Abstract
Coagulation factor IX deficiency causes hemophilia B in humans. We have used gene targeting to develop a coagulation factor IX-deficient (factor IX-knockout) mouse strain. Mouse embryonic stem (ES) cells were targeted by a socket-containing vector that replaces the promoter through exon 3 of the factor IX gene by neoΔHPRT, which is a functional neo gene plus a partially deleted hypoxanthine phosphoribosyl transferase minigene. Chimeric mice generated using these socket-containing ES cells transmitted the targeted factor IX gene to their female offspring. Male offspring from these females were characterized and shown to exhibit a phenotype similar to hemophilia B. This factor IX-deficient mouse strain will be useful for studying gene therapy methods and structure-function relationships of recombinant factor IX proteins in vivo.
FACTOR IX DEFICIENCY, resulting either from reduced production of the functional factor IX protein or from production of a defective protein, causes the bleeding disorder hemophilia B.1 Hemophilia B, which afflicts 1 of every 25,000 to 30,000 males, is responsible for almost 25% of all hemophilic patients and is classified as either severe (<1% activity), moderate (1% to 5%), or mild (>5% to 30%). Severe hemophilia B causes spontaneous bleeding and joint bleeding in affected patients.2
Hemophilia B is a good model for studying the efficacy of gene therapy because deficiency in coagulation factor IX alone is responsible for this bleeding disorder, the biochemistry of factor IX and the pathophysiology of the disease are well characterized, and factor IX levels as low as 5% result in significant amelioration of the disease.3-6
Currently, only the dog model carrying spontaneous mutation of the factor IX gene is available for studying hemophilia B.7-9 There are several potential advantages to a mouse model of hemophilia B over the dog model. The gestation period of mice is much shorter, ie, 21 days compared with 63 days for dogs, and much less space is required to maintain a mouse colony. Although several groups have used normal mice to test the efficacy of factor IX gene therapy,10-13 the presence of endogenous factor IX limits the application of this system.
A factor IX-deficient mouse strain would be useful not only for studying gene therapy, but also for studying the functions of mutant factor IX in vivo. Many mutant factor IX proteins generated using recombinant DNA techniques have been shown to have interesting characteristics in vitro,14 but information regarding their functions in vivo is lacking.
We here use the plug-socket gene targeting method15 to generate a factor IX-knockout mouse strain. DNA specifying the promoter through exon 3 of a mouse embryonic stem (ES) cell factor IX gene was replaced by a socket that includes DNA encoding a functional neo gene plus a partially deleted hypoxanthine phosphoribosyl transferase gene (neoΔHPRT). We chose to use the plug-socket strategy because the socket in the targeted ES cell line will allow future experiments in which different plugs can, with the help of positive selection for a functional HPRT gene, be used to generate a series of modified factor IX genes. Thus, different plugs can be designed that carry any desired modification in the promoter or exons 1 through 4 of the factor IX gene, thereby generating mouse strains with specific factor IX mutations.
MATERIALS AND METHODS
Isolation of murine factor IX genomic DNA. A cDNA fragment of 280 bp containing exon 1 to exon 3 of the murine factor IX gene was used to probe a genomic DNA library made from BamHI-digested ES cell strain E14TG2a (subclone BK4).15 A fragment of 14 kb was isolated and partially sequenced.
Socket targeting construct. Socket/F9 (Fig 1) was constructed in the pPNT vector (a kind gift from Dr R.C. Mulligan, Massachusetts Institute of Technology, Cambridge, MA)16 using standard recombinant DNA techniques.17 It contains 5′ and 3′ homology regions of the factor IX gene, the socket, and a negative selection marker. The 1-kb 5′ homology region containing 5′ untranscribed sequences of the factor IX gene is followed by the socket, neoΔHPRT, which consists of the neo gene and a partially deleted, nonfunctional HPRT minigene. The neoΔHPRT genes are followed by the 5.5-kb 3′ homology region, which contains part of intron 3, exon 4, and part of intron 4. The negative selection marker, HSV-tk gene,16 is joined to the 3′ end. The whole construct is about 20 kb and can be linearized with Not I.
Scheme for the homologous recombination between the targeting vector (socket/F9) and the endogenous factor IX gene of the murine ES cells. Homologous recombination causes replacement of a 5.5-kb segment that includes the promoter to exon 3 region of the factor IX gene with the socket (neoΔHPRT) in the ES cells. Restriction sites changed by the homologous recombination caused the targeted DNA to yield a 7-kb instead of the wild-type 14-kb fragment after BamHI digestion and a 12-kb instead of the wild-type 8.8-kb fragment after Bgl II digestion. The G418 resistance gene (neo ) and the thymidine kinase gene (TK ) are used for positive and negative selection, respectively. Locations of PCR primers and the PE449 probe for Southern blot analysis are indicated. Thick black lines indicate genomic DNA and regions of homology. Exons 1, 2, 3, and 4 of the murine factor IX gene are labeled. B, BamHI; B′, Bgl II.
Scheme for the homologous recombination between the targeting vector (socket/F9) and the endogenous factor IX gene of the murine ES cells. Homologous recombination causes replacement of a 5.5-kb segment that includes the promoter to exon 3 region of the factor IX gene with the socket (neoΔHPRT) in the ES cells. Restriction sites changed by the homologous recombination caused the targeted DNA to yield a 7-kb instead of the wild-type 14-kb fragment after BamHI digestion and a 12-kb instead of the wild-type 8.8-kb fragment after Bgl II digestion. The G418 resistance gene (neo ) and the thymidine kinase gene (TK ) are used for positive and negative selection, respectively. Locations of PCR primers and the PE449 probe for Southern blot analysis are indicated. Thick black lines indicate genomic DNA and regions of homology. Exons 1, 2, 3, and 4 of the murine factor IX gene are labeled. B, BamHI; B′, Bgl II.
Electroporation and selection of socket/F9-targeted ES cell clones. ES cells were transfected with 10 μg of the linearized socket/F9 construct (1.5 nmol/L) using electroporation as previously described.15 Transfected cells were selected in ES cell medium (Dulbecco's Modified Eagle Medium-H medium from GIBCO BRL [Gaithersburg, MD] with 15% fetal calf serum, 0.1 mmol/L β-mercaptoethanol, and 0.1 mmol/L L-glutamine) with G418 (200 μg/mL) and ganciclovir (2 μmol/L).
Isolation of ES cell DNA. Individual ES cell colonies were expanded in 24-well plates, washed with phosphate-buffered saline, and treated for 1 day at 37°C with 200 μL salt lysis buffer (100 mmol/L NaCl, 100 mmol/L EDTA, 50 mmol/L Tris-HCl pH 8.0, 1% sodium dodecyl sulfate [SDS]) in the presence of 100 μg/mL proteinase K. Seventy-five microliters of saturated NaCl solution was added, and the mixture was centrifuged in a microcentrifuge (CS-15; Shelton Scientific, Shelton, CT) for 5 to 10 minutes. The supernatant was ethanol precipitated and dissolved in TE (pH 8.0) buffer.
Isolation of tail DNA. One centimeter of mouse tail was treated with 400 μL tail lysis buffer (50 mmol/L Tris-HCl, 0.1 mol/L EDTA, 125 mmol/L NaCl, 1% SDS) and 100 μg/mL proteinase K at 55°C for 12 to 16 hours. Two hundred microliters of saturated NaCl solution was added, and the steps listed above for isolation of ES cell DNA were then followed.
Polymerase chain reaction (PCR) and Southern blot analysis on ES cell and tail DNA. For PCR, a forward primer, 5′-ATATACAGTTACCAAATTCAGA-3′, and a reverse primer, 5′-CAGTAATGTTGACTGTATTTTCCAA-3′, were used. These primers give a 1.4-kb PCR product from targeted DNA. For Southern analysis, 32P-labeled probe PE449 was used. PE449 is a 449-bp fragment from the cloned genomic DNA containing exon 4 of the murine factor IX gene (Fig 1). DNA, after BamHI or Bgl II digestion, was separated by electrophoresis in a 0.7% agarose gel and transferred to Hybond-N membrane (Amersham, Arlington Heights, IL). When probed with PE449, the BamHI digest of the DNA from wild-type cells showed a 14-kb fragment, whereas the targeted cells showed a 7-kb fragment. After the Bgl II digest, the wild-type showed a 8.8-kb fragment and the targeted DNA showed a 12-kb fragment. Both BamHI and Bgl II digestions were performed on the ES cell to confirm the socket/F9 targeting. BamHI digestions were performed on tail DNA to confirm the targeted factor IX mutation of the mice.
Production of the factor IX-knockout mouse strain. The socket/F9-targeted ES cell line was injected into blastocyst strain C57BL6 and implanted into a pseudopregnant mouse. Male chimeric offspring of these mice were crossed with C57BL6 females. Agouti female offspring of these chimeric mice were back-crossed with C57BL6 males to generate male mice lacking factor IX. Their wild-type litter mates were used as controls in the experiments.
Reverse transcriptase-PCR (RT-PCR). Total RNA was isolated from livers of normal and factor IX-knockout mice (total RNA isolation kit; Promega, Madison, WI). First-strand cDNA was synthesized using random primers and reverse transcriptase. Primers made from exon 4 (forward), 5′-ATGCTGGTGCCAAGTTGG-3′, and exon 6 (reverse), 5′-GGCACCATCAGTGATGTC-3′, of the murine factor IX cDNA were used. Primers from the murine factor VII gene, 5′-AGCCAGATGAGGTGTCCT-3′ (forward) and 5′-CGTAGTCAGTGAAGGTCA-3′ (reverse), and β-actin genes, 5′-CCTTCCTGTGCATGGAGTCCT-3′ (forward) and 5′-GGAGCAATGATCTTGATCTTC-3′ (reverse), were used.
Activated partial thromboplastin time (APTT) factor IX assay. Clotting activities of the normal and factor IX-knockout mice were measured using plasma from blood withdrawn from the retro-orbital area of anesthetized mice. Tested mice were 8 to 10 weeks old. Blood was collected and diluted into a final concentration of 0.4% sodium citrate within 5 to 10 seconds. Mouse plasma was diluted with 20 mmol/L Tris-HCl (pH 7.5) and 100 mmol/L NaCl. The APTT factor IX assay was performed as follows: 100 μL of APTT reagent (Sigma, St Louis, MO), 100 μL of human factor IX-deficient plasma (Sigma), and 100 μL of sample plasma were incubated at 37°C for 3 minutes. One hundred microliters of 25 mmol/L CaCl2 was then added and the time required for clot formation was recorded. A standard curve was established using pooled mouse plasma obtained from 5 normal male mice collected by the same method.
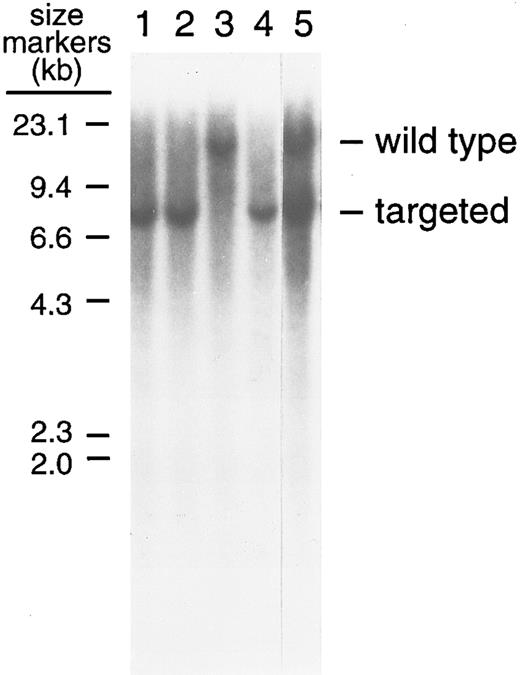
Southern blot analysis of the BamHI digest of tail DNA isolated from factor IX-knockout and normal mice. Analyses were performed as described in the Materials and Methods. After BamHI digestion, DNA was electrophoresed in a 0.7% agarose gel and transferred to a Hybond-N membrane. Factor IX-knockout mice gave the expected 7-kb and wild-type mice the expected 14-kb fragment after hybridization to the 32P-labeled probe PE449. Lanes 1, 2, and 4 show factor IX-knockout male mice; lane 3 shows a normal male mouse; and lane 5 shows a (female) hemophilia B carrier.
Southern blot analysis of the BamHI digest of tail DNA isolated from factor IX-knockout and normal mice. Analyses were performed as described in the Materials and Methods. After BamHI digestion, DNA was electrophoresed in a 0.7% agarose gel and transferred to a Hybond-N membrane. Factor IX-knockout mice gave the expected 7-kb and wild-type mice the expected 14-kb fragment after hybridization to the 32P-labeled probe PE449. Lanes 1, 2, and 4 show factor IX-knockout male mice; lane 3 shows a normal male mouse; and lane 5 shows a (female) hemophilia B carrier.
RESULTS
Production of the factor IX-knockout mouse strain. Cloned factor IX genomic DNA that contained the promoter and exons 1, 2, 3, and 4 of the murine factor IX gene was isolated and shown to have the same sequence as published earlier.18,19 In addition, the exon/intron boundaries are conserved relative to the sequence of the human factor IX gene.20
Targeting was performed as shown in Fig 1 and described in the Materials and Methods. Three of 300 clones resistant to G418 and ganciclovir were confirmed to have their factor IX gene targeted by the vector (socket/F9; Fig 1) using the PCR and Southern blot analysis (data not shown). The overall targeting frequency was 7 per 107 electroporated cells.
Four of five male chimeric mice generated from the socket/F9-targeted ES cells transmitted the ES cell genome to their offspring (indicated by their agouti coat color). Because the factor IX gene is on the X chromosome and the ES cell used was male, all agouti female offspring of the chimeras carry the targeted factor IX gene; they are equivalent to hemophilia B carriers (always female) in humans. Male factor IX-knockout mice were generated by back-crossing the agouti female offspring to C57BL6 male mice. Of the male mice characterized, 41% (n = 240) were factor IX-knockouts.
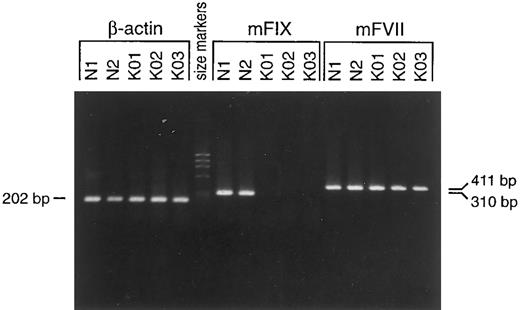
Phenotype of hemophilia B in the factor IX-knockout mice. Targeted mutation in the factor IX-knockout mice was confirmed by PCR (data not shown) and Southern blot analysis (Fig 2). There is a remote possibility that the remaining gene, including exons 4 to 8 (coding for the EGF-1, EGF-2, activation, and catalytic domains) could be transcribed using the promoter of the neo gene. To investigate this possibility, we isolated total RNA from mouse livers and performed an RT-PCR assay with primers specifically designed for those portions of the gene that remain after targeting. Although no RT-PCR product was detected in the knock-out strain, normal mice gave the expected band of 310 bp (Fig 3). The similar intensities of the RT-PCR products of the factor VII and β-actin genes (411 and 202 bp, respectively; Fig 3) in the normal and factor IX-knockout mice samples indicated normal transcription of the factor VII and β-actin genes in the livers of these mice. In addition, the integrity of the RNA samples was confirmed by the similar amounts of factor VII and β-actin gene transcription products in both the normal and factor IX-knockout mice. The possibility of low level transcription is unlikely, because the factor IX transcript remained detectable even at 40-fold diluted levels of RNA from livers of normal mice (data not shown). On the other hand, neo transcript in the livers of factor IX-deficient mice was detectable when primers specific for the neo gene were applied (data not shown). These results indicate that there is no transcription of the factor IX gene in the factor IX-knockout mice and, thus, that they are completely factor IX-deficient.
Analysis of total liver RNA for the murine factor IX gene in factor IX-knockout and normal mice by RT-PCR. RT-PCR was performed as described in the Materials and Methods. A 310-bp RT-PCR product was detected in total liver RNA from wild-type but not from the factor IX-knockout mice. RT-PCR products of the factor VII (411 bp) and β-actin (202 bp) genes for the factor IX-knockout mice were no different from those of normal mice. Lanes are as follows: N1 and N2, normal male mice; KO1, KO2, and KO3, factor IX-knockout male mice. The primers used are for the β-actin gene, the murine coagulation factor VII gene, and the murine coagulation factor IX gene as indicated.
Analysis of total liver RNA for the murine factor IX gene in factor IX-knockout and normal mice by RT-PCR. RT-PCR was performed as described in the Materials and Methods. A 310-bp RT-PCR product was detected in total liver RNA from wild-type but not from the factor IX-knockout mice. RT-PCR products of the factor VII (411 bp) and β-actin (202 bp) genes for the factor IX-knockout mice were no different from those of normal mice. Lanes are as follows: N1 and N2, normal male mice; KO1, KO2, and KO3, factor IX-knockout male mice. The primers used are for the β-actin gene, the murine coagulation factor VII gene, and the murine coagulation factor IX gene as indicated.
The factor IX-knockout mice are not visually different from normal mice until symptoms such as hemorrhagic swelling of the top of the feet or of the footpads or pale footpads occur. These features are caused by spontaneous bleeding in the factor IX-knockout mice. The factor IX-knockout mice survive well if no injury occurs, although sudden death of these mice was observed occasionally during growth, mostly as a consequence of internal hemorrhage after normal fighting with their cage mates. Physical examination and autopsy of the dead mice showed massive hemorrhages, mostly subcutaneous or in the dorsal surface musculature. Also, intra-cranial hemorrhages, hemorrhages in the axilliary and inguinal areas, hemorrhages around the salivary glands, and hemorrhages in the pericardium were observed. When tails of the factor IX-knockout mice were cut, they bled and died if the wound was not cauterized. Another commonly observed symptom was splenomegaly, indicating anemia caused by blood loss.
The relative clotting activities of plasma from factor IX-knockout mice and normal mice measured by APTT factor IX assays are shown in Table 1. The factor IX-knockout mouse plasma has reduced clotting activity compared with the normal mouse plasma, but exhibits about 8% residual activity.
Relative Clotting Activities of Plasma From Factor IX-Knockout Mice and Normal Mice Using APTT Factor IX Assay
| Mice . | Average Clotting Activity (%) . | Range (%) . |
|---|---|---|
| Normal* | 100 ± 32† | 55-185 (n = 22) |
| Factor IX-knockout | 8.4 ± 1.9† | 5.2-13.1 (n = 19) |
| Mice . | Average Clotting Activity (%) . | Range (%) . |
|---|---|---|
| Normal* | 100 ± 32† | 55-185 (n = 22) |
| Factor IX-knockout | 8.4 ± 1.9† | 5.2-13.1 (n = 19) |
APTT factor IX assay was performed as described in the Materials and Methods.
Defined by the clotting activity of 1:5 dilution of pooled normal mouse plasma.
Mean ± SD.
DISCUSSION
This study describes the generation of a coagulation factor IX-deficient mouse strain using gene targeting. Our results show that these mice carry disrupted factor IX genes, do not express the factor IX gene, and exhibit the phenotype characteristic of severe hemophilia B in humans.
In human severe hemophilia B, factor IX activity in clotting assays is always less than 1% of normal. Surprisingly, we detected about 8% clotting activity in the APTT assay in the factor IX-knockout mouse plasma. This result is inconsistent with the lack of factor IX expression and with the bleeding symptoms exhibited in these mice. The activity is likely an artifact of the assay due to one of the following reasons. Because plasma was collected from blood through the retro-orbital region using unsiliconized glass capillary tubes, blood samples may be exposed to tissue factor and, thus, partially activated through the extrinsic coagulation pathway. On the other hand, because the APTT assay was performed using human plasma, the discrepancy may result from species differences. For example, assays performed comparing normal mouse plasma and normal human plasma in human factor IX-deficient plasma showed that mouse plasma had approximately twice as much apparent activity as human plasma. Preliminary data from further characterization of this activity showed that it can be blocked by polyclonal antibodies against human factors V and X. However, at this time, we cannot fully explain the source of the activity.
Because hemophilia B is an X-linked disease, we expected that about 50% of the male offspring from the carriers would carry the factor IX mutation. However, only 41% of the male mice characterized were factor IX-knockouts, which is significantly (P < .05) lower than 50%. It is possible that the weakness of newborn factor IX-knockout pups causes some deaths; therefore, the birth rate of factor IX-knockout mice may not be different from that of normal mice. For example, carcasses were often eaten by cage mates, so we do not know if this contributes to the apparent difference in birth rates. In addition, it is possible that in utero deaths of factor IX knockout mice may contribute to the lower rate, but we have not investigated these issues.
It is known that factor IX and VIII deficiencies in humans are almost indistinguishable in bleeding pattern. Because factor VIII-deficient mice have also been developed,21 it is of interest to compare whether they have similiar phenotypes to factor IX-deficient mice. Interestingly, the factor VIII-knockout mice have residual clotting activity, measured by the APTT assay, similar to that of factor IX-knockout mice.21 On the other hand, factor IX-deficient mice appear to have a more severe phenotype than do factor VIII-deficient mice. Factor VIII-deficient mice have a mild phenotype compared with severe hemophilia A in humans; they show no spontaneous bleeding, illness, or reduced activity during the first year of life.22 In contrast, factor IX-knockout mice are essentially a phenocopy of the human disease. Spontaneous hemorrhage in footpad tissues and subcutaneous hemorrhages were common in the factor IX-knockout mice.
Based on the targeted defect in the factor IX gene, the lack of factor IX transcript in the livers, and the distinct physical characteristics of the mice, we conclude that a factor IX-deficient mouse strain has been successfully generated. This mouse strain should be useful for studying gene therapy methods for hemophilia B and for investigating structure-function relationships of recombinant factor IX proteins in vivo. In addition, the socket/F9-targeted ES cell line that we isolated, which is capable of germline transmission, can be used in plug targeting experiments to generate plug-targeted ES cell lines. Such experiments should allow generation of mouse strains having specific mutations of the factor IX gene.
ACKNOWLEDGMENT
We thank D. Lee and K. Kluckman for technical assistance; Drs S-M. Wu and D. Bellinger and the Smithies-Maeda laboratory group for helpful discussions; and H. Chang for proofreading the manuscript.
Supported by National Institutes of Health Grants No. GM20069 (O.S.), HL42630 (N.M.), and HL06350 (D.W.S.).
Address reprint requests to Darrel W. Stafford, PhD, CB#3280, Department of Biology, UNC-CH, Chapel Hill, NC 27599-3280.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal