To the Editor:
The Wilms' tumor gene (WT1) located on chromosome 11p131 encodes a transcription factor, which is involved in control of growth and differentiation of various cell types including hematopoietic cells.2 It activates or suppresses the transcription of target genes depending on their promoter structure and the presence of other transcriptional regulators. The WT1 gene is expressed in blasts of almost all acute leukemia patients, irrespective of lineage.3-5 Normal blood cells and CD34+ hematopoietic progenitors express the WT1 gene on a far lower level3 or not at all4,5 depending on the reverse transcriptase-polymerase chain reaction protocols used. Thus, detection of WT1 gene transcripts was implied as a diagnostic tool to mark minimal residual disease and imminent relapse in treated acute leukemia patients.3,4 However, recently increasing evidence suggests that a subset of normal CD34+ hematopoietic progenitors expresses the WT1 gene during not yet defined circumstances.6 7
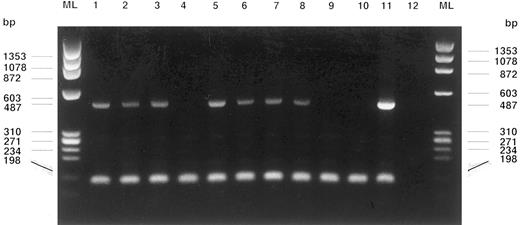
WT1 gene (487 bp) and cABL gene (198 bp, control of RNA-integrity) RT-PCR amplification products of fetal blood MNCs and different single soft agar colonies are shown on an electrophoresis gel stained with ethidium bromide. Lanes from left: Fetal blood MNCs from patients S.M. (1), K.B. (2), and K.Z. (3), MNCs from the leukapheresis product of a solid cancer patient treated with stem cell-mobilizing chemotherapy and G-CSF4, colonies at day 14: CFU-GEMM (5), BFU-E (6), CFU-E (7), CFU-GEMM (8), colonies at day 21: BFU-E (9), CFU-GM (10), HL60 cells (11, positive control), water control (12), and marker lanes (ML).
WT1 gene (487 bp) and cABL gene (198 bp, control of RNA-integrity) RT-PCR amplification products of fetal blood MNCs and different single soft agar colonies are shown on an electrophoresis gel stained with ethidium bromide. Lanes from left: Fetal blood MNCs from patients S.M. (1), K.B. (2), and K.Z. (3), MNCs from the leukapheresis product of a solid cancer patient treated with stem cell-mobilizing chemotherapy and G-CSF4, colonies at day 14: CFU-GEMM (5), BFU-E (6), CFU-E (7), CFU-GEMM (8), colonies at day 21: BFU-E (9), CFU-GM (10), HL60 cells (11, positive control), water control (12), and marker lanes (ML).
Here, we report on WT1 gene expression in umbilical cord blood cells of human fetuses aged between 19 and 34 weeks of gestation. Further, we found WT1 gene expression in normal hematopoietic progenitors only during the early exponential growth phase when propagated in clonal growth assays.
Blood of human fetuses (n = 6) was obtained by ultrasound-guided puncture of the umbilical cord vein for intrauterine transfusions. Umbilical cord blood mononuclear cells (MNCs, 1.0 mL) were prepared and subjected to the RT-PCR protocol for WT1 gene detection as described.5WT1 gene transcripts were found in five of six fetal MNC preparations (Table 1). MNCs of leukapheresis products from solid cancer patients (n = 3) were seeded onto methyl-cellulose agar plates enriched with growth factors at a concentration of 100,000 cells/μL. Colony formation was observed, qualified, and counted. At days 14, 21, and 28 after seeding representative colonies were picked using flame-bent glass-micropipets under light-microscopic control (2.5×). Each colony containing between 100 and 1,000 vital cells (trypan blue uptake in less than 5% of cells) was separately subjected to the WT1-RT-PCR protocol. WT1 gene expression was found in almost all colonies picked at day 14 (100 to 300 cells per colony), irrespective of lineage. Contrary, WT1 gene transcripts were not detectable in colonies picked at days 21 or 28. MNC preparations (106 cells) from leukapheresis products of limited-disease solid cancer patients who were pretreated with stem cell-mobilizing chemotherapy and granulocyte colony-stimulating factor (G-CSF ) (breast or esophageal cancer; n = 10; CD34+ hematopoietic progenitor content: 0.5% to 6.6%), including the preparations already used for the clonal growth assays, did consistently not express the WT1 gene (Table 1, Fig 1).
WT1 Gene Expression at Different Time Spots in Single Hematopoietic Colonies Grown in Soft Agar
| . | WT1 m-RNA . | c-ABL m-RNA . |
|---|---|---|
| . | Expression . | Expression . |
| Colonies at day 14 | 28/35 | 35/35 |
| CFU-GEMM | 2/3 | 3/3 |
| BFU-E | 7/7 | 7/7 |
| CFU-E | 6/6 | 6/6 |
| CGU-GM | 12/19 | 19/19 |
| Colonies at day 21 | 1/20 | 20/20 |
| CFU-GEMM | 0/1 | 1/1 |
| BFU-E | 0/7 | 7/7 |
| CFU-E | — | — |
| CFU-GM | 1/12 | 12/12 |
| Colonies at day 28 | 0/21 | 21/21 |
| CFU-GEMM | — | — |
| BFU-E | 0/3 | 3/3 |
| CFU-E | 0/6 | 6/6 |
| CFU-GM | 0/12 | 12/12 |
| Fetal blood MNC | 5/6 | 6/6 |
| Adult blood MNC | 0/20 | 20/20 |
| Reactive BM MNC | 0/4 | 4/4 |
| Leukapheresis product MNC | 0/10 | 10/10 |
| . | WT1 m-RNA . | c-ABL m-RNA . |
|---|---|---|
| . | Expression . | Expression . |
| Colonies at day 14 | 28/35 | 35/35 |
| CFU-GEMM | 2/3 | 3/3 |
| BFU-E | 7/7 | 7/7 |
| CFU-E | 6/6 | 6/6 |
| CGU-GM | 12/19 | 19/19 |
| Colonies at day 21 | 1/20 | 20/20 |
| CFU-GEMM | 0/1 | 1/1 |
| BFU-E | 0/7 | 7/7 |
| CFU-E | — | — |
| CFU-GM | 1/12 | 12/12 |
| Colonies at day 28 | 0/21 | 21/21 |
| CFU-GEMM | — | — |
| BFU-E | 0/3 | 3/3 |
| CFU-E | 0/6 | 6/6 |
| CFU-GM | 0/12 | 12/12 |
| Fetal blood MNC | 5/6 | 6/6 |
| Adult blood MNC | 0/20 | 20/20 |
| Reactive BM MNC | 0/4 | 4/4 |
| Leukapheresis product MNC | 0/10 | 10/10 |
MNC preparations of leukapheresis products obtained from three solid cancer patients were seeded onto soft agar plates. Before leukapheresis, patients were treated with stem cell-mobilizing chemotherapy and G-CSF. At days 14, 21, and 28 colonies of different lineages were separately subjected to the WT1-RT-PCR protocol to detect WT1 mRNA transcripts.
Abbreviations: BFU-E, burst-forming unit erythrocytes; CFU, colony-forming unit; GEMM, colonies containing granulocytes, erythrocytes, monocytes, and megakaryocytes; E, erythrocyte; GM, granulocyte and monocytes.
Due to a large first amplification product (1,745 base pairs [bp]), the nested-primer RT-PCR protocol we are using is not as sensitive as other protocols employed for WT1 mRNA detection. However, our RT-PCR protocol enabled us to detect WT1 gene expression in the same percentage of acute leukemia MNC preparations as compared to others.3,4 Since more sensitive RT-PCR protocols detect low WT1 gene expression levels in normal blood and bone marrow (BM) MNCs, quantitative RT-PCR had to be implemented to discriminate between a physiologic and a malignant, leukemia-associated expression level of this gene.3 Contrary to acute leukemia, we never detected the WT1 nuclear protein in MNC preparations from normal blood and BM, or from leukapheresis products of solid cancer patients, using a single cell indirect immunofluorescence assay with anti-WT1 monoclonal antibodies.8 Thus, it remains unclear, whether the detection of low-level WT1 gene expression in normal blood cells and hematopoietic progenitors by highly-sensitive RT-PCR protocols reflects “illegitimate or ectopic transcripts” or may have a physiologic significance. To our surprise, we found WT1 gene transcripts in almost all hematopoietic soft agar colonies at day 14 but not thereafter, although single colonies at day 14 contain only 100 to 300 as compared to 800 to 1,000 cells at day 28, indicating transient WT1 gene expression in hematopoietic progenitor cells during their early exponential growth.
Finally, we hypothesize that expression of the WT1 gene is relevant to the fetal development and physiologic expansion of immature CD34+ hematopoietic progenitors, and that the WT1 gene is functionally switched off on their determination and differentiation. This hypothesis explains acute leukemia as a proliferative disorder, which is at least partly arrested in a state of WT1 gene-expressing stem cell expansion. It further explains, why the WT1 gene is downregulated in differentiation-induced leukemia cell lines, why antisense-WT1 oligonucleotides reduce growth of acute leukemia cell lines, and why subsets of normal regenerating BM CD34+ hematopoietic progenitors express the WT1 gene on levels comparable to leukemia blasts.6

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal