To the Editor:
Lately, arsenic trioxide (As2O3 ) has been described in the treatment of acute myeloid leukemia. Experiments in vitro showed that As2O3 induced the acute promyelocytic leukemia (APL) cell line NB4 to downregulate bcl-2 expression, as well as to undergo apoptosis.1 Clinically efficacy has been shown in 14 of 15 patients with relapsed APL, where the use of intravenous As2O3 at a dose of 10 mg/d for 4 to 9 weeks resulted in complete morphologic remission without associated bone marrow suppression.2 In these cases, partial differentiation of the APL cells and downregulation of the fusion protein PML/RARα could also be shown, which might account for the pharmacologic action of the drug.3
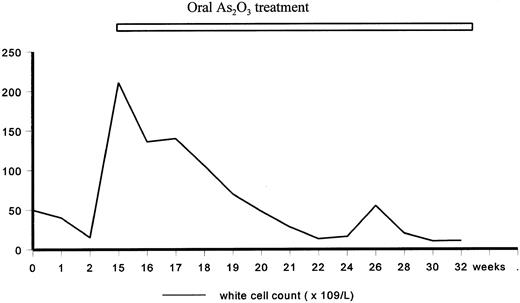
Arsenic has been known to be poisonous for centuries. Medicinal use of arsenic began in the 15th century. In the 18th century, Dr Thomas Fowler developed a solution preparation of As2O3 in potassium bicarbonate (1% wt/vol), known generally as Fowler's solution,4 which was used empirically for the treatment of a variety of infectious and malignant diseases. The effect of Fowler's solution on the reduction of white cells in two normal people and one patient with “leucocythemia” studied at Boston City Hospital, MA was first described in 1878.5 This lead to the use of As2O3 for the treatment of leukemia, until the advent of radiotherapy caused a decline in its clinical application. Its popularity waxed again when Forkner and Scott,6 also at Boston City Hospital, described nine of 10 patients with chronic myeloid leukemia (CML) who responded to As2O3 treatment. These results were subsequently confirmed by other reports,7 so that As2O3 was considered next to irradiation as the most effective treatment of CML before the development of modern chemotherapy.8 Clinical improvement of the leukemia, including the control of fever, reduction of white cell count, amelioration of anemia and decrease in the size of spleen, could often be achieved. Sometimes, a remission might be maintained for a long period. As expected, toxic side effects were observed in the majority of patients given long-term As2O3 , including skin pigmentation and keratosis, cirrhosis, polyneuritis, and gastrointestinal problems.9 In this department, As2O3 was used by hematologists in the 1950's for the treatment of a variety of leukemias. Figure 1 shows the typical course of a patient treated with As2O3 for CML in chronic phase. As As2O3 appeared to be effective for leukemias of different morphologic types, the action was probably related to an intrinsic toxicity of arsenic to marrow cells.
A 30-year-old man presented in March 1954 with splenomegaly and CML in chronic phase was diagnosed. No specific treatment was given until October 1954 when his splenomegaly increased to 5 cm and his white cell count increased to 50 × 109/L. Fowler's solution 5 minims (1 minim = 0.06 mL, equivalent to 0.6 mg As2O3 ) three times daily was administered, resulting in a satisfactory control of his white cell count to about 10 × 109/L. Treatment was stopped. Six months later, he was readmitted with progressive splenomegaly (10 cm) and leucocytosis (211 × 109/L). Fowler's solution was recommenced at 5 minims three times daily, and increased to 10 minims three times daily. This resulted in gradual control of his white cell count. The dose of As2O3 was decreased to a maintenance dose of 5 minims three times daily. However, 8 months later, signs and symptoms of chronic arsenic poisoning developed, including skin pigmentation, diarrhea, and chronic gastrointestinal hemorrhage. As2O3 was stopped and he was put on melphalan. Splenomegaly and leucocytosis progressed despite treatment, and he died 11 months later of pneumonia. The maximum daily dose (10 minims × 3) of As2O3 given orally was 18 mg, which is comparable to 10 mg/d when used intravenously for the treatment of relapsed APL.
A 30-year-old man presented in March 1954 with splenomegaly and CML in chronic phase was diagnosed. No specific treatment was given until October 1954 when his splenomegaly increased to 5 cm and his white cell count increased to 50 × 109/L. Fowler's solution 5 minims (1 minim = 0.06 mL, equivalent to 0.6 mg As2O3 ) three times daily was administered, resulting in a satisfactory control of his white cell count to about 10 × 109/L. Treatment was stopped. Six months later, he was readmitted with progressive splenomegaly (10 cm) and leucocytosis (211 × 109/L). Fowler's solution was recommenced at 5 minims three times daily, and increased to 10 minims three times daily. This resulted in gradual control of his white cell count. The dose of As2O3 was decreased to a maintenance dose of 5 minims three times daily. However, 8 months later, signs and symptoms of chronic arsenic poisoning developed, including skin pigmentation, diarrhea, and chronic gastrointestinal hemorrhage. As2O3 was stopped and he was put on melphalan. Splenomegaly and leucocytosis progressed despite treatment, and he died 11 months later of pneumonia. The maximum daily dose (10 minims × 3) of As2O3 given orally was 18 mg, which is comparable to 10 mg/d when used intravenously for the treatment of relapsed APL.
Therefore, while As2O3 induced apoptosis and differentiation of APL cells is a novel observation, its clinical use represents but a resurgence of the use of arsenicals in the treatment of leukemia. Because of the considerable toxicities and the possible and still undefined long-term sequelae, the usefulness of As2O3 in the modern treatment of leukemia is still unclear. For this reason, Fig 1 is only of historical interest. However, the use of heavy metals in the treatment of malignancies is not unprecedented, with platinum being a prominent example. Therefore, the rekindling of the interest in arsenicals is of potential importance, but the biological and pharmacological actions of arsenic must be further investigated to define its role in the treatment of leukemia and other types of malignancies.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal