Abstract
We have investigated the mechanism of action of the thrombopoietic cytokine, recombinant human interleukin-11 (rhIL-11), on megakaryocytopoiesis in vitro. We have shown that rhIL-11–induced murine and human megakaryocytopoiesis are not mediated by thrombopoietin (Tpo). Murine megakaryocytes (MKs) were produced from bone marrow (BM) mononuclear cells cultured with rhIL-11, IL-3, and a combination of the two cytokines. Conditioned media (CM) were collected and assayed for the presence of biologically active Tpo. Tpo activity was not detected in any of the CMs tested. Next, human BM CD34+ cells were cultured in serum-free fibrin clot medium with rhIL-11, IL-3, or rhIL-11 plus IL-3 and an antibody that neutralizes human Tpo activity. No inhibition of either burst-forming unit-MK– or colony-forming unit-MK–derived colony formation was observed. The antibody did partially inhibit steel factor-induced MK-colony formation, suggesting that the actions of this cytokine are mediated, in part, by Tpo. We determined that MKs can be direct targets of rhIL-11 by showing the expression of functional IL-11 receptor on these cells. Total RNA was prepared from cultured human BM CD41+CD14− cells (MKs) and IL-11 receptor α chain mRNA was detected in the MKs by reverse transcription-polymerase chain reaction. Analysis of single-sorted CD41+CD14− cells confirmed that the observed IL-11 receptor expression was not due to contaminating CD41− cells in the pool. The presence of rhIL-11 receptor α chain protein in the cells was established by Western blot analysis. After a short exposure of purified BM MKs to rhIL-11, enhanced phosphorylation of both its signal transduction subunit, gp130, and the transcription factor, STAT3 was detected, showing a direct activation of receptor signaling by the cytokine. Consistent with the lack of effect of rhIL-11 on platelets in vivo, IL-11 receptor α chain mRNA and protein were not detected in isolated human platelets. These data indicate that rhIL-11 acts directly on MKs and MK progenitors but not on platelets.
INTERLEUKIN-11 (IL-11) is a stromal cell-derived cytokine that has been shown to have potent thrombopoietic activity in vivo.1,2 Clinical studies with recombinant human IL-11 (rhIL-11) have shown it to be an effective therapeutic for ameliorating chemotherapy-induced thrombocytopenia. In a phase I trial, rhIL-11 was shown to be well tolerated and to improve platelet nadirs in women with breast cancer after chemotherapy.3 Administration of rhIL-11 significantly increased megakaryocyte (MK) frequencies, MK endoreduplication, and the proportion of proliferating cell nuclear antigen (PCNA) expressing MKs in the bone marrow (BM) in these patients.4 In two randomized placebo-controlled phase II clinical trials of cancer patients with severe thrombocytopenia, rhIL-11 significantly decreased the number of patients requiring platelet transfusions, as compared with those receiving placebo.5 6
Preclinical studies with rhIL-11 in normal and myelosuppressed animals have further shown the thrombopoietic potential of this cytokine. Administration of rhIL-11 to normal animals including mice, rats, rabbits, dogs, and nonhuman primates has been shown to increase peripheral platelet numbers.2,7-9 The mature MKs that develop in the BM of the animals appear ultrastructurally normal, with normal granule formation and demarcation membrane system development.2 Constant subcutaneous infusion of rhIL-11 into mice increased modal MK ploidy in the BM and colony-forming unit-MK (CFU-MK) number in the spleen.10 In myelosuppressed animals, treatment with rhIL-11 accelerated platelet recovery and improved platelet nadirs. Along with these effects, a large increase in the number of BM MKs was observed.9 11
Consistent with the effects of rhIL-11 in vivo are its effects on hematopoietic cells of the MK lineage in vitro. rhIL-11 has been shown to promote multiple stages of MK development.12-16 In combination with IL-3 or steel factor (SF ), rhIL-11 acted synergistically in stimulating human and murine CFU-MK–derived colony formation.12,13 When added along with IL-3 to human CD34+HLA-DR− BM cells, rhIL-11 enhanced burst-forming unit-MK (BFU-MK)–derived colony formation.14 rhIL-11 plus IL-3 increased MK number and acetylcholinesterase production in murine BM liquid cultures compared with IL-3 alone.15 As a single agent, rhIL-11 enhanced the size and ploidy of human BM MKs.16
IL-11 belongs to a family of cytokines including IL-6, leukemia inhibitory factor (LIF ), oncostatin M (OSM), and ciliary neurotropic factor (CNTF ) that share a common signal transducer, gp130. gp130 is ubiquitously expressed in many tissues and cell types.17 The recently cloned ligand binding IL-11 α chain is the member of the receptor complex that confers specificity.18 Cytokine-induced association of the α chain and gp130 initiates signal transduction by activation of the JAK/TYK tyrosine kinase family and the MAPK serine/threonine kinase family.19 The activation of these kinases results in the phosphorylation of a set of proteins including the transcription factors STAT1 and STAT3.19 20
In this study, we have investigated the mechanism of action of rhIL-11's effects on megakaryocytopoiesis. We show that the effects of rhIL-11 in vitro on MK progenitors and mature MKs are not mediated through a second factor, thrombopoietin (Tpo), the ligand for c-mpl receptor. In addition, we show that MKs express IL-11 receptor α chain mRNA and functional IL-11 receptor protein, establishing MKs as direct targets of rhIL-11 actions.
MATERIALS AND METHODS
Cytokines. The following cytokines used in this study were produced at Genetics Institute, Inc (Cambridge, MA): purified Escherichia coli-derived rhIL-11 and CHO cell-derived recombinant murine IL-3 (rmIL-3) in conditioned medium (CM; 1 U of activity is defined as the reciprocal of the dilution of CM needed to stimulate half-maximal proliferation of RB5 cells). The following purified recombinant cytokines were purchased from R&D Systems (Minneapolis, MN): E coli-derived human IL-3 and human SF and mouse NSO myeloma cell-derived human and murine Tpo.
Antibodies. Neutralizing rabbit polyclonal antisera to CHO-produced murine IL-3 was made at Genetics Institute, Inc. Neutralizing polyclonal antibody to human Tpo (goat IgG; R & D Systems), normal goat IgG (isotype control for anti-Tpo; R & D Systems), P2 anti-CD41 (mouse IgG1 ; Immunotech, Marseille, France), mouse IgG1 (isotype control for anti-CD41; Becton Dickinson, Mountain View, CA), and fluorescein isothiocyanate (FITC)-goat antimouse IgG (Southern Biotechnology, Birmingham, AL) were used in human MK colony assays. The anti-CD34 monoclonal antibody (MoAb) QBEND-10 (mouse IgG1 ; Miltenyi Biotech, Sunnyvale, CA) was used for immunomagnetic purification. Directly conjugated MoAbs phycoerythrin (PE)-679.1Mc7 and FITC-679.1Mc7 (mouse IgG1 isotype; Immunotech), FITC-anti-CD41 (mouse IgG1 ; Immunotech), and PE-anti-CD14 (mouse IgG1 ; Becton Dickinson) were used for flow cytometry. Polyclonal sheep antidigoxigenin, Fab fragments conjugated to alkaline phosphatase (anti-DIG-AP; Boehringer Mannheim, Indianapolis, IN) were used for the detection of platelet gene expression. Polyclonal antibody to human/mouse IL-11 receptor α chain (N-20, rabbit IgG; Santa Cruz Biotechnology, Santa Cruz, CA), SZ22 anti-CD41 (mouse IgG1 ; Immunotech), and horseradish peroxidase-conjugated donkey antirabbit IgG and antimouse IgG (Amersham Corp, Arlington Heights, IL) were used for Western blotting analysis. MoAb to human platelet glycoprotein Ib (Dako Corp, Carpinteria, CA) was used to isolate guinea pig MKs. The mouse monoclonal anti-guinea pig IIb-IIIa antibody used to determine the purity of isolated guinea pig MKs was a gift from Jonathan Miller (Department of Pathology, SUNY, Syracuse, NY). Polyclonal antibody to human gp130 (rabbit IgG; Upstate Biotechnology, Inc, Lake Placid, NY) and MoAb to rat STAT3 protein (clone #84, mouse IgG1 ; Transduction Laboratories, Lexington, KY) were used for protein phosphorylation analysis.
Generation and isolation of CD41+ cells. Human BM was purchased from Poietic Technologies, Inc (Germantown, MD). Mononuclear cells were isolated by density centrifugation (Ficoll-Hypaque; Pharmacia, Piscataway, NJ) at 1,200g at room temperature for 30 minutes. CD34+ cells were selected using the Miltenyi miniMACS magnetic microbead system (Miltenyi Biotech) according to the manufacturer's directions.
Liquid cultures were set up with 1 × 105 CD34+ cells/mL in EX-VIVO 10 medium (BioWhittaker, Walkersville, MD) with the following mixture of cytokines: 3.3 ng/mL rhIL-3, 50 ng/mL rmSF, 10 ng/mL rhTpo, and 20 ng/mL lipopolysaccharide (LPS) and incubated for 14 days at 37°C in 5% CO2 . The cultured cells were resuspended and washed in cold 3% CATCH buffer (0.38% sodium citrate, 2 mmol/L theophylline [Sigma, St Louis, MO], 1 mmol/L adenosine [Aldrich, Milwaukee, WI], 1 μg/mL prostaglandin E1 [Sigma], and 3% bovine serum albumin [BSA; Sigma] in Hanks' Balanced Salt Solution [HBSS]). The harvested cells were incubated with mouse IgG (1.5 μg/105 cells; Sigma) on ice for 15 minutes and then incubated simultaneously with the FITC-anti-CD41 and PE-anti-CD14 MoAbs on ice for 30 minutes. After two washings, cells were suspended in 3% CATCH buffer at a concentration of 5 × 105 cells/mL and separated by sorting.
Cells were sorted on a FACStarPlus (Becton Dickinson Immunocytometry Systems, San Jose, CA) using Cell Quest software. CD41+CD14− cells were identified on two-parameter histograms (side scatter [SSC] v forward scatter [FSC]). Compensation for two-color labeled samples was set up with single-stained samples. The brightest staining fraction (30%) of CD41+CD14− cells was sorted. For single-cell experiments, CD41+CD14− cells were directly sorted into 96-well V-bottom tissue culture plates (Corning, Corning, NY) using an automated cell deposition unit and CloneCyt software (Becton Dickinson Immunocytometry Systems).
Human MK colony assays. Isolated CD34+ human BM cells were assayed for their ability to form CFU-MK– or BFU-MK–derived colonies in serum-depleted fibrin clot cultures as previously described by Bruno et al.21 Ten thousand CD34+ cells/mL were cultured in serum-depleted media with specified cytokines and antibodies in Iscove's modified Dulbecco's medium (IMDM) at various concentrations. Four 1-mL cultures (triplicates plus an IgG1 isotype control) for each condition were cultured for 12 to 14 days (CFU-MK) or 21 days (BFU-MK) at 37°C in an humidified atmosphere of 5% CO2 . The cultures were stained with either anti-CD41 (2.6 μg/mL) or mouse IgG1 for 60 minutes at room temperature, followed by 20 μg/mL of FITC-goat antimouse IgG1 . The colonies were counterstained with 0.125% Evans Blue. Colonies were scored using an Olympus inverted IMT-2 microscope with a BH2-RFC reflected light fluorescence attachment (Tokyo, Japan). A CFU-MK–derived colony was defined as a cluster of 3 or more fluorescent cells and a BFU-MK–derived colony was defined as multifocal and greater than 100 fluorescent cells/colony.
Development of an MPL ligand-responsive cell line. The full-length cDNA for murine c-mpl was isolated from a murine IL-3–dependent cell line (DA-2) and inserted into the multiple cloning site of the retroviral expression vector pLNCX.22 A total of 5 × 106 FDCP-1 cells in 0.8 mL RPMI were transfected by electroporation at 960 μF and 200 V using a BioRad Gene Pulsar (Bio-Rad, Hercules, CA) with 20 μg of Acc I-linearized pLNC-mpl. Cells were recovered on ice and incubated in a T-25 flask for 2 days in RPMI1640 containing 10% fetal calf serum (FCS) and 5 U/mL murine IL-3 followed by selection in 0.5 mg/mL wt/vol G418 for 14 days.
Murine BM liquid culture analysis. Murine BM mononuclear cells were isolated as described above. The cells were seeded at 5 × 105 cells/mL in RPMI1640 containing 10% FCS. CHO rmIL-3 (20 U/mL), rhIL-11 (10 ng/mL), or mIL-3 and rhIL-11 were added and the cultures were incubated for 72 hours. After the collection of the CM by centrifugation at 400g to remove cells, cytospins were made and MKs were quantitated by staining for AchE activity. The CM were assayed for the presence of Tpo by a modification of the proliferation assay described by Paul et al23 using FDCP-MPL cells. Cells were cultured in microtiter plates at 5 × 103 cells per well with titration of growth factors or conditioned media with or without antibody to CHO rmIL-3 (1:100 dilution) for 72 hours. Before harvesting, cells were pulse-labeled with 3H-TdR for 4 hours.
MK RNA analysis. Total RNA was prepared from sorted human BM CD41+CD14− cells, HEL cells, and K562 cells using the Qiagen RNeasy Total RNA Kit (Hilden, Germany), and the first cDNA strands were synthesized (Superscript II Preamplification System; GIBCO/BRL, Gaithersburg, MD) using random hexamer primers according to the manufacturer's directions. The cDNA strands were purified using the QIAquick PCR Purification Kit as described by the manufacturer (Qiagen). Reverse transcription-polymerase chain reaction (RT-PCR) amplification was performed using the Perkin Elmer Cetus hot start technique (AmpliWax beads; Perkin Elmer Cetus/Roche Molecular Systems, Inc, Branchburg, NJ). Thirty cycles of amplification were performed as follows: denaturation for 1 minute at 94°C, annealing for 1 minute at 60°C, and elongation for 1 minute at 72°C. Specific oligonucleotide primers were used to generate the following fragments by PCR: human IL-11 receptor -(C C G G A T TAATGTGACTGAGGTG/GTAGCCG A G G G T G T G-GTTGGAG generates a 475-bp fragment); human c-mpl (GAGCCAGCTCCAGAAATCAGT/CAGGCAGGTCCACAGTCACAG generates a 379-bp fragment); and human GAPDH (AATCCCATCACCATCTTCCAG/AGGGGCCATCCACAGTCTTCT generates a 361-bp fragment). The reaction volumes were decreased to 10 μL on microcon 30 microconcentrators (Amicon, Inc, Beverly, MA) and the PCR fragments were visualized by staining with ethidium bromide after separation in 2% agarose gel.
Platelet RNA analysis. Human platelets were prepared from whole blood using standard techniques. Total RNA was isolated from platelets and gene expression was analyzed by RT-PCR as described above. The DNA fragments were visualized by including digoxigenin-11-dUTP (0.1 mmol/L; Boehringer Mannheim) in the PCR mix. PCR fragments were separated in 2% agarose gel, denatured, transferred to a nylon membrane, and detected using an immunochemiluminescent detection system (Genius 7; Boehringer Mannheim). Membranes were exposed to Hyperfilm-ECL (Amersham Corp) for 15 to 120 minutes.
Single-cell RT-PCR analysis. Single CD41+CD14− cells were sorted directly into lysis buffer, and cDNA was prepared and amplified as previously described.24 25 PCR was then performed for 50 amplification cycles (1 minute at 94°C, 2 minutes at 42°C, and 6 minutes at 72°C with a 10-second extension/cycle) with 5 μmol/L (dT)24X primer (ATG TCG TCC AGG CCG CTC TGG ACA AAA TAT GAA TTC dT24 ) and 5 U of Taq polymerase (Perkin-Elmer Cetus/Roche Molecular Systems, Inc). Cell-free and reverse transcriptase-free samples were used as negative controls. The PCR DNA fragments were electrophoresed through 1% agarose, stained with ethidium bromide, and transferred to a nylon membrane. The membranes were hybridized at 42°C for 18 hours to human IL-11 receptor α chain cDNA and human c-mpl cDNA radiolabeled probes (2 × 106 cpm/mL). Membranes were exposed for 18 hours to FUJI imaging plates that were developed in a FUJIX BAS 2000 Bio Imaging Analyzer (FUJI, Tokyo, Japan). Single cells from specified lines were included as negative and positive controls.
Western blot analysis. Platelets and cultured human BM MKs (CD41+CD14− cells) were isolated as described above. Cell lysates were prepared, separated in 10% acrylamide tricine gels (Novex, San Diego, CA), and analyzed with IL-11 receptor α chain antibodies as described by the manufacturer (Santa Cruz Biotechnology). Antibody to CD41 was used as a positive control for cell specificity. The proteins were visualized using ECL Western blotting detection reagents (Amersham Corp) and then exposure of the membranes to Hyperfilm-ECL for 30 seconds to 1 hour.
MK protein phosphorylation analysisA guinea pig BM cell suspension was separated on a 4-step BSA density gradient. The gradient was centrifuged at 10,000g for 30 minutes and all cells above the pellet were kept, washed to remove BSA, and then incubated for 1 hour at room temperature with MoAb to human platelet glycoprotein Ib. Immunomagnetic beads conjugated with antibody to mouse IgG were then added to the cells and the incubation was continued for another 30 minutes, at which time the MKs were collected on a magnet. The cells were washed 4 times on the magnet to remove the nonmegakaryocytic cells and the MKs were then suspended in culture medium.26 The MKs were approximately 97% pure based on staining with MoAb to guinea pig IIb-IIIa. Isolated guinea pig BM MKs (5 × 105 to 1 × 106 MKs) were incubated for 2 hours with 32P-orthophosphate (2.5 mCi/mL), followed by 10 minutes of exposure to rhIL-11 at 50 ng/mL.
Immunoprecipitation was then performed at 4°C as described by Upstate Biotechnology, Inc. Labeled MKs were lysed, insoluble material was removed by centrifugation, and cell supernatant was used for immunoprecipitation. Nonspecific material was removed by a preincubation with protein-A sepharose and the lysate was then incubated with primary antibody at a 1:100 dilution for 12 to 18 hours. The protein-antibody complexes were isolated with protein-A sepharose and then separated by polyacrylamide (10%) electrophoresis.27 Autoradiography was performed on the dried gels.
Statistical analysis. The Student's t-test was used to determine statistical significance. All P values were calculated from the data obtained in three separate experiments.
RESULTS
rhIL-11– and IL-3–stimulated MK colony formation is not mediated by Tpo. Experiments were performed to determine if rhIL-11, alone and in combination with IL-3, acts directly on megakaryocytopoiesis. As an initial approach to detect the presence of Tpo being produced in murine BM cultures after treatment with rhIL-11, we measured Tpo levels using a sensitive engineered cell line that detects as little as 15 pg/mL of mTpo (Fig 1A). At least 10-fold higher concentrations of mTpo were required for enhancement of CFU-MK proliferation in culture (data not shown). Mononuclear cells were isolated by Nycodenz density centrifugation from murine BM and MKs were produced by culturing the cells with rhIL-11, IL-3, and a combination of the two cytokines. CM was collected from 3-day cultures and assayed for the presence of biologically active Tpo. Because FDCP-MPL cells proliferate in response to IL-3 (Fig 1A), neutralizing antibodies to IL-3 were included in the assay. Tpo activity was not detected in any of the CMs tested (Fig 1B). All of the proliferation observed in response to CMs containing exogenous IL-3 was inhibited by the neutralizing antibodies. These results show that the levels of Tpo required to stimulate MK development (>15 pg/mL) were not generated, suggesting that Tpo induction is not likely to be involved in rhIL-11 and IL-3 stimulation of murine BM MK progenitors in culture.
Tpo is not detected in murine BM cultures induced with rhIL-11 and IL-3. PCR-generated DNA encoding full-length murine c-mpl was subcloned and transfected into FDCP-1 cells by electroporation as described in the Materials and Methods. FDC-MPL cells detected murine Tpo to concentrations of 15 pg/mL. CMs from murine liquid BM cultures were generated by separating out the low-density cells by centrifugation and culturing them with various growth factor combinations for 3 days (20 U/mL rmIL-3 and 10 ng/mL rhIL-11). CMs for assay were collected and cells were stained for AchE activity. FDCP-MPL cells were assayed in microtiter plates at 5 × 103 cells per well with (A) titration of growth factors ([▪] rmIL-3; [○] rmTpo; [□] media control) or (B) CM with or without antibody to mIL-3 at a 1:100 dilution ([▪] IL-3 CM; [•] IL-3 CM + anti–IL-3; [×] rhIL-11 CM; [□] IL-3 + rhIL-11 CM; [▵] IL-3 + rhIL-11 CM + anti–IL-3) for 72 hours. An enlarged view of the activity detected with (•) IL-3 CM + anti–IL-3, (×) rhIL-11 CM, and the (▵) IL-3 + rhIL-11 CM + anti–IL-3 is shown in the insert graph of (B).
Tpo is not detected in murine BM cultures induced with rhIL-11 and IL-3. PCR-generated DNA encoding full-length murine c-mpl was subcloned and transfected into FDCP-1 cells by electroporation as described in the Materials and Methods. FDC-MPL cells detected murine Tpo to concentrations of 15 pg/mL. CMs from murine liquid BM cultures were generated by separating out the low-density cells by centrifugation and culturing them with various growth factor combinations for 3 days (20 U/mL rmIL-3 and 10 ng/mL rhIL-11). CMs for assay were collected and cells were stained for AchE activity. FDCP-MPL cells were assayed in microtiter plates at 5 × 103 cells per well with (A) titration of growth factors ([▪] rmIL-3; [○] rmTpo; [□] media control) or (B) CM with or without antibody to mIL-3 at a 1:100 dilution ([▪] IL-3 CM; [•] IL-3 CM + anti–IL-3; [×] rhIL-11 CM; [□] IL-3 + rhIL-11 CM; [▵] IL-3 + rhIL-11 CM + anti–IL-3) for 72 hours. An enlarged view of the activity detected with (•) IL-3 CM + anti–IL-3, (×) rhIL-11 CM, and the (▵) IL-3 + rhIL-11 CM + anti–IL-3 is shown in the insert graph of (B).
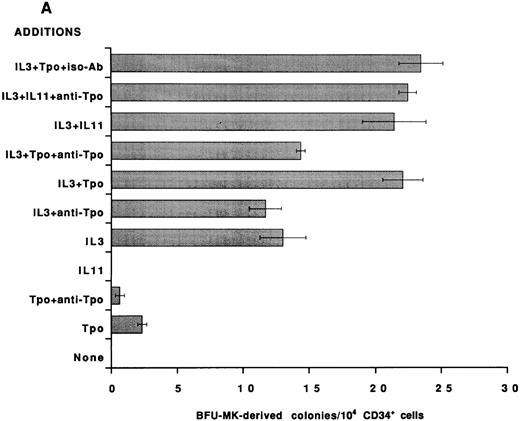
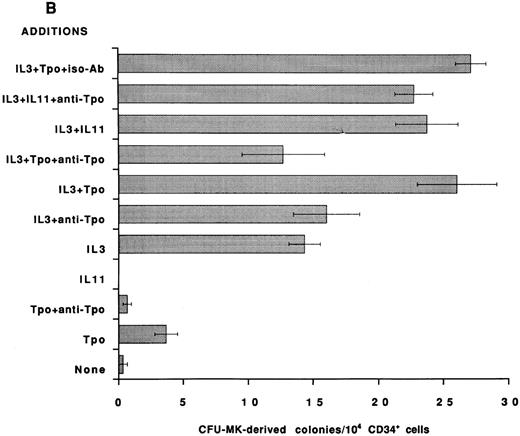
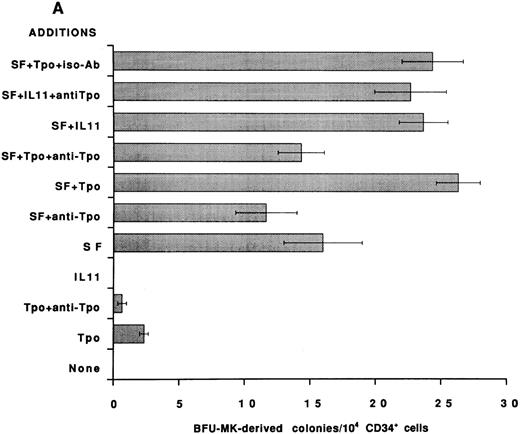
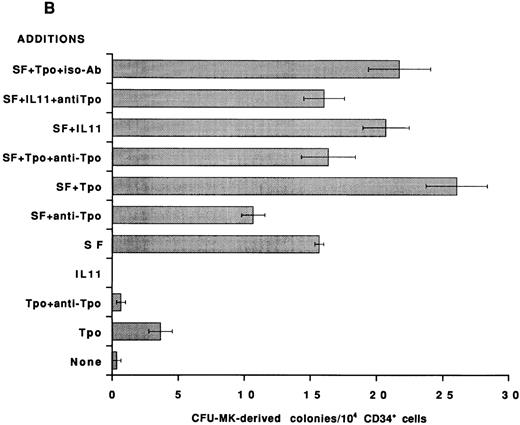
To establish that the Tpo-independent actions of rhIL-11 on MK development in vitro are not restricted to the murine system, an antibody that neutralizes human Tpo activity was added to human BFU-MK and CFU-MK cultures and colony formation was evaluated. CD34+ cells were isolated from normal human BM and cultured in serum-free semisolid fibrin clot media.21 Tpo neutralizing antibody was included, as indicated in Figs 2 and 3. rhIL-11 alone did not support human MK colony formation; however, when it was added in combination with IL-3 and SF, it synergized to stimulate the formation of both BFU-MK–derived (Figs 2A and 3A; P < .01 for IL-3 and P < .02 for SF ) and CFU-MK–derived (Figs 2B and 3B; P < .001 for IL-3 and P < .05 for SF ) colonies. Tpo alone supported the formation of a small number of MK colonies. The addition of Tpo neutralizing antibody to the cultures reduced BFU-MK–derived colony formation (Figs 2A and 3A; P < .01) and CFU-MK–derived colony formation (Figs 2B and 3B; P < .001) to background levels. The antibody reduced the number of colonies formed in cultures containing IL-3 + Tpo to that observed in cultures with IL-3 alone (P < .001 for BFU-MK and CFU-MK), whereas IL-3 and IL-3 + rhIL-11 induced BFU-MK–derived (Fig 2A) and CFU-MK–derived (Fig 2B) colony formation were not inhibited (P = .8 for BFU-MK and P = .6 for CFU-MK). These data show that the mechanism of action of IL-3 and rhIL-11 in human MK colony formation is not mediated by Tpo.
Effect of neutralizing anti-TPO antibody on rhIL-11– and IL-3–induced human MK colony formation. CD34+ cells were isolated from human BM and assayed in serum-free media for BFU-MK–derived (A) and CFU-MK–derived (B) colonies. The following cytokine concentrations were used: rhIL-11 (50 ng/mL), rhIL-3 (10 ng/mL), and rhTpo (10 ng/mL). Both the anti-Tpo antibody and isotype antibody were added into the cultures at 75 μg/mL. Suboptimal concentrations of growth factors and optimal concentrations of antibody were used to guarantee antibody excess. Clusters of 3 or more CD41+ cells were scored as a CFU-MK–derived colony, whereas multifoci and greater than 100 fluorescent cells were scored as a BFU-MK–derived colony. Each column with bar corresponds to the mean ± SEM of triplicate cultures from one experiment representative of three separate experiments.
Effect of neutralizing anti-TPO antibody on rhIL-11– and IL-3–induced human MK colony formation. CD34+ cells were isolated from human BM and assayed in serum-free media for BFU-MK–derived (A) and CFU-MK–derived (B) colonies. The following cytokine concentrations were used: rhIL-11 (50 ng/mL), rhIL-3 (10 ng/mL), and rhTpo (10 ng/mL). Both the anti-Tpo antibody and isotype antibody were added into the cultures at 75 μg/mL. Suboptimal concentrations of growth factors and optimal concentrations of antibody were used to guarantee antibody excess. Clusters of 3 or more CD41+ cells were scored as a CFU-MK–derived colony, whereas multifoci and greater than 100 fluorescent cells were scored as a BFU-MK–derived colony. Each column with bar corresponds to the mean ± SEM of triplicate cultures from one experiment representative of three separate experiments.
Effect of neutralizing anti-Tpo antibody on rhIL-11– and SF-induced human MK colony formation. CD34+ cells were isolated from human BM, assayed in serum-free media for BFU-MK–derived (A) and CFU-MK–derived (B) colonies, and scored as described in Fig 2. The following cytokine concentrations were used: rhIL-11 (50 ng/mL), rhSF (50 ng/mL), and rhTpo (10 ng/mL). The anti-Tpo antibody was added into the cultures at 75 to 100 μg/mL, and the isotype antibody was added at 75 to 100 μg/mL. Each column with bar corresponds to the mean ± SEM of triplicate cultures from an experiment representative of three separate experiments.
Effect of neutralizing anti-Tpo antibody on rhIL-11– and SF-induced human MK colony formation. CD34+ cells were isolated from human BM, assayed in serum-free media for BFU-MK–derived (A) and CFU-MK–derived (B) colonies, and scored as described in Fig 2. The following cytokine concentrations were used: rhIL-11 (50 ng/mL), rhSF (50 ng/mL), and rhTpo (10 ng/mL). The anti-Tpo antibody was added into the cultures at 75 to 100 μg/mL, and the isotype antibody was added at 75 to 100 μg/mL. Each column with bar corresponds to the mean ± SEM of triplicate cultures from an experiment representative of three separate experiments.
The addition of Tpo neutralizing antibody to BFU-MK (Fig 3A) cultures containing SF resulted in very little inhibition of colony formation (P = .4). In contrast, colony formation in CFU-MK cultures containing SF (Fig 3B) was significantly inhibited by the addition of anti-Tpo antibody (P < .001). Therefore, as would be expected, CFU-MK–derived colony formation induced by cytokine combinations that include SF is inhibited in part by the neutralizing Tpo activity (Fig 3B).
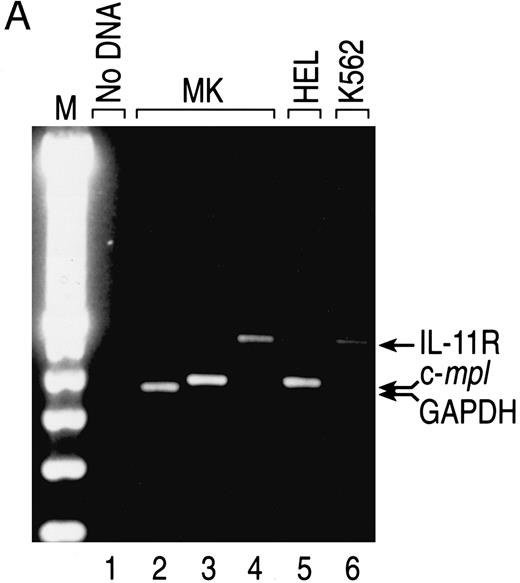
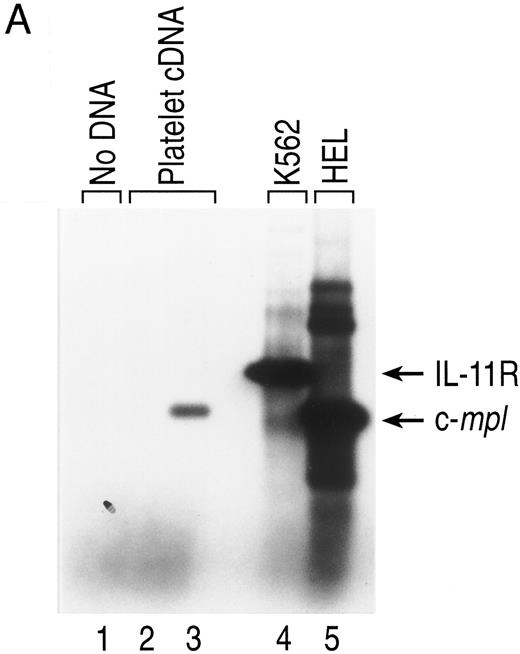
IL-11 receptor is expressed on human MKs. A population of human CD41+CD14− cells was analyzed for IL-11 receptor expression to determine if MKs are a direct target for the actions of rhIL-11. CD34+ cells were selected from human BM and cultured with IL-3, SF, Tpo, and LPS for 14 days. CD41+CD14− cells were isolated from the cultures by positive selection and gene expression was analyzed by RT-PCR. A DNA fragment of appropriate size for IL-11 receptor α chain (Fig 4, lane 4), as determined by comparison with the fragment generated from K562 cells, an IL-11 receptor-positive cell line18 (Fig 4, lane 6), was produced from the MK mRNA. C-mpl, a gene shown to be expressed by MKs, was detected in the cells (Fig 4, lane 3).28
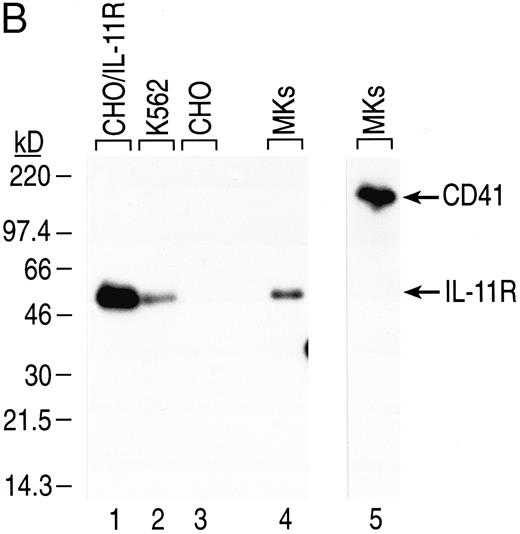
Expression of IL-11 receptor α chain in human BM CD41+CD14− cells. (A) CD34+ human BM cells were isolated and cultured as described in the Materials and Methods. Total RNA from sorted cultured CD41+CD14− cells and K562 and HEL cells (positive controls for IL-11 receptor α chain and c-mpl mRNAs, respectively) was isolated, first-strand cDNA was synthesized, and gene expression was analyzed via RT-PCR. The fragments were visualized by staining with ethidium bromide after separation in 2% agarose gel. The primers used generated the following fragments: human IL-11R, 475 bp (lanes 1, 4, and 6); human c-mpl, 379 bp (lanes 3 and 5); and human GAPDH, 361 bp (lane 2). Lane 1, no cDNA; lane 2, MK cDNA from 2.5 × 104 cell equivalents; lanes 3 and 4, MK cDNA from 105 cell equivalents; lane 5, HEL cDNA from 37.5 ng of polyA+ RNA; lane 6, K562 cDNA from 37.5 ng of polyA+ RNA. (B) Western blot analysis was performed on total cell lysate from human cultured BM CD41+CD14− cells and K562 cells (positive control). CHO/IL11R (James Tobin, Genetics Institute, Inc) cells were used as a positive control for IL-11 receptor α chain antibody specificity and CHO cells as the negative control. Total cell protein from CD41+CD14− cells (3 × 106 cells for IL-11R and 5 × 105 cells for CD41) and from cell lines (2 × 106 K562 cells for IL-11R, 5 × 103 CHO/IL-11R cells for IL-11R, and 5 × 103 CHO cells for IL-11R) was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. IL-11 receptor α chain, 48-kD protein (lanes 1 through 4), and CD41, 135-kD protein (lane 5), were detected with the appropriate antibodies (1.0 μg/mL). Autoradiography was performed on the membranes (CD41 for 4 minutes and IL-11 receptor for 1 hour).
Expression of IL-11 receptor α chain in human BM CD41+CD14− cells. (A) CD34+ human BM cells were isolated and cultured as described in the Materials and Methods. Total RNA from sorted cultured CD41+CD14− cells and K562 and HEL cells (positive controls for IL-11 receptor α chain and c-mpl mRNAs, respectively) was isolated, first-strand cDNA was synthesized, and gene expression was analyzed via RT-PCR. The fragments were visualized by staining with ethidium bromide after separation in 2% agarose gel. The primers used generated the following fragments: human IL-11R, 475 bp (lanes 1, 4, and 6); human c-mpl, 379 bp (lanes 3 and 5); and human GAPDH, 361 bp (lane 2). Lane 1, no cDNA; lane 2, MK cDNA from 2.5 × 104 cell equivalents; lanes 3 and 4, MK cDNA from 105 cell equivalents; lane 5, HEL cDNA from 37.5 ng of polyA+ RNA; lane 6, K562 cDNA from 37.5 ng of polyA+ RNA. (B) Western blot analysis was performed on total cell lysate from human cultured BM CD41+CD14− cells and K562 cells (positive control). CHO/IL11R (James Tobin, Genetics Institute, Inc) cells were used as a positive control for IL-11 receptor α chain antibody specificity and CHO cells as the negative control. Total cell protein from CD41+CD14− cells (3 × 106 cells for IL-11R and 5 × 105 cells for CD41) and from cell lines (2 × 106 K562 cells for IL-11R, 5 × 103 CHO/IL-11R cells for IL-11R, and 5 × 103 CHO cells for IL-11R) was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. IL-11 receptor α chain, 48-kD protein (lanes 1 through 4), and CD41, 135-kD protein (lane 5), were detected with the appropriate antibodies (1.0 μg/mL). Autoradiography was performed on the membranes (CD41 for 4 minutes and IL-11 receptor for 1 hour).
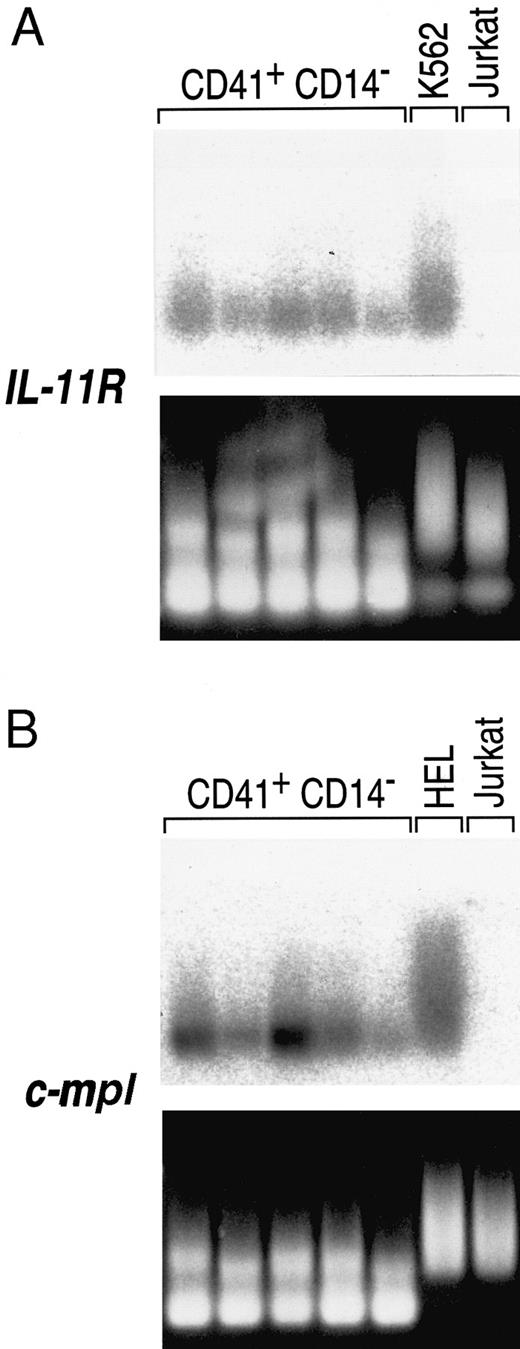
To rule out the possibility that the observed IL-11 receptor expression was due to contaminating CD41− cells in the population, individual cells were analyzed. Single CD41+CD14− cells were isolated by sorting into microtiter wells. Complementary DNA was generated from single-cell mRNA and then amplified nonspecifically by a modified PCR protocol. The cDNAs were probed via Southern analysis for IL-11 receptor α chain expression. All of the single CD41+CD14− cells analyzed expressed IL-11 receptor α chain mRNA (Fig 5A). Expression of the c-mpl gene was also detected in all of the cells (Fig 5B). These results show that the IL-11 receptor α chain gene is expressed in CD41+ cells.
Expression of IL-11 receptor α chain in single human BM CD41+CD14− cells. The lower panels are the ethidium bromide-stained amplified MK cDNAs that were transferred to nylon membranes, probed with the specified receptor cDNAs, and subjected to FUJI bio imaging analysis (upper panels). Five MKs were analyzed for IL-11 receptor α chain expression (A) to avoid individual cell artifacts, and the cell differences may represent heterogeneity in the MK population. The cDNAs were also probed for c-mpl gene expression (B), a receptor known to be MK lineage-specific. K562 cells and HEL cells were used as positive controls for IL-11 receptor α chain and c-mpl expression, respectively. Jurkat cells were used as a negative control for both genes. The amplification protocol produces variable length cDNAs from any given transcript, so that smears rather than bands result when the membranes are hybridized to a corresponding probe.24
Expression of IL-11 receptor α chain in single human BM CD41+CD14− cells. The lower panels are the ethidium bromide-stained amplified MK cDNAs that were transferred to nylon membranes, probed with the specified receptor cDNAs, and subjected to FUJI bio imaging analysis (upper panels). Five MKs were analyzed for IL-11 receptor α chain expression (A) to avoid individual cell artifacts, and the cell differences may represent heterogeneity in the MK population. The cDNAs were also probed for c-mpl gene expression (B), a receptor known to be MK lineage-specific. K562 cells and HEL cells were used as positive controls for IL-11 receptor α chain and c-mpl expression, respectively. Jurkat cells were used as a negative control for both genes. The amplification protocol produces variable length cDNAs from any given transcript, so that smears rather than bands result when the membranes are hybridized to a corresponding probe.24
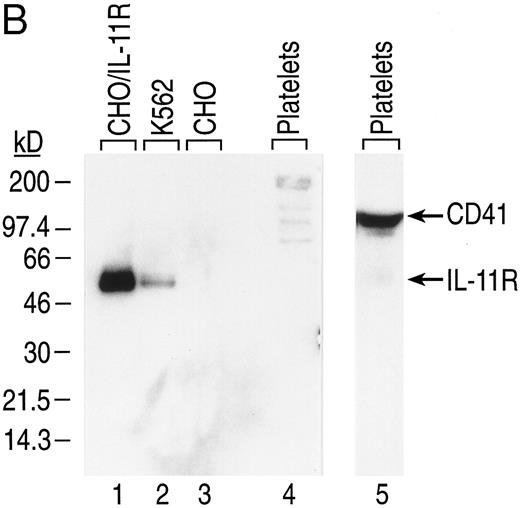
The presence of rhIL-11 receptor α chain protein on CD41+CD14− cells was confirmed by Western blot analysis (Fig 4B). Human CD41+CD14− cells were generated in culture from BM CD34+ cells and isolated by positive selection. Total cell protein was prepared, separated by electrophoresis, and transferred to nitrocellulose membranes. IL-11 receptor α chain (48 kD) and CD41 (135 kD) were detected with antibodies having reactivity to the proteins. Specificity of the antihuman IL-11 receptor α chain antibody was shown by its reactivity to protein isolated from CHO/IL-11R cells that have been transfected with the cDNA that encodes the receptor and its lack of reactivity to nontransfected CHO cells (Fig 4B, lanes 1 and 3). Consistent with the expression of IL-11 receptor α chain mRNA in CD41+CD14− cells, IL-11 receptor α chain protein was detected in the CD41+CD14− cell lysate (Fig 4B, lane 4). IL-11 receptor α chain protein was detected in the K562 cell lysate, a positive control, which expresses IL-11 receptor α chain at physiologic levels (Fig 4B, lane 2). GPIIb (CD41), an MK/platelet-specific protein, was detected in the CD41+CD14− cell lysate (Fig 4, lane 5).
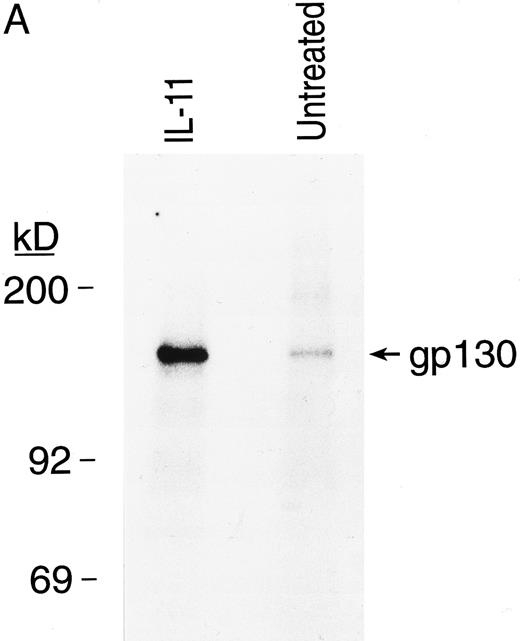
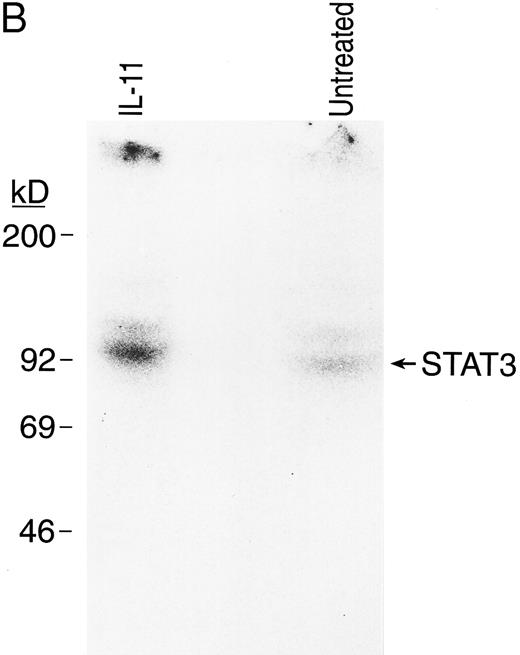
IL-11 directly activates signal transduction in BM MKs. The ability of the IL11 receptor protein on the surface of MKs to initiate signaling was investigated by evaluating the phosphorylation status of its signal transduction subunit, gp130, and STAT3, a transcription factor previously shown to be part of the rhIL-11 signaling pathway17 29 (Fig 6). Purified BM MKs were incubated with 32P-orthophosphate. After 10 minutes of exposure to rhIL-11, gp130 and STAT3 proteins were immunoprecipitated. The immunoprecipitated proteins were then separated by gel electrophoresis and detected by autoradiography. rhIL-11 enhanced phosphorylation of both gp130 (Fig 6A) and STAT3 (Fig 6B) proteins in the MKs, showing a direct activation of the receptor signaling pathway by the cytokine.
rhIL-11 induced tyrosine phosphorylation of gp130 and STAT3 proteins in BM MKs. Isolated guinea pig BM MKs were incubated with or without rhIL-11 after a preincubation with 32P-orthophosphate. Cells were lysed and supernatants were incubated with anti-gp130 antibody (A) and anti-STAT3 antibody (B). Immunoprecipitates were separated by SDS-PAGE and autoradiography of the dried gels was performed.
rhIL-11 induced tyrosine phosphorylation of gp130 and STAT3 proteins in BM MKs. Isolated guinea pig BM MKs were incubated with or without rhIL-11 after a preincubation with 32P-orthophosphate. Cells were lysed and supernatants were incubated with anti-gp130 antibody (A) and anti-STAT3 antibody (B). Immunoprecipitates were separated by SDS-PAGE and autoradiography of the dried gels was performed.
IL-11 receptor gene expression is not detected in platelets. During a human clinical trial in which rhIL-11 was administered to patients either before or after receiving chemotherapy treatment, no rhIL-11–related effect on platelet function was observed.3 To determine if this lack of effect was due to the absence of IL-11 receptor on platelets, IL-11 receptor mRNA expression was evaluated. Total RNA was made from human platelets prepared from platelet-rich plasma, and IL-11 receptor expression was analyzed by RT-PCR. No DNA fragment corresponding to IL-11 receptor α chain was generated from human platelet RNA, whereas a fragment corresponding to c-mpl was detected (Fig 7A, lanes 2 and 3).
Expression of IL-11 receptor α chain in human platelets. (A) Total RNA from human platelets, K562 cells, and HEL cells was isolated and analyzed via RT-PCR as described in Fig 4. The DNA fragments were visualized by including digoxigenin-11-dUTP in the PCR mix and using an immunochemiluminescent detection system. Autoradiography of the membrane filter was performed for 2 hours. The primers used generated the fragments described in Fig 4: human IL-11R (lanes 1, 2, and 4) and human c-mpl (lanes 3 and 5). Lane 1, no cDNA; lanes 2 and 3, platelet cDNA from 1 × 106 cell equivalents; lane 4, K562 cDNA from 37.5 ng of polyA+ RNA; lane 5, HEL cDNA from 37.5 ng of polyA+ RNA. (B) Western blot analysis of IL-11 receptor α chain expression in human platelets. Total protein from platelets (7.5 × 107 for IL-11R and 5 × 107 for CD41) and cells (5 × 103 CHO cells, 5 × 103 CHO/IL-11R cells, and 2 × 106 K562 cells) was separated by SDS-PAGE and transferred to nitrocellulose membranes. IL-11 receptor α chain (lanes 1 through 4) and CD41 (lanes 5 and 6) were detected with 1.0 μg/mL of primary antibody. Autoradiography was performed on the membranes (CD41 for 30 seconds and IL-11 receptor for 1 hour).
Expression of IL-11 receptor α chain in human platelets. (A) Total RNA from human platelets, K562 cells, and HEL cells was isolated and analyzed via RT-PCR as described in Fig 4. The DNA fragments were visualized by including digoxigenin-11-dUTP in the PCR mix and using an immunochemiluminescent detection system. Autoradiography of the membrane filter was performed for 2 hours. The primers used generated the fragments described in Fig 4: human IL-11R (lanes 1, 2, and 4) and human c-mpl (lanes 3 and 5). Lane 1, no cDNA; lanes 2 and 3, platelet cDNA from 1 × 106 cell equivalents; lane 4, K562 cDNA from 37.5 ng of polyA+ RNA; lane 5, HEL cDNA from 37.5 ng of polyA+ RNA. (B) Western blot analysis of IL-11 receptor α chain expression in human platelets. Total protein from platelets (7.5 × 107 for IL-11R and 5 × 107 for CD41) and cells (5 × 103 CHO cells, 5 × 103 CHO/IL-11R cells, and 2 × 106 K562 cells) was separated by SDS-PAGE and transferred to nitrocellulose membranes. IL-11 receptor α chain (lanes 1 through 4) and CD41 (lanes 5 and 6) were detected with 1.0 μg/mL of primary antibody. Autoradiography was performed on the membranes (CD41 for 30 seconds and IL-11 receptor for 1 hour).
We next performed Western blot analysis on platelet lysates. Human platelets were isolated from platelet-rich plasma. Total platelet protein was prepared, separated by electrophoresis, and transferred to nitrocellulose membranes. Human IL-11 receptor α chain was detected in the positive controls, CHO/IL-11R cells, and K562 cells (Fig 7B, lanes 1 and 2), but not in the negative control, nontransfected CHO cells (Fig 7B, lane 3). Consistent with the inability to detect IL-11 receptor α chain mRNA in platelets, protein was not detected in platelet lysate after extensive exposure of the membrane to film (Fig 7B, lane 4).
DISCUSSION
rhIL-11 has been shown to have multiple effects on MK development.1,2 In vivo, rhIL-11 increases peripheral platelets in normal animals and humans. Administration of rhIL-11 to patients undergoing chemotherapy has shown its ability to induce the formation of platelets in these myelosuppressed patients and to decrease their need for platelet transfusions. In vitro, rhIL-11 synergizes with other cytokines, including IL-3 and SF, to enhance the formation of both murine and human MK colonies. It also has previously been shown to increase the size and ploidy of immature MKs.16 Various investigators have proposed that these effects of rhIL-11 are mediated, in part, by endogenous Tpo.30 31
To address the issue of whether rhIL-11's effects on MK development and platelet production are indirect, we assessed Tpo activity during in vitro MK development. The results of these experiments clearly show that the actions of rhIL-11 as well as IL-3 in stimulating the production of MKs from murine and human progenitors does not require endogenous Tpo, whereas the actions of SF are, in part, Tpo-mediated. Using a Tpo-responsive cell line that is able to detect physiologic levels of cytokine (≥15 pg/mL), no activity was detected in murine BM cultures in which rhIL-11, IL-3, and a combination of the two cytokines promote the production of MKs from mononuclear cells. This level of Tpo is less than that required in cultures of murine BM to promote enhanced CFU-MK–derived colony formation. It is possible that low levels of Tpo produced in these cultures were consumed by MK progenitors and, therefore, not detected when the CM was assayed. Nevertheless, consistent with what was observed in the murine system, rhIL-11– and IL-3–induced human BFU-MK– and CFU-MK–derived colony formation was shown to be independent of Tpo. Antibody that was able to completely neutralize significant levels of Tpo activity did not inhibit rhIL-11– or IL-3–stimulated human MK colony formation. In addition, the generation of MKs (CD41+ cells) from human CD34+ cells in liquid cultures containing rhIL-11, IL-3, or a combination of the two cytokines was not inhibited by a distinct neutralizing anti-Tpo antibody (data not shown). A similar finding for IL-3–plus rhIL-11–stimulated murine CFU-MK–derived colony formation was reported with murine BM cells using soluble c-mpl to neutralize endogenous Tpo activity.30 Our results are also in agreement with a study by Carver-Moore et al32 that establishes that the megakaryocytopoietic activity of these cytokines does not require the presence of endogenous murine Tpo. Using BM from mice deficient in c-mpl (Tpo receptor) expression, they demonstrated the ability of rhIL-11 and IL-3 to stimulate the growth of MKs in liquid culture. Compatible with their in vitro data is an in vivo experiment that showed an increase in platelet counts when rhIL-11 or IL-3 was administered to c-mpl−/− and Tpo−/− mice. Their results show that these cytokines promote murine MK development and platelet production in the absence of a functional c-mpl/Tpo system.
Although rhIL-11 is able to work independently of Tpo, recent investigations as well as our own results show that the two cytokines synergize to promote MK maturation and platelet production. We have detected synergy between rhIL-11 and Tpo in promoting MK-colony formation from murine BM mononuclear cells in a serum-containing agar culture system (data not shown). Ku et al33 observed synergy between rhIL-11 and Tpo in supporting MK-colony formation from primitive murine hematopoietic progenitors cultured in serum. They went on to demonstrate that, although SF present in the fetal calf serum could account for the synergistic effects of rhIL-11 and Tpo on granulocyte/erythrocyte/macrophage/MK-colony (GEMM-colony), granulocyte/macrophage/MK-colony (GMM-colony), and granulocyte/macrophage-colony (GM-colony) formation, factors other than SF were required for the synergistic effects on MK-colony formation. Synergy between rhIL-11 and Tpo has also been shown in vivo (S. Goldman, personal communication, January 1997). Administration of both rhIL-11 and Tpo to mice receiving a myelosuppressive regimen of carboplatin and irradiation was more effective than either cytokine administered alone. The cytokine combination greatly increased absolute reticulated platelet numbers and completely abrogated the thrombocytopenia that follows myelosuppression.
We also established that MKs are a direct target of rhIL-11. IL-11 receptor α chain mRNA and protein were shown to be present in a population of isolated human BM-derived CD41+ CD14− cells. When individual cells were analyzed, all were determined to express IL-11 receptor α chain message. The receptor protein was then shown to be functional, as exposure of purified BM MKs to rhIL-11 induced activation of its signaling pathway. Increased phosphorylation of the receptor signaling subunit, gp130, and the downstream transcription factor, STAT3, was observed in response to rhIL-11. Earlier work has shown that, upon binding of rhIL-11 to the α chain, gp130 is phosphorylated and recruited into the receptor complex and signaling is initiated.34-36 As signal transduction proceeds, cytoplasmic STAT proteins, including STAT3, are phosphorylated by the JAK/TYK kinase family and then translocated to the nucleus. A ligand-specific pattern of gene expression is subsequently observed.18,31,37 38
The results obtained in this report are consistent with previous studies that have suggested rhIL-11 was capable of acting directly on MKs and committed MK progenitors. Burstein et al15 and Carver-Moore et al32 observed that optimal concentrations of rhIL-11 alone stimulated AchE activity and increased the MK numbers in murine serum-free liquid BM cultures using normal and c-mpl deficient BM, respectively. Teramura et al,16 using purified human BM CD41+ cells, reported that rhIL-11 as a single cytokine stimulated a shift in the cells to a higher ploidy. Our findings provide evidence to link initiation of signal transduction by the binding of rhIL-11 to its receptor on MKs with rhIL-11's ability to directly promote MK maturation and growth.
Although functional IL-11 receptor α chain mRNA and protein is present in MKs, neither could be detected in platelets, suggesting an inverse correlation between α chain expression and the stages of MK maturation that lead to and include platelet formation. This type of inverse correlation has previously been described for IL-6 expression during human megakaryocytopoiesis. Navarro et al39 demonstrated the presence of IL-6 mRNA and IL-6 protein in MKs, whereas no IL-6 message, activity, or protein was detectable in normal platelets. In situ hybridization showed higher levels of IL-6 message in immature MKs than in mature MKs. However, it is interesting to note that IL-6 receptor protein is present in both MKs and platelets (Navarro et al39 and N.S. Weich, unpublished results). Nevertheless, our results show that a difference exists in the expression of IL-11 receptor protein between MKs and platelets. At this time it is unclear if the loss of IL-11 receptor α chain expression occurs as MKs mature or as platelets are formed. Further investigation will be needed to resolve this issue. This finding supports an earlier study by Zucker-Franklin and Petursson40 that presented evidence that the freeze-fracture morphology of the platelet membrane differs from that of the MK. The investigators proposed that MKs and platelets may have distinct surface properties and that they may be responsive to different external stimuli. rhIL-11 appears to be one of those external stimuli, as the absence of detectable ligand binding chain of the receptor on platelets and its presence on MKs most likely accounts for the observations made in a recent clinical trial. In this study, patients treated with rhIL-11 both before and after myelosuppressive chemotherapy showed an increase in MK numbers and maturation, whereas no significant changes in the mean platelet volume or in platelet aggregation characteristics were observed.4
Now that we have shown rhIL-11 has the potential to directly affect megakaryocytopoiesis, it is important to understand its physiologic role in this process. A recent study in humans has begun to define the relationship between rhIL-11 and platelet production.41 In this study, endogenous levels of IL-11 were determined in patients exposed to myeloablative therapy and BM/peripheral blood stem cell transplantation. During these situations of stress, circulating IL-11 levels were observed to be significantly increased above those in controls with normal platelet counts, showing an inverse correlation between IL-11 and circulating platelet numbers. The investigators proposed that megakaryocytopoiesis during acute thrombocytopenia is regulated, in part, by IL-11. It will now be interesting to investigate the role of rhIL-11 and the expression pattern of the IL-11 receptor during early stages of MK commitment.
ACKNOWLEDGMENT
The authors thank Drs S. Clark and S. Goldman for their critical reviews of the manuscript, Dr John Loewy for his help with the statistical analysis, and Dr Edward Bruno for his assistance in setting up the human MK colony assays.
Address reprint requests to Nadine S. Weich, PhD, Department of Immunology and Hematopoiesis, Genetics Institute, Inc, 87 Cambridge Park Dr, Cambridge, MA 02140.

![Fig. 1. Tpo is not detected in murine BM cultures induced with rhIL-11 and IL-3. PCR-generated DNA encoding full-length murine c-mpl was subcloned and transfected into FDCP-1 cells by electroporation as described in the Materials and Methods. FDC-MPL cells detected murine Tpo to concentrations of 15 pg/mL. CMs from murine liquid BM cultures were generated by separating out the low-density cells by centrifugation and culturing them with various growth factor combinations for 3 days (20 U/mL rmIL-3 and 10 ng/mL rhIL-11). CMs for assay were collected and cells were stained for AchE activity. FDCP-MPL cells were assayed in microtiter plates at 5 × 103 cells per well with (A) titration of growth factors ([▪] rmIL-3; [○] rmTpo; [□] media control) or (B) CM with or without antibody to mIL-3 at a 1:100 dilution ([▪] IL-3 CM; [•] IL-3 CM + anti–IL-3; [×] rhIL-11 CM; [□] IL-3 + rhIL-11 CM; [▵] IL-3 + rhIL-11 CM + anti–IL-3) for 72 hours. An enlarged view of the activity detected with (•) IL-3 CM + anti–IL-3, (×) rhIL-11 CM, and the (▵) IL-3 + rhIL-11 CM + anti–IL-3 is shown in the insert graph of (B).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/10/10.1182_blood.v90.10.3893/3/m_bl_0022f1a.jpeg?Expires=1763629710&Signature=gqMOT7OdcgwPSU0ZNw9MjhjNpsl6vL1nyEt-flq3NdjNNK5Pp8AoUD6Uamj445thkMpqKtqkat~tVjYxMcJkF2wabhXMXEq6fB3wE6DAvgaj53ITD3O4qhruZuNzBBf2gPb6rJHoW3XrF4R5bAGB9XmWB2Mwblp0VkTteZvWT22DLt92MEMAAzD5Wbjw7VFwlU1zxSPcvMla-K1dI4fs7b7-L8CcnJ4wYbBJxQQg1II~2WhrT4L~Z76DEmgxp22nlsANMB1c8clazdlD-O~yhUG71yeLQftbgkoZVukrcm~4gFCQ~UfEGFxA0Jr5NHI4fFRfnB-D5n4CgnNYBPcM3A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. Tpo is not detected in murine BM cultures induced with rhIL-11 and IL-3. PCR-generated DNA encoding full-length murine c-mpl was subcloned and transfected into FDCP-1 cells by electroporation as described in the Materials and Methods. FDC-MPL cells detected murine Tpo to concentrations of 15 pg/mL. CMs from murine liquid BM cultures were generated by separating out the low-density cells by centrifugation and culturing them with various growth factor combinations for 3 days (20 U/mL rmIL-3 and 10 ng/mL rhIL-11). CMs for assay were collected and cells were stained for AchE activity. FDCP-MPL cells were assayed in microtiter plates at 5 × 103 cells per well with (A) titration of growth factors ([▪] rmIL-3; [○] rmTpo; [□] media control) or (B) CM with or without antibody to mIL-3 at a 1:100 dilution ([▪] IL-3 CM; [•] IL-3 CM + anti–IL-3; [×] rhIL-11 CM; [□] IL-3 + rhIL-11 CM; [▵] IL-3 + rhIL-11 CM + anti–IL-3) for 72 hours. An enlarged view of the activity detected with (•) IL-3 CM + anti–IL-3, (×) rhIL-11 CM, and the (▵) IL-3 + rhIL-11 CM + anti–IL-3 is shown in the insert graph of (B).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/10/10.1182_blood.v90.10.3893/3/m_bl_0022f1b.jpeg?Expires=1763629710&Signature=w74O1skGHwu2pdEaYs2fETyX6OORZIvqvrwT5Bc0IJbNAy6YsLA364MsWyo8eOPOHF3hrvXV98SNMnhVN6ItFDlvEg8owvQrcG46NIJCa-bRHFHSdPt6u~QJqVPAcKoMhMZf4Hrl4IjI4k2txrPPWhnFs2VfZUI5Xf~yxf7kMyoEUM4VITKQxiqPK59UatY6Sh0z5omLf~AuuXp5ida-cNwSytLhC5qSHlwcL5FglDplKmh-0FVY0y5Ah~JxMgRk5qZKhHtmQoAw3CkvWMK0-KOUQbfiDO6wcD~d3XfBh6~wkDFQja4kwI-IsWj4trFSNXIQ~vrFyVFrfuy8ytOtwA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal