any evidence of leukemia were killed at 1 year after infection. Splenic cells of mice killed in the terminal stages of leukemia or that survived for 1 year without evidence of leukemia were cultured at 2 × 105 viable cells/mL in a factor-independent, 14-day methylcellulose colony-forming assay.20 For each mouse, 5 × 106 viable, nucleated splenic cells were assayed. Leukemic cell lines were generated by in vitro expansion of individual colonies that developed in these colony-forming assays.
The leukemia cell lines derived from these mice were analyzed for provirus integration near the Spi-1 gene by Southern blotting of extracted DNA samples that were digested with the restriction enzymes BamHI, HindIII, or EcoRV.22 The Southern blots were hybridized with the cloned Pst I-Pst I restriction fragment “B” from the Spi-1 locus,22 which was a gift from Dr Sandra Ruscetti (National Cancer Institute). Alteration in the pattern of restriction fragment lengths of DNA that hybridized with the Spi-1 probe were considered to represent provirus integrations.
Total RNA extracted from each cell line was used to detect alterations in the p53 gene. Using a modification of the previously reported method,32 cDNA was generated from total RNA using Superscript II reverse transcriptase (GIBCO/BRL, Gaithersburg, MD). An aliquot of cDNA was amplified by polymerase chain reaction (PCR) using TaKaRa LA Taq polymerase. Primers used in the PCR reaction were generated using mouse p53 coding sequences33 as follows: primer 1, 5′ ATCTGTTGCTGCCCCAGGATGTTG 3′ (positions 285-308); primer 2, 5′ CCTGTCTTCCAGATACTCGGGATAC 3′ (positions 772-751); primer 3, 5′ AGAAGTCACAGCACATGACGGAGG 3′ (positions 639-662); primer 4, 5′ TGGTTTTTTCTTTTGCGGGGGAGAGG 3′ (positions 1114-1089). These two sets of primers amplified 829 bases of the murine p53 coding region, including the most frequently mutated exons. Dideoxyfingerprinting of the amplified cDNA was performed as described previously.32
Peripheral blood cell counts of mice during the preleukemic phase of Friend virus infection. The (A) leukocytes (WBC), (B) hematocrit, and (C) platelets were measured at various times after infection on day 0. The mice had been fed either folate-free diet or control diet at 2 weeks before infection. The folate-free diet was switched to control diet at 2 weeks after infection in the folate-deficient 1 group and at 6 weeks after infection in the folate-deficient 2 groups. The mice in each group that survived for 52 weeks without signs of leukemia were killed at that time. Data are ± 1 standard error of the mean (SEM) for 10 to 50 mice.
Peripheral blood cell counts of mice during the preleukemic phase of Friend virus infection. The (A) leukocytes (WBC), (B) hematocrit, and (C) platelets were measured at various times after infection on day 0. The mice had been fed either folate-free diet or control diet at 2 weeks before infection. The folate-free diet was switched to control diet at 2 weeks after infection in the folate-deficient 1 group and at 6 weeks after infection in the folate-deficient 2 groups. The mice in each group that survived for 52 weeks without signs of leukemia were killed at that time. Data are ± 1 standard error of the mean (SEM) for 10 to 50 mice.
Spleen and liver size and total liver folate from mice in the preleukemic phase. Mice were fed folate-free and control diets as described in the Materials and Methods and the Fig 1 legend. From 10 to 30 mice were killed at 2, 6, 9, 12, and 52 weeks. Those killed at 52 weeks had no signs of leukemia. (A) Nucleated spleen cells. (B) Liver weights. (C) Total liver folate. The early increase in size of the livers is due to growth from weanling mice into adults. Data are ± 1 SEM. of 10 to 30 mice.
Spleen and liver size and total liver folate from mice in the preleukemic phase. Mice were fed folate-free and control diets as described in the Materials and Methods and the Fig 1 legend. From 10 to 30 mice were killed at 2, 6, 9, 12, and 52 weeks. Those killed at 52 weeks had no signs of leukemia. (A) Nucleated spleen cells. (B) Liver weights. (C) Total liver folate. The early increase in size of the livers is due to growth from weanling mice into adults. Data are ± 1 SEM. of 10 to 30 mice.
RESULTS
The folate-free diet had effects on both the preleukemic phase and the subsequent malignant leukemic phase of Friend virus-infected mice. Figure 2 shows the blood cell counts and Fig 3 shows organ sizes and total folate content of the livers during the preleukemic phase of Friend virus infection. The 52-week data in Figs 2 and 3 are from mice that survived 1 year without evidence of leukemia. Each group of mice was followed over the course of 1 year and all deaths except one (see Fig 4 legend) were due to leukemia. Hepatomegaly was not found in any group in the preleukemic phase (compare liver weights of preleukemic mice in Fig 3B with those of leukemic mice in Table 1). Folate deficiency and prompt recovery after the switch to the control diet was documented in both folate-deficient groups (Fig 3C).
Leukemia incidence for mice in the latent phase of Friend virus disease. Mice were fed folate-free and control diets as described in the Materials and Methods and the Fig 1 legend. Mice were infected with Friend virus on day 0 and monitored for the subsequent year for the development of acute leukemia. The numbers of mice in each group that entered the latent part of the preleukemic phase as defined in results were 63 for controls, 63 for folate-deficient 1, and 44 for folate-deficient 2. All deaths were due to leukemia except for 1 mouse of the folate-deficient 2 group that was killed on day 238 (shown as +) with severe ascites and cystic kidneys, but normal liver, spleen, and WBC. This mouse was censored for statistical analysis.
Leukemia incidence for mice in the latent phase of Friend virus disease. Mice were fed folate-free and control diets as described in the Materials and Methods and the Fig 1 legend. Mice were infected with Friend virus on day 0 and monitored for the subsequent year for the development of acute leukemia. The numbers of mice in each group that entered the latent part of the preleukemic phase as defined in results were 63 for controls, 63 for folate-deficient 1, and 44 for folate-deficient 2. All deaths were due to leukemia except for 1 mouse of the folate-deficient 2 group that was killed on day 238 (shown as +) with severe ascites and cystic kidneys, but normal liver, spleen, and WBC. This mouse was censored for statistical analysis.
Hematologic Parameters and Liver Weights of Leukemic and Nonleukemic Mice
| . | WBC/mL (×10−3) . | Hematocrit (%) . | Platelets/mL (×10−3) . | Nucleated Spleen Cells (×10−6) . | Liver Weight (g) . |
|---|---|---|---|---|---|
| Control nonleukemic (n = 27) | 5.7 ± 1.7 | 45.0 ± 0.9 | 1,348 ± 38 | 359 ± 78 | 1.42 ± 0.05 |
| Control leukemic (n = 19) | 62.2 ± 9.0 | 31.5 ± 1.8 | 572 ± 55 | 1,124 ± 111 | 4.52 ± 0.30 |
| Folate-deficient 1 nonleukemic (n = 16) | 4.0 ± 0.6 | 44.9 ± 0.4 | 1,373 ± 53 | 337 ± 5 | 1.30 ± 0.05 |
| Folate-deficient 1 leukemic (n = 32) | 95.5 ± 16.8 | 31.1 ± 1.7 | 775 ± 86 | 1,369 ± 132 | 4.44 ± 0.26 |
| Folate-deficient 2 nonleukemic (n = 10) | 3.1 ± 0.0 | 45.0 ± 0.6 | 1,458 ± 54 | 228 ± 28 | 1.26 ± 0.05 |
| Folate-deficient 2 leukemic (n = 27) | 82.4 ± 9.7 | 28.0 ± 2.1 | 609 ± 61 | 1,428 ± 173 | 4.50 ± 0.38 |
| . | WBC/mL (×10−3) . | Hematocrit (%) . | Platelets/mL (×10−3) . | Nucleated Spleen Cells (×10−6) . | Liver Weight (g) . |
|---|---|---|---|---|---|
| Control nonleukemic (n = 27) | 5.7 ± 1.7 | 45.0 ± 0.9 | 1,348 ± 38 | 359 ± 78 | 1.42 ± 0.05 |
| Control leukemic (n = 19) | 62.2 ± 9.0 | 31.5 ± 1.8 | 572 ± 55 | 1,124 ± 111 | 4.52 ± 0.30 |
| Folate-deficient 1 nonleukemic (n = 16) | 4.0 ± 0.6 | 44.9 ± 0.4 | 1,373 ± 53 | 337 ± 5 | 1.30 ± 0.05 |
| Folate-deficient 1 leukemic (n = 32) | 95.5 ± 16.8 | 31.1 ± 1.7 | 775 ± 86 | 1,369 ± 132 | 4.44 ± 0.26 |
| Folate-deficient 2 nonleukemic (n = 10) | 3.1 ± 0.0 | 45.0 ± 0.6 | 1,458 ± 54 | 228 ± 28 | 1.26 ± 0.05 |
| Folate-deficient 2 leukemic (n = 27) | 82.4 ± 9.7 | 28.0 ± 2.1 | 609 ± 61 | 1,428 ± 173 | 4.50 ± 0.38 |
Nonleukemic mice are all mice that survived 1 year without developing evidence of leukemia.
The control group of mice in Figs 2 and 3 show the typical changes of the acute erythroblastosis after infection with the anemia-inducing stain of FVA. Folate deficiency inhibited the development of the acute erythroblastosis and delayed the sequence of events, as shown, for the folate-deficiency groups in Figs 2 and 3. The decrease in WBC for the folate-deficient 2 group at 6 weeks in Fig 2 is due to severely decreased erythroblasts and moderately decreased granulocytes, whereas lymphocyte numbers were not affected. However, once the folate-deficient mice were switched to the control diet, they promptly resumed the sequence of events that characterizes the acute erythroblastosis phase of the disease. The WBC and platelets had a “rebound” overshoot after the switch to control diet (Fig 2A and C) that was similar to rebound effects in the WBC and platelets that are found when uninfected, folate-deficient mice are switched from the folate-free to control diet.28 The splenic erythroblastosis also resumed its delayed expansion (Fig 3A) after the switch to control diet. This rebounding splenic erythroblastosis was rapid and accompanied by an increased number of deaths associated with hemorrhage, as compared with the control group. The rates of death in the acute erythroblastic phase of the second experiment, when mice were observed long term, was 2 of 67 (3%) in controls, 4 of 67 (6%) in the folate-deficient 1, and 17 of 61 (28%) in the folate-deficient 2 groups. This increased death rate associated with the rebound acute erythroblastosis phase is due to Friend virus disease because it was never found in normal, uninfected mice that were made folate-deficient and then re-fed the control diet.
In each group of mice, the period of deaths in the acute erythroblastosis phase was followed by a characteristic period of resolution when splenic erythroblastosis and WBC began to decrease and no deaths occurred. This resolution period is the beginning of the latent premalignant phase during which the malignant leukemias developed. This resolution period was used as the demarcation period between the acute erythroblastosis phase deaths and the malignant leukemia deaths. In the control group, the two acute-phase deaths occurred in week 4 (after infection) and the subsequent resolution period lasted until the first leukemic death in week 9; in the folate-deficient 1 group, the four acute-phase deaths were all in weeks 5 to 7 and the resolution period lasted until the first leukemic death in week 11; in the folate-deficient 2 group, the 17 acute-phase deaths were all in weeks 7 to 10 and the resolution period lasted until the first leukemic death in week 13.
Figure 4 shows the incidence of leukemia in the three groups of mice. Analysis by Fisher's exact test of leukemia incidence at 1 year shows a significant (P = .045) difference among the groups, with 75% leukemia incidence in both folate-deficient 1 and folate-deficient 2 groups and 57% in the control group. If the folate-deficient 1 and folate-deficient 2 groups are combined and compared with the control group, then P = .01. The usual log rank test cannot be used to compare the leukemia incidence because relative risk is clearly a function of time. Using a proportional hazards regression procedure in SAS (SAS version 6.12, SAS Institute, Inc, Cary, NC) a proportional hazards model with a time component was evaluated. Indicator variables at day 150 were used for folate-deficient 1 and folate-deficient 2 groups. This estimated separate relative risks before and after day 150 that were clearly indicated by the data. With this model both folate-deficient 1 and folate-deficient 2 leukemia incidence curves (hazard functions) were significantly different than the control group (P ≤ .01 for all comparisons) before day 150 and after day 150. The estimated risk ratio is approximately 0.8 before 150 days and 2.7 after 150 days for folate-deficient 1 and folate-deficient 2 groups. Thus, Fig 4 shows that, compared with the control group, both folate-deficient groups had a delayed onset but an increased incidence of leukemia.
The mice that developed leukemia (Table 1) had a very elevated WBC due to large numbers of leukemic blasts. These mice also had moderate anemia, thrombocytopenia, and extremely enlarged livers as compared with the nonleukemic mice. Using the Kruskal-Wallis test, the leukemic mice in each group varied significantly (P = .0001) from the nonleukemic ones in all groups for each hematologic parameter and organ size determined. By the same test, all leukemic subgroups were the same and all nonleukemic subgroups were the same for each hematologic parameter and organ size. Microscopic analyses of the livers showed extensive malignant cell infiltration in all of the leukemic mice. The histological preparations of the brains of mice with neurological deficits that were examined showed evidence of cerebral vascular occlusion with leukemic cells and nearby areas of inflammation and edema consistent with hypoxic damage. A few mice in each group had microscopic evidence of leukemic infiltration of other organs such as the kidneys, ovaries, or lymph nodes.
Clonal continuous leukemia cell lines were successfully established from individual leukemic mice at the following rates: 10 of 19 control mice (53%), 16 of 27 folate-deficient 1 mice (59%), and 9 of 20 folate-deficient 2 mice (45%). In all three groups, these cell lines were developed from leukemias that occurred throughout the year of the experiment. Factor-independent colony growth did not occur in the spleen cell cultures of the other killed mice despite leukemia in the blood, spleen, and liver of each mouse. Phenotypic analyses of the cell lines, including electron microscopy in several cases, have shown that none of the leukemia cell lines have phenotypic markers of lymphoid, granulocyte, or monocytic differentiation. Although none of the leukemic cell lines shows any evidence of hemoglobin synthesis, electron microscopy of the leukemic cells in situ in the spleens of mice from each group showed pinocytotic vesicles consistent with erythroleukemia.
We analyzed the leukemia cell lines for Spi-1 gene rearrangements by provirus integration. Six of 10 (60%) leukemia cell lines from control mice, 13 of 16 (81%) leukemia cell lines from folate-deficient 1 mice, and 5 of 9 (56%) leukemia cell lines from folate-deficient 2 mice had Spi-1 rearrangement (Table 2). Each cell line in Table 2 is from a different individual mouse. We also examined the frequency of p53 gene mutation in the leukemia cell lines derived from folate-deficient mice and control mice. Five of 10 (50%) cell lines from control mice, 11 of 16 (69%) cell lines from folate-deficient 1 mice, and 5 of 9 (56%) cell lines from the folate-deficient 2 group had p53 alterations (Table 2). Comparing cell lines from controls with those from the folate-deficient groups, none of the differences was significant in the frequency of Spi-1 rearrangement or p53 alterations. Thus, alterations of either Spi-1 or p53 did not account for the increased incidence of leukemia in the folate-deficient groups of Friend virus-infected mice.
Spi-1 Rearrangement and p53 Alterations in Leukemic Cell Lines
| Leukemic Cell Line . | Spi-1 Rearrangement . | p53 Alterations . | ||
|---|---|---|---|---|
| . | . | Base Change . | Codon . | Amino Acid Change . |
| Control-A | − | G → A | 170 | Val → Met |
| Control-B | + | |||
| Control-C | − | |||
| Control-D | + | G → T | 271 | Arg → Leu |
| Control-E | + | |||
| Control-F | + | |||
| Control-G | − | Deletion or disruption | ||
| Control-H | + | Deletion or disruption | ||
| Control-I | + | A → G | 213 | Ser → Gly |
| Control-J | − | |||
| Folate deficient 1 A | − | A → G | 278 | Arg → Gly |
| Folate deficient 1 B | + | C deletion | 311 | Frame shift |
| Folate deficient 1 C | + | T → G | 275 | Cys → Gly |
| Folate deficient 1 D | + | T → G | 275 | Cys → Gly |
| Folate deficient 1 E | + | G → A | 246 | Arg → His |
| Folate deficient 1 F | − | |||
| Folate deficient 1 G | + | G → A | 173 | Cys → Tyr |
| Folate deficient 1 H | + | |||
| Folate deficient 1 I | + | Deletion or disruption | ||
| Folate deficient 1 J | + | T → C | 108 | Leu → Pro |
| Folate deficient 1 K | + | Deletion or disruption | ||
| Folate deficient 1 L | + | |||
| Folate deficient 1 M | + | G → A | 256 | Glu → Lys |
| Folate deficient 1 N | − | C → T | 276 | Pro → Ser |
| Folate deficient 1 O | + | |||
| Folate deficient 1 P | + | |||
| Folate deficient 2 A | + | |||
| Folate deficient 2 B | − | T → A | 232 | Tyr → Asn |
| Folate deficient 2 C | − | |||
| Folate deficient 2 D | + | T → G | 275 | Cys → Gly |
| Folate deficient 2 E | + | A → C | 266 | Asp → Ala |
| Folate deficient 2 F | + | |||
| Folate deficient 2 G | − | Deletion or disruption | ||
| Folate deficient 2 H | + | |||
| Folate deficient 2 I | − | A → G | 152 | Ser → Gly |
| Leukemic Cell Line . | Spi-1 Rearrangement . | p53 Alterations . | ||
|---|---|---|---|---|
| . | . | Base Change . | Codon . | Amino Acid Change . |
| Control-A | − | G → A | 170 | Val → Met |
| Control-B | + | |||
| Control-C | − | |||
| Control-D | + | G → T | 271 | Arg → Leu |
| Control-E | + | |||
| Control-F | + | |||
| Control-G | − | Deletion or disruption | ||
| Control-H | + | Deletion or disruption | ||
| Control-I | + | A → G | 213 | Ser → Gly |
| Control-J | − | |||
| Folate deficient 1 A | − | A → G | 278 | Arg → Gly |
| Folate deficient 1 B | + | C deletion | 311 | Frame shift |
| Folate deficient 1 C | + | T → G | 275 | Cys → Gly |
| Folate deficient 1 D | + | T → G | 275 | Cys → Gly |
| Folate deficient 1 E | + | G → A | 246 | Arg → His |
| Folate deficient 1 F | − | |||
| Folate deficient 1 G | + | G → A | 173 | Cys → Tyr |
| Folate deficient 1 H | + | |||
| Folate deficient 1 I | + | Deletion or disruption | ||
| Folate deficient 1 J | + | T → C | 108 | Leu → Pro |
| Folate deficient 1 K | + | Deletion or disruption | ||
| Folate deficient 1 L | + | |||
| Folate deficient 1 M | + | G → A | 256 | Glu → Lys |
| Folate deficient 1 N | − | C → T | 276 | Pro → Ser |
| Folate deficient 1 O | + | |||
| Folate deficient 1 P | + | |||
| Folate deficient 2 A | + | |||
| Folate deficient 2 B | − | T → A | 232 | Tyr → Asn |
| Folate deficient 2 C | − | |||
| Folate deficient 2 D | + | T → G | 275 | Cys → Gly |
| Folate deficient 2 E | + | A → C | 266 | Asp → Ala |
| Folate deficient 2 F | + | |||
| Folate deficient 2 G | − | Deletion or disruption | ||
| Folate deficient 2 H | + | |||
| Folate deficient 2 I | − | A → G | 152 | Ser → Gly |
DISCUSSION
During the year after infection with FVA, the incidence of leukemia was increased in mice that were folate deficient around the time of infection as compared with normal controls. These results are consistent with epidemiological studies showing an increased incidence of leukemia in patients treated for cobalamin deficiency2,3 and in a family with impaired cellular transport of folate.4 Likewise, they are consistent with animal studies of mammary9 and colon carcinomas10 in rats. The increased incidence of leukemia in our studies appears to contradict other animal studies in which folate deficiency inhibited the growth of malignant tumors.11-14 However, the delayed onset of the leukemias in the folate-deficient groups during the first 150 days after infection (Fig 4) is consistent with these previous studies.11-14
The delayed onset of leukemia after a period of folate deficiency may be explained by results from our previous studies of splenic erythroblasts from folate-deficient mice at 2 weeks after FVA infection (ie, cells from the folate-deficient 1 group just before the switch to control diet1,34 ). When these folate-deficient erythroblasts were placed in short-term cultures under folate-deficient conditions, they had increased uracil misincorporation into DNA34 and increased programmed cell death (apoptosis).1 Increased uracil misincorporation into DNA in folate-deficient hematopoietic cells has been associated with decreased deoxyuridylate methylation due to decreased 5,10-methylenetetrahydrofolate. The increased ratio of deoxyuridylate to thymidylate ultimately leads to uracil misincorporation into DNA in place of the normal thymine.35-37 When the uracil misincorporation into DNA is slight, the uracil is removed and the DNA repair is successfully completed. When the misincorporation is more prevalent, the uracil removal and DNA repair occurring simultaneously in the same area but on opposite DNA strands can create a double-stranded cleavage of DNA.38 Thus, slight folate deficiency may have no significant effect on cells because DNA damage would be limited and cellular repair mechanisms could correct the damage. In severe folate deficiency, the extensive DNA damage could overwhelm the repair mechanisms and apoptosis would be triggered. Thus, severely folate-deficient hematopoietic cells with genetic changes that normally would lead to leukemic transformation could be destroyed by apoptosis before they were able to proliferate. These premature deaths of potentially leukemic cells would result in the delayed onset of leukemias found in the first 150 days after infection in mice that were folate deficient during the preleukemic phase of Friend virus disease. In moderately folate-deficient cells, the DNA damage would be sufficient to cause dysfunction of specific genes but yet the damage might not be sufficient to trigger apoptosis. In these cases, the hematopoietic cells acquire their genetic damage during the period of folate deficiency and then later, after folate levels have returned to normal, their progeny can become the leukemic cells.
The mechanism by which folate deficiency around the time of FVA infection leads to increased leukemic transformation at later times is unknown. The DNA damage associated with folate deficiency appears to interact with the leukemogenic action of the FVA infection. The sites of provirus integration into the genome have been shown to be important in the leukemias that have developed in Friend virus-infected mice. The gene most frequently affected by provirus integration in leukemia cell lines derived from Friend virus-infected mice is Spi-1.22 Integrations in this region have been reported in 72% and 95% of these leukemia cell lines.22,24 Our results show that a majority of the leukemias that developed in each group (folate-deficient 1, folate-deficient 2, and control) had provirus integration near the Spi-1 locus (Table 2). Although the percentages of Spi-1 rearrangements in our cell lines were slightly less than reported previously, they were similar in all groups indicating that folate deficiency did not affect the frequency of integration in this area of the genome. Although provirus integrations near oncogenes other than the Spi-1 are uncommon in leukemia cells,27 the rate of such provirus integrations may be greater in folate-deficient mice for at least three reasons. First, proviruses integrate into the genome during the S-phase of the cell cycle. Because a prolongation of S-phase has been associated with folate deficiency,39 such a prolongation may permit more provirus integration in folate-deficient cells and thus more chance for oncogene activation. Second, DNA damage and repair associated with increased uracil misincorporation due to folate deficiency in Friend virus-infected erythroid progenitor cells34 may provide increased sites for provirus integration near an oncogene. Third, hematopoietic progenitor cells that are the likely targets for leukemic transformation, as opposed to more differentiated cells, are increased by folate deficiency in mice.28
In leukemia cell lines derived from Friend virus-infected mice, p53 can be altered by point mutations, deletions, or disruption by provirus integration.25,26 The p53 protein is a transcription regulator of genes that appear to have roles in the regulation of DNA replication and cell cycle progression.40,41 The normally low levels of p53 protein in cells increase with stimuli that induce DNA damage such as γ-irradiation and chemotherapeutic agents.42-45 In the preleukemic phase of Friend virus disease, p53 protein and the product of its target gene, p21CIP1/WAF1 (p21 ), both accumulate in folate-deficient erythroblasts.34 These p53 and p21 proteins appear to be induced by DNA damage in the folate-deficient cells and are involved in cellular attempts to repair the DNA damage before cell replication. When p53 protein is absent or mutant, DNA damage that would normally be repaired is not corrected. Inability to repair DNA damage would, in turn, allow more leukemogenic alterations in DNA to persist and ultimately lead to enhanced malignant transformation. Although folate deficiency in rats can lead to DNA strand breaks within p53 in the liver,46 our data in Table 2 show that p53 alterations per se are not increased by folate deficiency in leukemia cell lines from Friend virus-infected mice. However, some target gene(s) of p53 or other genes involved in cell cycle progression or DNA repair that perform a tumor-suppressor function may be altered by folate deficiency and, thereby, account for some of the increased incidence of leukemic transformation in the folate-deficient, Friend virus-infected mice.
Friend virus disease with its preleukemic period of variable length and its high rate of transformation into acute leukemia can be considered as a model for those human acute leukemias that are preceded by a preleukemic period. These acute leukemias include those that arise from bone marrow diseases such as chronic granulocytic leukemia and myelodysplasia. They also include treatment-related leukemias that arise in patients that have previously been exposed to chemotherapy and/or radiation therapy during the treatment of another malignancy.47,48 Chemotherapy and/or radiation therapy are weaker leukemogenic stimuli than Friend virus, but they have been estimated to account for 10% to 15% of acute leukemia and myelodysplasia.47 Our in vivo studies presented here show that transient folate deficiency in Friend virus-infected mice leads to increased leukemic transformation. Previous in vitro studies showed that folate deficiency can act synergistically with DNA damage caused by chemotherapy and irradiation.49 Together, these studies suggest that patients with chronic granulocytic leukemia or myelodysplasia and patients receiving chemotherapy and/or radiation therapy would benefit from close examination of folate and cobalamin status with aggressive supplementation when indicated.
ACKNOWLEDGMENT
The authors thank Sarvadana Rana, Judith Luna, Carol Howard, Rosalind Hollaway, and Joe Boyd for technical assistance. We also thank Dr. George Reed for statistical analyses and Dr. John Cousar for assistance in analyzing pathological specimens.
Supported by Grant No. 94-B80 from the American Institute of Cancer Research (M.J.K.), Merit Review Grants from the Department of Veterans Affairs (M.J.K. and D.W.H.), NIDDK Grant No. DK-32189 (D.W.H.), and the Turner Foundation (J.A.W.). D.J.P. is the recipient of a Florence A. Carter Fellowship in Leukemia Research from the American Medical Association Education and Research Foundation.
Address reprint requests to Mark J. Koury, MD, 547 Medical Research Bldg II, 2220 Pierce Ave, Vanderbilt University, Nashville, TN 37232-6305.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal