Abstract
To characterize L-selectin–dependent cell adhesion to human vascular endothelium, human cardiac microvascular endothelial cells (HCMEC) and human coronary endothelial cells (HCEC) were isolated from explanted human hearts. The adhesion behavior of human (NALM-6) and mouse (300.19) pre-B cells transfected with cDNA encoding for human L-selectin was compared with that of the respective nontransfected cells in a flow chamber in vitro. More than 80% of the adhesion to tumor necrosis factor-α (TNF-α)–stimulated HCMEC at shear stresses <2 dyne/cm2 was L-selectin dependent and could be equally well blocked by an anti–L-selectin antibody or a L-selectin-IgG-chimera. No L-selectin dependent adhesion to HCEC could be shown. The L-selectin dependent adhesion to HCMEC was insensitive to neuraminidase, but greatly inhibited by addition of NaClO3 , which inhibits posttranslational sulfation and remained elevated for at least 24 hours of stimulation. E-selectin dependent adhesion of HL60 cells to HCMEC was blocked by neuraminidase, but not by NaClO3 and returned to control levels within 18 hours of HCMEC stimulation. It is concluded that microvascular, but not macrovascular endothelial cells express TNF-α–inducible sulfated ligand(s) for L-selectin, which differ from known L-selectin ligands, because sialylation is not required. The prolonged time course of L-selectin dependent adhesion suggests a role in sustained leukocyte recruitment into inflammatory sites in vivo.
THE SELECTIN family of adhesion molecules consists of three members: two vascular selectins (E- and P-selectin) and one leukocyte selectin (L-selectin).1 All three selectins play a role in mediating leukocyte rolling type adhesion in postcapillary venules, which is an early step of leukocyte recruitment during inflammatory reactions.2 This is concluded from studies in different models, which showed rolling to occur in the presence of any one of the selectins alone.3-5
For a better understanding of the in situ phenomenon, the interplay of the three selectins as well as possible temporal differences in their involvement are of interest. While the kinetics of P- and E-selectin presentation on the endothelium exhibits a characteristic maximum at 4 to 6 hours after tumor necrosis factor-α (TNF-α) stimulation,6-8 L-selectin is constitutively expressed on most leukocytes.9 Therefore, the time course of L-selectin mediated function depends on the kinetics of surface expression of L-selectin ligands on endothelial cells.
Further insight into the interplay of the selectins comes from studies using selectin-deficient mice.10 Early leukocyte rolling seen after surgery is absent in P-selectin deficient, but not impaired in L-selectin deficient mice in which it ceases after 1 hour.11 Leukocyte migration into the peritoneal cavity during the first 4 hours after thioglycollate instillation is significantly reduced in P- as well as L-selectin deficient mice. At later time points, it remains diminished in L-selectin deficient, but is near normal in P-selectin deficient mice. These observations suggest a special role of L-selectin during the later time points of the inflammatory process. The normal inflammatory responses to thioglycollate instillation seen in E-selectin deficient mice can be completely inhibited by antibodies to P-selectin. The same effect can be achieved in P-selectin deficient mice by antibodies to E-selectin. Thus, in these models L-selectin alone seems to be insufficient to mediate leukocyte rolling.
L-selectin develops its function as an adhesion molecule in conjunction with its counter ligand. Three specific L-selectin ligands have previously been isolated from high endothelial venules of peripheral lymph nodes PLN-HEV of mice12: GlyCAM-1, CD34, and MadCAM-1. Sialylation, sulfation, and fucosylation of O-linked carbohydrate side chains of these ligands are required to provide full-binding activity. A fourth L-selectin ligand of 200,000 Mr has been isolated from HEV and shown to need sulfation to develop its binding activity.13,14 Although certain inflammatory mediators, especially TNF-α and interleukin-4, have been shown to induce functional ligands for L-selectin on cultured HUVEC,15 16 L-selectin ligands from postcapillary venules have not yet been identified.
The present study was designed to characterize L-selectin ligands on cultured human microvascular and macrovascular endothelial cells from the same vascular bed. Human cardiac microvascular endothelial cells (HCMEC) and human coronary endothelial cells (HCEC) were isolated and compared with respect to the expression of L-selectin ligands. The time course of cytokine induced expression of L-selectin ligands was studied in comparison with that of E-selectin. Further experiments were performed to reveal whether sialylation and/or sulfation of putative L-selectin ligand(s) are necessary for L-selectin–dependent adhesive interactions in the presence of flow.
MATERIALS AND METHODS
Isolation and cultivation of endothelial cells.HCMEC were obtained from explanted human hearts as described previously.17 Briefly, perfusion of heart muscle segments (approximately 25 g) for 30 minutes with Krebs-Henseleit buffer, containing 0.074% collagenase II (Sigma, Deisenhofen, Germany), 0.012% dispase (5,000 U/mL, Collaborative Research Inc, Bedford, MA), 0.012% trypsin (1:250, Serva, Heidelberg, Germany), and 0.27% bovine serum albumin (BSA; fraction V, 7.5%, Sigma) was followed by further disaggregation of the tissue by incubation for 20 minutes in the enzyme solution. Capillary fragments were cleared from undigested tissue by filtration through a nylon net (200 μm, Recker, Berlin, Germany), pelleted and seeded on gelatin coated culture flasks. Further purification of endothelial cells was achieved with paramagnetic beads (Dynal, Hamburg, Germany) linked to the Ulex europaeus-I lectin (UEA-I, Sigma). A strong magnetic field was used to separate the endothelial cells coupled to the beads from nonbinding cells. The cells were cultured with medium 199 containing fetal calf serum (20%), streptomycin (100 μg/mL, Sigma), penicillin (100 U/mL, Sigma), and endothelial cell growth factor (10 ng/mL, Boehringer, Mannheim, Germany) in 5% CO2 at 37°C.
Cell cultures of HCEC were prepared as described previously.17 Briefly, segments of epicardial coronary arteries were obtained from explanted hearts, stored in ice cold medium 199 and cleared of the surrounding tissue. Arterial segments were incubated with collagenase II (0.2%, Roth, Karlsruhe, Germany) at 37°C for 30 minutes. Detached cells were rinsed out of the vessel with medium 199, pelleted and seeded on gelatine-coated cell culture flasks. HCEC were cultured under the same conditions as described above for HCMEC.
Preparation of endothelial cells for adhesion experiments.Endothelial cells of 3rd passage were grown on fibronectin (10 μg/mL, Sigma) coated coverslips and stimulated by incubation with human TNF-α (100 U/mL, Costar GmbH, Bodenheim, Germany) for up to 24 hours. Digestion of activated HCMEC with neuraminidase (type X from Clostridium perfringens, Sigma) was performed with 0.1 U/mL in phosphate-buffered saline (PBS) (pH 6.4) supplemented with BSA (0.1%) and glucose (0.25%) for 60 minutes at 37°C. Inhibition of sulfation reactions was achieved by cultivating HCMEC in the presence of sodium chlorate (10 mmol/L, Aldrich, Steinheim, Germany) for 48 hours.18 In additional experiments, HCMEC were incubated either with function blocking anti–E-selectin monoclonal antibody (MoAb) BBA2 (10 μg/mL, Hermann Biermann GmbH, Bad Nauheim, Germany) or with a L-selectin-IgG-chimera19 (10 μg/mL, a kind gift from S. Watson and L. Lasky, Genetech, South San Francisco, CA) for 60 minutes at 37°C, before the coverslips were mounted in the flow chamber.
Flow chamber adhesion assay.Endothelial cells of either type were grown on fibonectin-coated coverslips and exposed to near physiologic flow conditions using a flow chamber with parallel-plate geometry. The wall shear stress produced with a constant flow rate is a function of the width of the flow channel, which widens along the flow direction in a hyperbolic manner. Therefore, the shear stress decreases linearly with increasing distance from the flow chamber entrance. At flow rates of 116 μL/min and 47 μL/min, the wall shear stress varied between 0.6 and 2.9 dyne/cm2 or 0.2 and 1.07 dyne/cm2, respectively.
The assembled flow chamber was placed on the stage of an inverted Nikon microscope (Nikon GmbH, Düsseldorf, Germany) and its temperature was set at 37°C throughout the experiment by feedback controlled warm air flow. An injection port at the entrance permitted the addition of cell suspensions to the main stream of the perfusion medium (medium 199 containing 20 mmol/L HEPES and 0.1% BSA). The perfusion medium was prewarmed to 37°C by passing through a waterbath and the flow rates were controlled by a Harvard syringe pump (Harvard Apparatus Co Inc, Dover, MA). Usually 100 μL of cell suspension (106 cells per mL) were added and cell adhesion was allowed to take place during a 5-minute run. The number of firmly attached cells was counted after 5 minutes with the perfusion medium still flowing. It represents a composite measure of attachment rate, detachment rate, and rolling velocity, referred to as rolling adhesion. Numbers given in the following text represent mean values (±SEM) of firmly attached cells under flow conditions per mm2 of endothelial surface in n experiments.
Cell systems.Four different cell species were used in the experiments. These cells were selected on the basis of the surface expression of receptors or ligands required for selectin mediated adhesion.
(1) Mouse pre-B cells 300.19 and stable transfectants expressing L-selectin (300.19-L)5 9 were grown in suspension culture in RPMI-1640 with 10% fetal calf serum, 10 μmol/L 2-mercaptoethanol and antibiotics in 5% CO2 at 37°C (all cell culture reagents were obtained from Biochrom KG, Berlin, Germany).
(2) Human pre-B cells NALM-6 and stable transfectants expressing L-selectin (NALM-6-L) were grown under the same culture conditions without the addition of 2-mercaptoethanol.
In some experiments L-selectin transfectants in a concentration of 106/mL were incubated with MoAb LAM1-3 or LAM1-12 (both MoAb dilluted 1:200 from ascites) 10 minute before the adhesion assay. LAM1-3 binds to the lectin domain of L-selectin and was used to block L-selectin specific adhesion. LAM1-12 binds to an epitope located at the CR-repeats. Thus, it was used as a negative control compared to LAM1-3. Both antibodies are of the IgG1 subtype.20
(3) HL60 cells with high expression of sLex 21 were grown under the same culture conditions as NALM-6 cells. Neuraminidase (type X from Clostridium perfringens, Sigma) digestion of HL60 was performed with 0.1 U/mL neuraminidase in PBS (pH 6.4) supplemented with 0.1% BSA and 0.25% glucose for 30 minutes at 37°C.
(4) Human polymorphonuclear neutrophils (PMN) were isolated by routine methods. Briefly, heparinized venous blood of healthy subjects was taken. Autologous plasma sedimentation was followed by centrifugation on a discontinous percoll gradient of 74% and 55% isotonic percoll (Sigma), respectively. After 20 minutes centrifugation at 600g, the cell layer containing 98% of granulocytes (as judged by microscopic analysis following Turk's staining procedure) was collected and washed once with 10× excess of PBS without divalent cations supplemented with 0.25% glucose and 0.1% BSA. To avoid preactivation of the cells, the pellet was gently resuspended in this solution without any lysis procedure of the few remaining erythrocytes. The viability of PMN was always well above 99% as judged by Trypan blue exclusion.
Flow cytometry.Endothelial cells were suspended by incubation in divalent cation free PBS with additional EDTA (0.2% wt/vol) for 10 minutes at 4°C. This treament allowed the cells to be shaken off from the culture flask. Suspended cells (106/mL) were incubated for 30 minutes at 4°C with antibody and washed twice. Cells labeled with L-selectin–specific rhodamine-conjugated MoAb TQ1-RD (1:100; Coulter, Krefeld, Germany) were directly subjected to flow cytometry. Binding of sialyl-Lewisx-specific MoAbs AM-322 (as hybridoma culture supernatant diluted 1:35) or CSLEX23 (as hybridoma culture supernatant diluted 1:25), binding of E-selectin specific MoAb BBA2 (10 μg/mL, Hermann Biermann GmbH as well as binding of CD34 specific MoAb BW 905/151 (10 μg/mL, a kind gift of Behringwerke AG, Marburg, Germany) was visualized by incubation with fluorescein isothyiocyanate-conjugated rabbit-antimouse Ig (1:40, DAKO, Hamburg, Germany). Antigen expression was measured using a FacScan flow cytometer and analysed using the lysis II software (Becton Dickinson, Heidelberg, Germany).
RESULTS
Flow cytometry data are shown in Table 1. Although 300.19-L cells express L-selectin to a similar amount as PMN, the expression on NALM-6-L cells is significantly higher. Thus, the latter cells provide an ideal tool for the investigation of L-selectin dependent adhesion, whereas both the nontransfected NALM-6 cells and HL60 cells are negative for L-selectin expression. Pre-B cells NALM-6 express only small amounts of sLex, whereas HL60 cells express huge amounts of sLex. Thus, comparison of the adhesion behavior of HL60 cells and L-selectin–transfected pre-B cells to vascular endothelium is a suitable approach to distinguish between adhesion mechanisms mediated by either vascular or leukocyte selectins. HCMEC express a substantial amount of CD34, but are negative for sLex expression as shown by two MoAbs, AM-3 and CSLEX. Stimulation by TNF-α (100 U/mL, 5 hours) does neither alter the expression of CD34 nor that of sLex but E-selectin expression is strongly enhanced.
Cell Surface Expression of L-Selectin, Sialyl-Lewisx, E-Selectin, and CD34 as Measured by Flow Cytometry
| . | TQ1 . | AM-3 . | CSLEX . | BBA2 . | BW 905/151 . | Neg. Control . |
|---|---|---|---|---|---|---|
| PMN | 542 (21) | 480 (10) | nt | nt | nt | 10 (50) |
| 300.19 | 15 (66) | nt | nt | nt | nt | 10 (50) |
| 300.19-L | 340 (164) | nt | nt | nt | nt | 11 (154) |
| NALM-6 | 16 (62) | 116 (433) | nt | nt | nt | 10 (60) |
| NALM-6-L | 1,608 (43) | 115 (443) | nt | nt | nt | 18 (38) |
| HL60 | 16 (62) | nt | nt | nt | nt | 15 (33) |
| HCMEC, unstim. | nt | 16 (56) | 21 (76) | 192 (77) | 105 (286) | 17 (82) |
| HCMEC, TNF-α | nt | 13 (55) | 22 (77) | 4,684 (54) | 88 (277) | 18 (54) |
| . | TQ1 . | AM-3 . | CSLEX . | BBA2 . | BW 905/151 . | Neg. Control . |
|---|---|---|---|---|---|---|
| PMN | 542 (21) | 480 (10) | nt | nt | nt | 10 (50) |
| 300.19 | 15 (66) | nt | nt | nt | nt | 10 (50) |
| 300.19-L | 340 (164) | nt | nt | nt | nt | 11 (154) |
| NALM-6 | 16 (62) | 116 (433) | nt | nt | nt | 10 (60) |
| NALM-6-L | 1,608 (43) | 115 (443) | nt | nt | nt | 18 (38) |
| HL60 | 16 (62) | nt | nt | nt | nt | 15 (33) |
| HCMEC, unstim. | nt | 16 (56) | 21 (76) | 192 (77) | 105 (286) | 17 (82) |
| HCMEC, TNF-α | nt | 13 (55) | 22 (77) | 4,684 (54) | 88 (277) | 18 (54) |
Abbreviations: nt, not tested; unstim, unstimulated.
Measurements of fluorescence intensity obtained with 5 MoAbs (TQ1 for L-selectin, AM3 and CSLEX for sLex, BW 905/151 for CD34, BBA2 for E-selectin). Numbers given represent geometric mean values of 5,000 events on a 4-decade logarithmic scale in 3 independent experiments; coefficients of variation (in %) are given in parentheses.
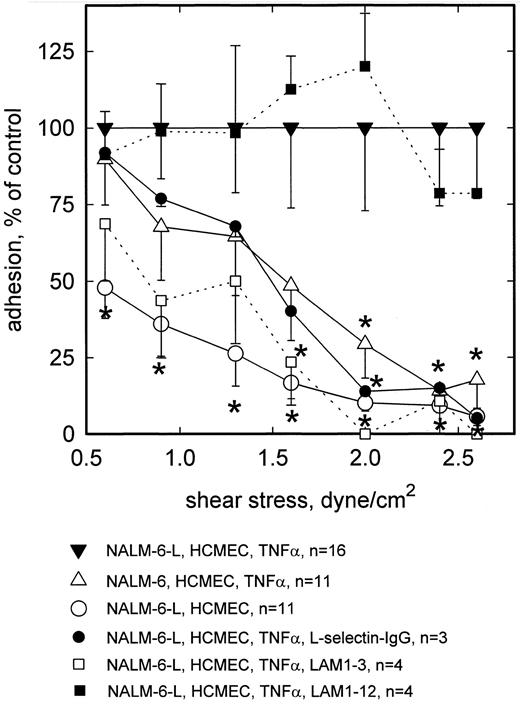
In all flow chamber experiments, rolling adhesion was observed to decline with increasing wall shear stress. In n = 16 experiments with HCMEC incubated for 5 hours with TNF-α (100 U/mL), the number of firmly attached NALM-6-L cells per mm2 of TNF-α–activated HCMEC averaged 194 (±21) at 0.6 dyne/cm2, 138 (±22) at 0.9 dyne/cm2, 90 (±19) at 1.3 dyne/cm2, 64 (±16) at 1.6 dyne/cm2, 40 (±11) at 2.0 dyne/cm2, 17 (±4) at 2.4 dyne/cm2, and 10 (±2) at 2.6 dyne/cm2. In n = 11 experiments the number of firmly attached L-selectin negative NALM-6 cells per mm2 of TNF-α–activated HCMEC averaged 175 (±29) at 0.6 dyne/cm2, 94 (±24) at 0.9 dyne/cm2, 58 (±17) at 1.3 dyne/cm2, 31 (±11) at 1.6 dyne/cm2, 12 (±4) at 2.0 dyne/cm2, 2 (±0.6) at 2.4 dyne/cm2, and 1.7 (±1) at 2.6 dyne/cm2. No higher shear stresses were applied. On nonactivated HCMEC, rolling adhesion of NALM-6-L cells was substantially lower over the whole shear stress range tested (Fig 1). At 0.6 dyne/cm2, adhesion of L-selectin− NALM-6 to activated HCMEC was not significantly different from that of NALM-6-L. By contrast, at >2 dyne/cm2 adhesion of the nontransfected cells to TNF-α–stimulated HCMEC was less than 20% of that of transfected cells, thus showing that about 80% of rolling adhesion was L-selectin dependent at these higher shear stresses. This was confirmed blocking L-selectin dependent adhesion of NALM-6-L cells by MoAb LAM1-3, whereas MoAb LAM1-12 as an isotype matched binding control had no effect. Furthermore, the rolling adhesion of L-selectin transfected NALM-6-L cells was reduced by preincubation of the activated HCMEC monolayer with a L-selectin-IgG-chimera. Under these conditions, adhesion of NALM-6-L was similar to that of L-selectin− NALM-6 cells. NALM-6-L cells showed a low level of unspecific adhesion to nonactivated HCMEC, particularly at the lower wall shear stresses.
Rolling adhesion of L-selectin+ (NALM-6-L) and L-selectin− (NALM-6) cells to activated HCMEC as a function of shear stress. At low shear wall stress (0.6 dyne/cm2 ), NALM-6 cells adhere to TNF-α–activated HCMEC equally well as NALM-6-L cells. At high shear stress (<2.0 dyne/cm2 ), adhesion of NALM-6 cells to activated HCMEC is as low as that of NALM-6-L cells to nonactivated HCMEC. L-selectin dependence to TNF-α–stimulated HCMEC is confirmed by inhibition with anti–L-selectin MoAb LAM1-3 and by a L-selectin-IgG-chimera. MoAb LAM1-12 as a binding control antibody does not reduce NALM-6-L adhesion to activated HCMEC. Mean values ±SEM; *P < .05 versus positive control.
Rolling adhesion of L-selectin+ (NALM-6-L) and L-selectin− (NALM-6) cells to activated HCMEC as a function of shear stress. At low shear wall stress (0.6 dyne/cm2 ), NALM-6 cells adhere to TNF-α–activated HCMEC equally well as NALM-6-L cells. At high shear stress (<2.0 dyne/cm2 ), adhesion of NALM-6 cells to activated HCMEC is as low as that of NALM-6-L cells to nonactivated HCMEC. L-selectin dependence to TNF-α–stimulated HCMEC is confirmed by inhibition with anti–L-selectin MoAb LAM1-3 and by a L-selectin-IgG-chimera. MoAb LAM1-12 as a binding control antibody does not reduce NALM-6-L adhesion to activated HCMEC. Mean values ±SEM; *P < .05 versus positive control.
This pattern of shear stress dependent rolling adhesion, which is characterized by a major contribution of L-selectin at >2 dyne/cm2 could not be shown on several preparations of HCEC (Fig 2). There was no difference in adhesion of NALM-6 or NALM-6-L cells to TNF-α–activated HCEC, neither at lower (0.9 dyne/cm2 ) nor at higher (2.4 dyne/cm2 ) shear stresses. However, stimulation of HCEC with TNF-α did result in some elevation of adhesion of both cell types at low wall shear stress.
Adhesion to HCEC is independent of L-selectin. At 0.9 dyne/cm2, adhesion of NALM-6-L as well as NALM-6 cells is equally enhanced by activation of HCEC with TNF-α. At 2.4 dyne/cm2, no difference exists between adhesion of NALM-6-L and NALM-6 cells to activated HCEC. Mean values ±SEM.
Adhesion to HCEC is independent of L-selectin. At 0.9 dyne/cm2, adhesion of NALM-6-L as well as NALM-6 cells is equally enhanced by activation of HCEC with TNF-α. At 2.4 dyne/cm2, no difference exists between adhesion of NALM-6-L and NALM-6 cells to activated HCEC. Mean values ±SEM.
Rolling adhesion of HL60 cells to activated HCMEC (TNF-α for 5 hours) under flow conditions was completely E-selectin dependent, because preincubation of HCMEC with the anti–E-selectin antibody BBA2 greatly inhibited adhesion of HL60 cells at the higher range of shear stresses applied (Fig 3). Adhesion at low shear stress or near static conditions was not affected by anti–E-selectin antibody. Since the same observation was made with NALM-6 and NALM-6-L cells (Fig 1), E- as well as L-selectin–mediated cell to cell interactions seem to contribute to adhesion only under conditions of enhanced flow.
Adhesion of HL60 to HCMEC is blocked by MoAb BBA2 against E-selectin. At shear stresses <1.0 dyne/cm2, adhesion of HL60 cells to TNF-α–stimulated HCMEC was significantly blocked by preincubation with MoAb BBA2. No adhesion is seen on unstimulated HCMEC. Mean values ±SEM; * P < .05 versus positive control.
Adhesion of HL60 to HCMEC is blocked by MoAb BBA2 against E-selectin. At shear stresses <1.0 dyne/cm2, adhesion of HL60 cells to TNF-α–stimulated HCMEC was significantly blocked by preincubation with MoAb BBA2. No adhesion is seen on unstimulated HCMEC. Mean values ±SEM; * P < .05 versus positive control.
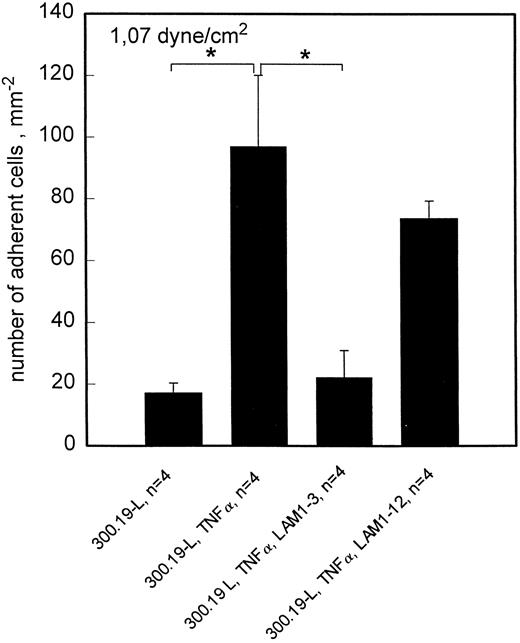
To investigate the time course of L-selectin ligand expression in TNF-α–activated HCMEC, L-selectin transfected 300.19 cells were used. Because the attachment of these cells to endothelial cells is less resistant to flow, the highest shear stress applied in these experiments was 1.07 dyne/cm2. This shear stress was chosen to strike a balance between a reasonable amount of adhesion and specificity of interaction for both L- and E-selectin mediated adhesion. At this shear stress, rolling adhesion of L-selectin transfected 300.19 cells to HCMEC is elevated for at least 24 hours of TNF-α treatment (Fig 4). By contrast, sLex+, but L-selectin− HL60 cells showed a transient pattern of adhesion to HCMEC, which was maximal at 6 hours and returned to control levels at 18 hours of stimulation with TNF-α. To confirm L-selectin–dependent rolling adhesion of 300.19-L cells under these experimental conditions, adhesion blocking experiments were done with MoAb LAM1-3, which reduced the number of adherent cells to negative control values. LAM1-12 as an isotype matched binding control MoAb had no significant effect (Fig 5).
Rolling adhesion of L-selectin+ (300.19-L, top panel) and L-selectin−, but sLex+ (HL60, bottom panel) cells to TNF-α–stimulated HCMEC as a function of the duration of TNF-α incubation. At 1.07 dyne/cm2, adhesion of 300.19-L increases at 4 to 5 hours of stimulation and remains elevated for at least 24 hours. Adhesion of HL60 increases at 3 to 4 hours of stimulation and returns to the control level within 18 hours. Mean values ±SEM, n = 2.
Rolling adhesion of L-selectin+ (300.19-L, top panel) and L-selectin−, but sLex+ (HL60, bottom panel) cells to TNF-α–stimulated HCMEC as a function of the duration of TNF-α incubation. At 1.07 dyne/cm2, adhesion of 300.19-L increases at 4 to 5 hours of stimulation and remains elevated for at least 24 hours. Adhesion of HL60 increases at 3 to 4 hours of stimulation and returns to the control level within 18 hours. Mean values ±SEM, n = 2.
Rolling adhesion of 300.19-L cells to HCMEC is L-selectin dependent. At 1.07 dyne/cm2, adhesion of 300.19-L cells is significantly enhanced by activation of HCMEC with TNF-α. The enhanced adhesion is completely blocked by anti–L-selectin MoAb LAM1-3 but not by LAM1-12. Mean values ±SEM; *P < .05.
Rolling adhesion of 300.19-L cells to HCMEC is L-selectin dependent. At 1.07 dyne/cm2, adhesion of 300.19-L cells is significantly enhanced by activation of HCMEC with TNF-α. The enhanced adhesion is completely blocked by anti–L-selectin MoAb LAM1-3 but not by LAM1-12. Mean values ±SEM; *P < .05.
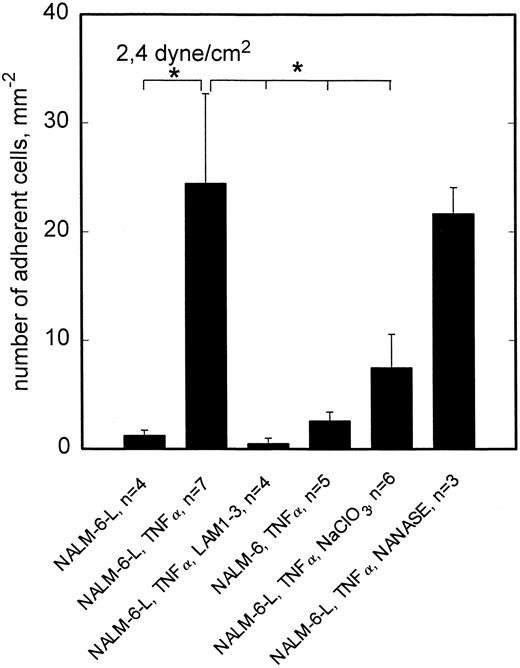
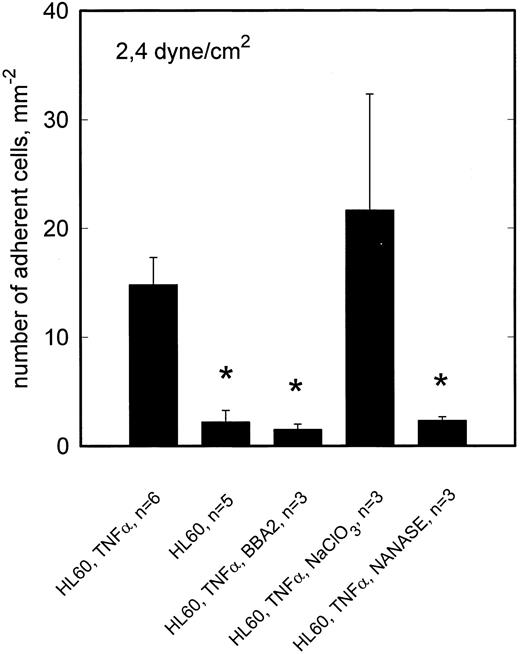
To reveal whether the observed L-selectin mediated adhesion of NALM-6-L cells requires sialylation of the putative ligand(s) involved, activated HCMEC (TNF-α, 5 hours) were incubated with neuraminidase. As shown in Fig 6, neuraminidase digestion had no effect on rolling adhesion of NALM-6-L to activated HCMEC, not even at 2.4 dyne/cm2, indicating that sialylation of the L-selectin ligand is not required for adhesive interaction. On the other hand, neuraminidase treatment of HL60 cells completely abrogated the adhesion of HL60 cells to TNF-α–activated HCMEC at 2.4 dyne/cm2 (Fig 7). HL60 cells express huge amounts of sLex as shown by a FacScan analysis, which showed a fluorescence intensity of 4280 (C.V. 54%) (negative control 133 [C.V. 58%]) when using AM-3 as a primary MoAb. This level of fluorescence is diminished to 117 (C.V. 37%) by incubating the cells with NANASE. These findings are consistent with the observation that E-selectin, which became upregulated on HCMEC after TNF-α activation (Table 1), contributed to adhesion of HL60 cells to activated HCMEC at >1 dyne/cm2 (Fig 3) and show that sialylation of E-selectin ligands is required to provide binding function.
L-selectin–dependent rolling adhesion to TNF-α–stimulated HCMEC is reduced by NaClO3 but not by neuraminidase. At 2.4 dyne/cm2, adhesion of L-selectin+ NALM-6-L cells to HCMEC is significantly enhanced by TNF-α stimulation of endothelial cells. This stimulated adhesion is completely blocked by anti–L-selectin MoAb LAM1-3 and cannot be observed in L-selectin− NALM-6 cells. This L-selectin dependent adhesion can be reduced to one third, if posttranslational sulfation reactions are inhibited by cultivation of HCMEC in the presence of NaClO3 . Neuraminidase treatment of HCMEC shows no effect on the adhesion behavior. Mean values ±SEM; *P < .05 versus positive control (first column).
L-selectin–dependent rolling adhesion to TNF-α–stimulated HCMEC is reduced by NaClO3 but not by neuraminidase. At 2.4 dyne/cm2, adhesion of L-selectin+ NALM-6-L cells to HCMEC is significantly enhanced by TNF-α stimulation of endothelial cells. This stimulated adhesion is completely blocked by anti–L-selectin MoAb LAM1-3 and cannot be observed in L-selectin− NALM-6 cells. This L-selectin dependent adhesion can be reduced to one third, if posttranslational sulfation reactions are inhibited by cultivation of HCMEC in the presence of NaClO3 . Neuraminidase treatment of HCMEC shows no effect on the adhesion behavior. Mean values ±SEM; *P < .05 versus positive control (first column).
TNF-α–stimulated adhesion of HL60 cells to HCMEC is inhibited by neuraminidase but not by NaClO3 . At 2.4 dyne/cm2, adhesion of HL60 cells to activated HCMEC is reduced by anti–E-selectin MoAb BBA2 to the level of adhesion on nonactivated HCMEC. E-selectin dependent adhesion is inhibited by neuraminidase digestion of sLex from the HL60 cell surface. Cultivation of HCMEC in the presence of NaClO3 shows no effect on the adhesion behavior. Mean values ±SEM; *P < .05 versus positive control.
TNF-α–stimulated adhesion of HL60 cells to HCMEC is inhibited by neuraminidase but not by NaClO3 . At 2.4 dyne/cm2, adhesion of HL60 cells to activated HCMEC is reduced by anti–E-selectin MoAb BBA2 to the level of adhesion on nonactivated HCMEC. E-selectin dependent adhesion is inhibited by neuraminidase digestion of sLex from the HL60 cell surface. Cultivation of HCMEC in the presence of NaClO3 shows no effect on the adhesion behavior. Mean values ±SEM; *P < .05 versus positive control.
To elucidate whether sulfation of putative L-selectin ligand(s) on activated HCMEC is required to provide full adhesive activity, HCMEC were cultivated in the presence of 10 mmol/L sodium chlorate for 48 hours for inhibition of posttranslational sulfation reactions. This treatment reduced NALM-6-L rolling adhesion to activated HCMEC at 2.4 dyne/cm2 to one third (Fig 5). Consistent with the absence of L-selectin specific adhesion at 0.5 dyne/cm2, sodium chlorate treatment of HCMEC showed no effect at this lower shear stress (data not shown). By contrast, rolling adhesion of HL60 cells to activated HCMEC was not reduced at 2.4 dyne/cm2 by sodium chlorate treatment of HCMEC (Fig 6).
DISCUSSION
The data presented in this study show clear evidence for TNF-α inducible L-selectin–dependent cell adhesion on monolayers of human cardiac microvascular endothelial cells (HCMEC). L-selectin dependence was shown by the difference in adhesion behavior between L-selectin+ and L-selectin− cells. This model differs from the in vivo situation as leukocytes do not only express L-selectin, but ligands for the vascular selectins as well. Therefore, leukocyte rolling in vivo can be mediated by one, two, or all three selectins in combination. The advantage of the different model cells used in the present study is that the interpretation of adhesive interactions can be restricted to L-selectin. In addition, the adhesion behavior of L-selectin+ cells could be changed to that of L-selectin− cells in the presence of a L-selectin-IgG-chimera, which competes for the same endothelial ligand(s). Furthermore, L-selectin–specific rolling adhesion could be reduced to negative control level by adhesion blocking MoAb LAM1-3 but not by MoAb LAM1-12, which served as an control. Taking the presented evidence together, we conclude that TNF-α–inducible L-selectin ligand(s) are expressed on HCMEC. By contrast, we failed to show any contribution of L-selectin to the adhesion to activated HCEC. Because little is known about molecular differences between large and small vessel endothelium,24 the present observations contribute to a better understanding of functional differences between coronary microvascular and macrovascular endothelium. Although E-selectin can be expressed equally well on both types of endothelium, the different support of L-selectin mediated adhesion could be of functional relevance in vivo either with respect to the different time course or to different signal events to the rolling leukocyte. But until now this remains a matter of speculation, because selectin induced signal transduction just starts to be investigated.
In the present experiments, rolling adhesion of NALM-6-L to HCMEC under flow conditions was observed in a limited range of shear stresses up to 2.6 dyne/cm2. By contrast, leukocyte rolling in postcapillary venules takes place at shear stresses of and exceeding 8 dyne/cm2. This might be explained by additional adhesive interactions of leukocyte and vascular selectins in vivo. The contribution of L-selectin to adhesion under flow conditions in the present experiments was positively correlated with the shear stress applied: although no contribution of L-selectin to adhesion could be elucidated at the lower end of the shear stress range achieved in the flow chamber (approximately 0.5 dyne/cm2 ), adhesion of L-selectin+ cells was approximately 5 times greater than that of L-selectin−cells at >2 dyne/cm2. L-selectin mediated rolling at the higher shear stresses obviously serves as a necessary first step to slow down the freely flowing leukocytes before firm adhesion via β2 -integrins can occur as a second step.
Previously, rolling interactions under reduced flow conditions have also been shown to be mediated by β2 -25 as well as β4 -integrins.26-28 As pre-B cells express some amounts of VLA-4, and VCAM-1 is expressed by endothelial cells, this may contribute to cell adhesion under flow conditions in our model. However, at about 2 dyne/cm2 this contribution seems to be rather low, because 80% of rolling adhesion at this shear stress level was L-selectin mediated. In addition, adhesion of NALM-6 cells to HCEC at 2.4 dyne/cm2 could not be enhanced by TNF-α. Therefore it is unlikely that VLA-4/VCAM-1 interactions significantly contributed to NALM-6 cell rolling adhesion at the higher shear stresses used here. Contribution of VLA-4/VCAM-1 interactions to 300.19-L cell rolling adhesion to TNF-α–activated HCMEC at 1.07 dyne/cm2 can be ruled out, because it was completely inhibited by blocking L-selectin function.
E-selectin mediated adhesion to HCMEC could be shown in the present study using HL60 cells and an anti–E-selectin MoAb. These cells express high amounts of sLex, which is an element of the ligands for both vascular selectins and contributes to their binding function. The shear stress dependence of E-selectin mediated adhesion was very similar to that seen for L-selectin dependent mechanisms supporting the hypothesis that different selectins are able to contribute to leukocyte rolling.
If different selectins are involved in the first step of leukocyte recruitment, the kinetics of their involvement is a matter of relevance. Comparison of the adhesion behavior of L-selectin+ cells (300.19-L) on one hand, and L-selectin−cells, which are strongly positive for sLex expression (HL60) on the other hand provides a suitable approach to address this question. In our model, E-selectin mediated adhesion, which accounted for rolling adhesion of HL60 to activated HCMEC at higher flow peaked at 6 hours and returned to the control level within 18 hours, while L-selectin–mediated rolling adhesion remained elevated for at least 24 hours. These findings show different regulation of vascular and leukocyte selectin mediated adhesion. They may also explain the impaired leukocyte recruitment into sites of inflammation in L-selectin deficient mice in which diminished leukocyte emigration at this later time point has been shown,29 in contrast to P-selectin deficient animals.30
All known L-selectin ligands are sialylated and this contributes to their binding activity.12 These ligands have been exclusively isolated from HEV of murine peripheral lymph nodes. One of these ligands, CD34, is more generally distributed to endothelial cells.31 Therefore, the constitutive expression of CD34 on HCMEC, shown by flow cytometry in the present study is not unexpected. However, we failed to show any surface expression of sLex on these cells, even though we used two different MoAbs, which recognize monomeric (AM-3, CSLEX) as well as dimeric (CSLEX) variants of the sLex epitope.22 To exclude the possibility, that CD34 might be upregulated or glycosyltransferases for sLex expression be induced by TNF-α, surface expression of both epitopes was analysed following TNF-α treatment of HCMEC, but none of them increased. Consistent with the observed pattern of CD34 expression and lack of sialylation on HCMEC, neuraminidase treatment had no effect on L-selectin–dependent rolling adhesion. Although CD34 functions as a L-selectin ligand on HEV, it seems to be unable to play this role on HCMEC, probably because of differences in glycosylation. By contrast, E-selectin–mediated rolling adhesion to activated HCMEC occurs via sialylated ligands on HL60 cells. These ligands have recently been cloned and designated as ESL-1, which exclusively binds to E-selectin, and PSGL-1, which binds to both E- and P-selectin.32-34 In agreement with this, preincubation with neuraminidase reduced rolling adhesion of HL60 cells to activated HCMEC to control levels.
The present data obtained with sodium chlorate incubation reveal sulfation dependent function of L-selectin ligand(s) on HCMEC. Thus, L-selectin ligand interaction on HCMEC depends on a negative surface charge of the ligand(s), which is not linked to sialic acid. Control experiments with HL60 cells showed that none but the L-selectin–dependent adhesion mechanisms were inhibited by sodium chlorate.
L-selectin specific binding had already been shown to HUVEC15 and microvascular glomerular endothelial cells from bovine calf kidney.35 It could clearly be specified using inhibitors of L-selectin dependent adhesion ie, adhesion blocking antibodies and phosphomannan ester15 as well as L-selectin transfected cells,35 though these studies were not done under controlled shear conditions. Therefore, they were performed at 4°C to diminish integrin dependent binding. In agreement with the data presented in this report, both studies showed, that L-selectin dependent adhesion lasts for at least 48 hours after endothelial activation. But in contrast to the present data, TNF-α–induced L-selectin dependent binding to HUVEC and microvascular glomerular endothelial cells from bovine calf kidney, was at least partly inhibited by neuraminidase treatment. Thus, the L-selectin ligand(s) on both types of endothelium are supposed to differ from those on HCMEC. As all three types of endothelial cells investigated so far come from highly specialized vascular beds, further endothelial cells of different origin need to be investigated.
In conclusion, the present study shows that L-selectin dependent adhesion in the presence of shear stress is unique to microvascular and absent in large vessel endothelial cells of the coronary system, even after TNF-α stimulation. The inducible endothelial ligand(s) for L-selectin is (are) resistant to neuraminidase treatment, but require(s) posttranslational sulfation. Therefore, a significant difference appears to exist from the L-selectin ligands found in HEV. Compared to leukocyte adhesion mediated by P- or E-selectin, L-selectin–dependent rolling adhesion shows a prolonged time course and remains high for at least 24 hours. Therefore, the present data elucidate an endothelial adhesion mechanism, which explains the sustained relevance of L-selectin for leukocyte recruitment into inflammatory sites in vivo.
ACKNOWLEDGMENT
The authors thank Dr T. Tedder (Durham, NC) and Dr G. Kansas, (Chicago, IL) who provided the L-selectin transfectants and MoAb LAM1-3 and LAM1-12. The HL60 cells were a kind gift from Dr Stoolman (Ann Arbor, MI). The MoAb AM-3 was provided by Dr Hanski (Berlin, Germany). G. Beyer and M. Ehrlich are acknowledged for excellent technical assistance.
Supported by Deutsche Forschungsgemeinschaft, Ga 225/15-3 (Bonn, Germany).
Address reprint requests to A. Zakrzewicz, MD, Freie Universität Berlin, Department of Physiology, Arnimallee 22, D-14195 Berlin, Germany.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal