Abstract
Long-term heparin treatment causes osteoporosis through, an as yet, undefined mechanism. To investigate this phenomenon and to determine the relative benefits of low-molecular-weight heparin (LMWH) use, we treated rats with once daily subcutaneous injections of either unfractionated heparin (1.0 U/g or 0.5 U/g), the LMWH, Tinzaparin (1.0 U/g or 0.5 U/g), or placebo (saline) for a period of 32 days. The effects on bone were then compared both histomorphometrically and biochemically by measuring urinary type I collagen cross-linked pyridinoline (PYD) and serum alkaline phosphatase, markers of bone resorption and formation, respectively. Histomorphometric analysis of the distal third of the right femur, in the region proximal to the epiphyseal growth plate, demonstrated that both heparin and LMWH decrease cancellous bone volume in a dose-dependent fashion, but that heparin causes significantly more cancellous bone loss than does LMWH. Although both heparin and LMWH decrease osteoblast and osteoid surface to a similar extent, only heparin increases osteoclast surface. In support of these histomorphometric findings, biochemical markers of bone turnover demonstrated that both heparin and LMWH treatment produce a dose-dependent decrease in serum alkaline phosphatase, consistent with reduced bone formation, whereas only heparin causes a transient increase in urinary PYD, consistent with an increase in bone resorption. Based on these observations, we conclude that heparin decreases cancellous bone volume both by decreasing the rate of bone formation and increasing the rate of bone resorption. In contrast, LMWH, causes less osteopenia than heparin because it only decreases the rate of bone formation.
ALTHOUGH HEPARIN is an effective antithrombotic agent, it has limitations due to its pharmacokinetic properties and its side effects. While the major side effect of heparin is bleeding,1-3 other less common side effects include heparin-induced thrombocytopenia4 and osteoporosis.5-14 Some of the limitations of heparin are overcome by use of low molecular weight preparations. Thus, low-molecular-weight heparins (LMWHs) have a much more predictable dose response relationship than unfractionated heparin,15,16 a property that is thought to be related to their reduced binding to plasma proteins and endothelium.17-19 LMWHs are also associated with a lower risk of heparin-induced thrombocytopenia,20,21 but information on the relative effects of heparin and LMWHs on osteoporosis is very limited.13 Therefore, more definitive data comparing the effects of heparin and LMWH on bone are required.
In a previous study, we used cultured fetal rat calvariae to demonstrate that heparin increases bone resorption in a concentration-dependent fashion, whereas low molecular weight heparin does not.22 In contrast, using cultured mouse calvariae other investigators failed to demonstrate a heparin effect.23,24 Recently, however, we confirmed our initial findings by demonstrating that heparin administration to rats increases bone resorption and decreases bone formation, thereby causing a significant loss of cancellous bone.25
In the present study, we extend these findings by using our rat model of heparin-induced osteopenia to compare the effects of heparin and the LMWH, Tinzaparin, on bone. Accordingly, rats were given pharmacologically relevant doses of heparin or LMWH for 32 days and their effect on bone evaluated histomorphometrically and by obtaining serial measurements of urinary cross-linked pyridinoline (PYD) and serum alkaline phosphatase as biochemical markers of bone resorption and bone formation, respectively.
MATERIALS AND METHODS
Materials.Specific pathogen-free female Sprague-Dawley rats approximately 2 months old (180 to 185 g) were purchased from Charles River Laboratories (St Constant, Quebec, Canada). Unfractionated heparin was provided by Rhone-Poulenc Rorer (Montreal, Quebec, Canada), while Tinzaparin was a generous gift from Novo Nordisk (Gentofte, Denmark). Urinary type I collagen-derived cross-linked pyridinoline (PYD) and creatinine were assayed using commercially available enzyme immunoassays (Metra Biosystems, Palo Alto, CA), whereas serum alkaline phosphatase was measured using an assay from Sigma Chemical Co (St Louis, MO).
Experimental design.To examine the effect of heparin on bone morphometry, a total of 40 rats were studied. The animals were randomized into five treatment groups each consisting of eight rats. Four groups were given daily subcutaneous injections of either heparin or the LMWH, Tinzaparin, at doses of either 0.5 U/g or 1.0 U/g for a total of 32 days. The fifth group served as age-matched controls and were given an equivalent volume of saline instead of heparin. Eight rats were also killed at a body weight of 185 g to serve as baseline controls. To permit measurements of bone apposition rates and the calculation of dynamic parameters, all animals received two intraperitoneal injections of tetracycline, at 10 and 3 days before being killed (20 mg/kg). On day 32, all rats were killed with 5% isofluorane, and after exsanguination, the right femur was removed for histologic evaluation.
Biochemical markers of bone turnover.Tail vein blood samples were collected from each rat before the start of treatment and on day 32. After sedimentation of the red blood cells at 2,000g, the serum was removed and assayed for alkaline phosphatase (ALP) activity as an index of bone formation. All animals were housed in metabolic cages. Urine was collected daily between 1400 hours and 1600 hours and was assayed for PYD as an index of bone resorption. Creatinine concentrations also were measured in the same urine samples and the levels of PYD were then expressed as nmol/L PYD per mmol/L of urinary creatinine.
Bone histomorphometry.Bone histomorphometry was performed as described previously.25 Briefly, the undecalcified distal third of the right femur of each rat was embedded in glycolmethacrylate (JB-4 embedding medium; Analychem, Toronto, Ontario, Canada). Histologic sections (6 to 8 μm) were obtained using a Riechert Jung microtome (model K4; Riechert Jung Canada, Toronto, Ontario), mounted, and then stained with either 1% toluidine blue or hematoxylin and eosin (H & E) before being subjected to morphometric analysis. In each case, a region 800 μm below the epiphyseal growth plate that included the entire metaphysis was subjected to light microscopy using a Merz grid (Carl Zeiss Canada, Don Mills, Ontario).25 Sections examined in this fashion encompassed a total tissue area of approximately 10 to 15 mm2. The following parameters were determined: (1) cancellous bone volume, (2) osteoblast surface, (3) osteoid surface, and (4) osteoclast surface. For each section, cancellous bone volume was calculated from a total of >1,600 point measurements (45 fields; 400× magnification), which were selected at random using the Merz grid. The percent osteoblast, osteoid, or osteoclast surface was calculated under oil immersion (1,000×) by recording the presence or absence of each where the hemispherical grid of the Merz radicule crossed cancellous bone. Osteoblasts were identified morphologically as distinct cuboidal-shaped cells lining the cancellous bone surface, whereas osteoclasts were identified morphologically as large multinucleated cells in close proximity to the cancellous bone surface, which stained for tartrate-resistant acid phosphatase (Sigma Chemical Co, St Louis, MO; Procedure No.386).26 The histomorphometric parameters of trabecular width, number, and separation were measured directly with an epifluorescent microscope (Leica Laborlux; Willowdale, Ontario, Canada) coupled to an IBM computer (Empix Inc, Mississauga, Ontario, Canada). Images were captured using a CCD video camera module electronically linked to a computer imaging software system (Northern Exposure; Empix Inc, Mississauga, Ontario, Canada). Measurements of erosion depth were also determined from captured images by measuring at random the depth of resorption lacunae that were associated with tartrate-resistant acid phosphate (TRAP)-positive cells. All histological analyses were done by a single investigator who was blinded to treatment allocation.
Bone mineralization was quantitated as follows. Using the Merz radicule, cancellous bone surface was scored as either labeled or unlabeled, depending on the presence or absence of fluorescence at the cancellous bone surface. Fluorescent bone surface was further characterized as having either single or double label, according to the number of distinct lines that were observed on the labeled surface. Double-labeled perimeter was then used to calculate the dynamic variables of mineral apposition rate and bone formation rate (surface based) according to the standard nomenclature described by Jee et al27 and Parfitt et al.28
Statistical analysis.Analysis of variance was used to compare the results between the experimental and control groups. If a significant difference between experimental and control groups was detected, an unpaired Student's t-test was performed at each point. Significance levels were adjusted using a Bonferroni correction factor for multiple comparisons.
RESULTS
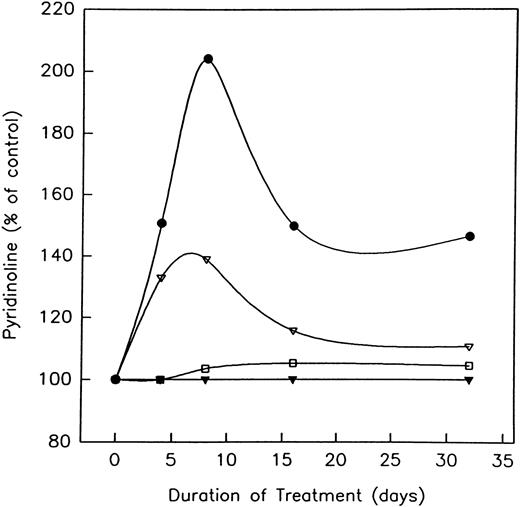
Effect of heparin on body weight, alkaline phosphatase, and pyridinoline levels.During the 32 days of this study, both LMWH and heparin-treated rats gained weight. No significant differences with respect to weight gain were found between those rats treated with either LMWH or heparin (1.0 U/g) and age-matched controls (73.2 ± 6.8 g and 66.9 ± 8.5 g v 71.7 ± 5.9 g, respectively). However, treatment with either unfractionated heparin or the LMWH, Tinzaparin, did result in a time-dependent decrease in serum ALP activity (Table 1). Maximum reduction occurred at day 32 in rats treated with high doses (1.0 U/g) of either agent. When compared with baseline values at day 0, both heparin and LMWH produced a significant (P < .005) decrease in serum ALP (30.3% ± 6.3% and 39.9% ± 5.4%, respectively). In contrast to ALP, heparin transiently increased the urinary excretion of PYD in a time- and dose-dependent fashion, whereas LMWH had no effect (Fig 1). Thus, by day 8, the excretion of urinary PYD in rats treated with 1.0 and 0.5 U/g of heparin had increased by 204% ± 10% and 139% ± 6.0%, respectively (P < .01). By day 32, PYD levels had returned to baseline values in the animals given 0.5 U/g heparin, but were still elevated in those given the higher heparin dose (Fig 1).
Effect of Unfractionated Heparin and the LMWH, Tinzaparin, on Serum Alkaline Phosphatase Levels
| . | Serum Alkaline Phosphatase . | ||
|---|---|---|---|
| . | (U/L) . | . | |
| . | Day 0 . | Day 32 . | . |
| Control | 79.5 ± 7.1 | 75.0 ± 5.5 | |
| LMWH (0.5 U/g) | 81.4 ± 6.8 | 61.3 ± 3.4*† | |
| LMWH (1.0 U/g) | 86.0 ± 3.0 | 51.7 ± 4.5*† | |
| Heparin (0.5 U/g) | 87.5 ± 5.2 | 63.3 ± 4.8*† | |
| Heparin (1.0 U/g) | 84.8 ± 6.5 | 59.1 ± 6.2*† | |
| . | Serum Alkaline Phosphatase . | ||
|---|---|---|---|
| . | (U/L) . | . | |
| . | Day 0 . | Day 32 . | . |
| Control | 79.5 ± 7.1 | 75.0 ± 5.5 | |
| LMWH (0.5 U/g) | 81.4 ± 6.8 | 61.3 ± 3.4*† | |
| LMWH (1.0 U/g) | 86.0 ± 3.0 | 51.7 ± 4.5*† | |
| Heparin (0.5 U/g) | 87.5 ± 5.2 | 63.3 ± 4.8*† | |
| Heparin (1.0 U/g) | 84.8 ± 6.5 | 59.1 ± 6.2*† | |
Rats were injected with either unfractionated heparin or LMWH at a concentration of either 0.5 anti-Xa U/g or 1.0 anti-Xa U/g. One group of rats also served as a control and were given equivalent volumes of saline instead of heparin. Serum samples were taken at days 0 and 32 from both treated and nontreated rats and assayed for alkaline phosphatase activity. Data are expressed as mean ± SEM.
P < .001 when compared with control values at day 32.
P < .005 when compared with baseline values at day 0.
The effect of unfractionated heparin and the LMWH, Tinzaparin, on the excretion of urinary pyridinoline. Rats were injected with vehicle alone; unfractionated heparin at a concentration of 0.5 U/g (▵) or 1.0 U/g (•), or the LMWH, Tinzaparin, at a concentration of 0.5 U/g (▴) or 1.0 U/g (□), and the excretion of pyridinoline determined. To account for variations in urine concentration, results are calculated in terms of nmol/mL of urinary creatinine. Values are expressed as a percentage of the controls at either days 0, 4, 8, 16, or 32. Control values routinely varied between 400 and 200 nmol/L PYD/mmol/L creatinine. P < .01 when compared with control values.
The effect of unfractionated heparin and the LMWH, Tinzaparin, on the excretion of urinary pyridinoline. Rats were injected with vehicle alone; unfractionated heparin at a concentration of 0.5 U/g (▵) or 1.0 U/g (•), or the LMWH, Tinzaparin, at a concentration of 0.5 U/g (▴) or 1.0 U/g (□), and the excretion of pyridinoline determined. To account for variations in urine concentration, results are calculated in terms of nmol/mL of urinary creatinine. Values are expressed as a percentage of the controls at either days 0, 4, 8, 16, or 32. Control values routinely varied between 400 and 200 nmol/L PYD/mmol/L creatinine. P < .01 when compared with control values.
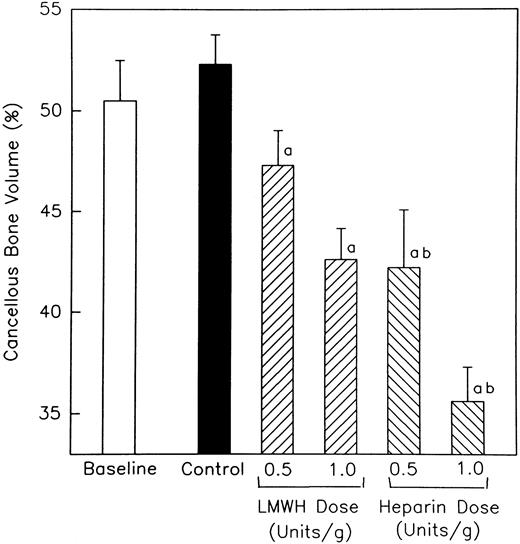
Effect of heparin on cancellous bone.Sections obtained from the undecalcified right femur of heparin- and LMWH-treated rats, as well as nontreated rats, were subjected to morphometric analysis. A region 800 μm below the epiphyseal growth plate that included the entire metaphysis was analyzed for cancellous bone. As shown in Fig 2, no significant difference in cancellous bone volume (BV/TV) was found between baseline and age-matched control rats. However, both heparin and LMWH produced a concentration-dependent reduction in cancellous bone volume when compared with either control. The effect of heparin on cancellous bone was significantly greater than that of LMWH (P < .001). Thus, at a dose of 1.0 U/g, heparin produced a 31.9% ± 3.2% reduction in cancellous bone volume, whereas an equivalent dose of LMWH produced only a 18.5% ± 3.0% decrease in cancellous bone volume, when compared with age-matched controls (P < .001).
The percentage of the total epiphyseal area occupied by cancellous bone in both heparin and LMWH-treated rats. Rats were injected daily with either unfractionated heparin or the LMWH, Tinzaparin, and the area occupied by cancellous bone determined at day 32. Data are expressed as mean ± standard error of mean (SEM). a P < .005 when compared with either baseline or control values. b P < .01 when compared with values obtained from LMWH-treated animals.
The percentage of the total epiphyseal area occupied by cancellous bone in both heparin and LMWH-treated rats. Rats were injected daily with either unfractionated heparin or the LMWH, Tinzaparin, and the area occupied by cancellous bone determined at day 32. Data are expressed as mean ± standard error of mean (SEM). a P < .005 when compared with either baseline or control values. b P < .01 when compared with values obtained from LMWH-treated animals.
Because a reduction in cancellous bone volume can result from a decrease in either trabecular width and/or trabecular number, we examined the effect of heparin and LMWH on both of these parameters (Table 2). Neither baseline nor age-matched control rats were found to be significantly different with respect to trabecular width or number. However, heparin was found to significantly decrease both trabecular width and number, when compared with either control. Age-matched control rats had a mean of 10.2 ± 0.5 trabeculae/mm2 with a mean width of 60.5 ± 3.7 μm. Over the course of 32 days, heparin treatment (1.0 U/g) reduced the number of trabeculae to a mean of 7.1 ± 0.3 trabeculae/mm2, with the remaining trabeculae having a mean width of 49.1 ± 1.6 μm. This resulted in a 55.8% ± 5.4% increase in the distance between adjacent trabeculae (Table 2). In rats treated with LMWH, the mean number of trabeculae was reduced to 8.0 ± 0.2 trabeculae/mm2 with the remaining trabeculae having a mean width of 54.4 ± 0.5 μm. The decrease in both trabecular bone width and trabecular number, produced by LMWH, was significantly less than that caused by heparin (P < .01).
Effect of Unfractionated Heparin and the LMWH, Tinzaparin, on Trabecular Thickness, Number, and Separation
| . | Trabecular . | Trabecular . | Trabecular . |
|---|---|---|---|
| . | Width (μm) . | No. (no./mm2 ) . | Separation (μm) . |
| Baseline | 63.2 ± 1.7 | 11.0 ± 0.6 | 101.2 ± 8.2 |
| Control | 60.5 ± 3.7 | 10.2 ± 0.5 | 110.9 ± 7.6 |
| LMWH (0.5 U/g) | 58.2 ± 2.9 | 7.9 ± 0.3* | 147.8 ± 10.7* |
| LMWH (1.0 U/g) | 54.4 ± 0.5* | 8.0 ± 0.2* | 157.7 ± 4.4* |
| Heparin (0.5 U/g) | 57.5 ± 1.0 | 7.2 ± 0.2*† | 164.8 ± 12.4* |
| Heparin (1.0 U/g) | 49.1 ± 1.6*† | 7.1 ± 0.3*† | 172.8 ± 6.0*† |
| . | Trabecular . | Trabecular . | Trabecular . |
|---|---|---|---|
| . | Width (μm) . | No. (no./mm2 ) . | Separation (μm) . |
| Baseline | 63.2 ± 1.7 | 11.0 ± 0.6 | 101.2 ± 8.2 |
| Control | 60.5 ± 3.7 | 10.2 ± 0.5 | 110.9 ± 7.6 |
| LMWH (0.5 U/g) | 58.2 ± 2.9 | 7.9 ± 0.3* | 147.8 ± 10.7* |
| LMWH (1.0 U/g) | 54.4 ± 0.5* | 8.0 ± 0.2* | 157.7 ± 4.4* |
| Heparin (0.5 U/g) | 57.5 ± 1.0 | 7.2 ± 0.2*† | 164.8 ± 12.4* |
| Heparin (1.0 U/g) | 49.1 ± 1.6*† | 7.1 ± 0.3*† | 172.8 ± 6.0*† |
Rats were injected daily with vehicle alone or either LMWH and/or unfractionated heparin, at doses of 0.5 and 1.0 U/g, and the region extending from the epiphyseal growth plate and including the entire metaphysis was analyzed at day 32 for: (1) trabecular width; (2) trabecular number and; (3) trabecular separation. Data are expressed as mean ± SEM.
P < .005 when compared with either baseline or control values.
P < .01 when compared with values obtained from LMWH-treated animals.
Effect of heparin on surface-based data.Sections were stained with toluidine blue to quantify surface-based data. The parameters measured were: (1) percent osteoblast surface (Ob.S/BS), (2) percent osteoid surface (OS/BS), and (3) percent osteoclast surface (Oc.S/BS). Figure 3A shows that the parameters of osteoblast and osteoid surface were not significantly different when comparing baseline and age-matched controls. However, both heparin and LMWH decreased the percentage of cancellous bone covered by osteoblasts as compared with either control. A dose-dependent decrease in the percentage of cancellous bone covered by osteoid also was observed (Fig 3B). Compared with age-matched controls, heparin (1.0 U/g) caused a significant (P < .001) decrease in osteoblast surface and the amount of osteoid (54.9% ± 11.0% and 78.7% ± 10.6%, respectively). LMWH (1.0 U/g) also significantly (P < .001) reduced osteoblast surface and osteoid surface (48.0% ± 9.2% and 64.7% ± 10.5%, respectively). The effects of LMWH and heparin on osteoblast and osteoid surface were not significantly different (P > .05). Osteoid thickness was also significantly decreased by both heparin and LMWH treatment from 4.63 ± 0.17 μm to 2.65 ± 0.14 μm, and 2.32 ± 0.13 μm, respectively (P < .005).
The effect of heparin and the LMWH, Tinzaparin, on the percentage of cancellous bone surface length occupied by either osteoblasts or osteoid. Rats were injected with either unfractionated heparin or LMWH, and the cancellous bone surface, proximal to the epiphyseal growth plate, characterized as being lined with either osteoblasts (A) or osteoid (B). Data are expressed as mean ± SEM. a P < .005 when compared with either baseline or control values.
The effect of heparin and the LMWH, Tinzaparin, on the percentage of cancellous bone surface length occupied by either osteoblasts or osteoid. Rats were injected with either unfractionated heparin or LMWH, and the cancellous bone surface, proximal to the epiphyseal growth plate, characterized as being lined with either osteoblasts (A) or osteoid (B). Data are expressed as mean ± SEM. a P < .005 when compared with either baseline or control values.
As illustrated in Fig 4A, only heparin produced a dose-dependent increase in the percentage of cancellous bone covered by osteoclasts. Whereas, rats given 1.0 U/g heparin demonstrated a 59.0% ± 9.8% (P < .001) increase in osteoclast surface, osteoclast surface was unaffected by treatment with 1.0 U/g LMWH (Fig 4). Similar results were obtained when erosion depth was measured (Fig 4B). Thus, erosion depth was increased by 55.4% ± 13.2% (P < .001) in rats given 1.0 U/g heparin whereas, treatment with 1.0 U/g LMWH had no effect.
The effect of heparin and the LMWH, Tinzaparin, on osteoclast surface and erosion depth. Rats were injected with increasing concentrations of either unfractionated heparin or LMWH and the percentage of cancellous bone surface covered with osteoclasts (A) and/or erosion depth (B) determined. Data are expressed as mean ± SEM. aP < .005 when compared with either baseline or control values. bP < .01 when compared with values obtained from LMWH-treated animals.
The effect of heparin and the LMWH, Tinzaparin, on osteoclast surface and erosion depth. Rats were injected with increasing concentrations of either unfractionated heparin or LMWH and the percentage of cancellous bone surface covered with osteoclasts (A) and/or erosion depth (B) determined. Data are expressed as mean ± SEM. aP < .005 when compared with either baseline or control values. bP < .01 when compared with values obtained from LMWH-treated animals.
Effects of heparin on dynamic tetracycline-based measurements.Bone mineralization was measured by dual tetracycline labeling over a 7-day period. Table 3 demonstrates that, both heparin and LMWH cause a decrease in the percentage of cancellous bone covered by double-labeled surface. Double-labeled surface (MS/BS) was significantly (P < .001) decreased by a mean of 48.9% ± 7.4% in rats administered 1.0 U/g heparin. LMWH (1.0 U/g) reduced the percentage of cancellous bone covered with double labels by 36.2% ± 9.6% (P < .001). This reduction in double-labeled surface was accompanied by a reduction in bone formation rate (BFR/BS). Thus, both heparin and LMWH were found to significantly (P < .001) reduce bone formation rates when compared with age-matched controls (54.1% ± 7.5% and 43.5% ± 9.1%, respectively). In contrast, the mineral apposition rate (MAR) was unaffected by either treatment (Table 3).
Effect of Unfractionated Heparin and the LMWH, Tinzaparin, on Dynamic Tetracycline-Based Measurements
| . | Labeled . | Mineral . | Bone Formation . |
|---|---|---|---|
| . | Surface (%) . | Apposition . | Rate Bone-Surface- . |
| . | . | Rate (μm/d) . | Based (μm3/μm2/d) . |
| Baseline | — | — | — |
| Control | 9.4 ± 2.2 | 1.10 ± 0.14 | 10.38 ± 2.61 |
| LMWH (0.5 U/g) | 6.3 ± 1.1 | 1.00 ± 0.09 | 6.30 ± 1.183-150 |
| LMWH (1.0 U/g) | 6.0 ± 0.93-150 | 0.98 ± 0.19 | 5.86 ± 0.943-150 |
| Heparin (0.5 U/g) | 5.5 ± 0.93-150 | 1.03 ± 0.18 | 5.70 ± 1.033-150 |
| Heparin (1.0 U/g) | 4.8 ± 0.73-150 | 0.99 ± 0.14 | 4.76 ± 0.783-150 |
| . | Labeled . | Mineral . | Bone Formation . |
|---|---|---|---|
| . | Surface (%) . | Apposition . | Rate Bone-Surface- . |
| . | . | Rate (μm/d) . | Based (μm3/μm2/d) . |
| Baseline | — | — | — |
| Control | 9.4 ± 2.2 | 1.10 ± 0.14 | 10.38 ± 2.61 |
| LMWH (0.5 U/g) | 6.3 ± 1.1 | 1.00 ± 0.09 | 6.30 ± 1.183-150 |
| LMWH (1.0 U/g) | 6.0 ± 0.93-150 | 0.98 ± 0.19 | 5.86 ± 0.943-150 |
| Heparin (0.5 U/g) | 5.5 ± 0.93-150 | 1.03 ± 0.18 | 5.70 ± 1.033-150 |
| Heparin (1.0 U/g) | 4.8 ± 0.73-150 | 0.99 ± 0.14 | 4.76 ± 0.783-150 |
Rats were injected with either unfractionated heparin or LMWH, at a concentration of either 0.5 or 1.0 U/g for 32 days. One group of rats also served as a control and were given equivalent volumes of saline in place of heparin. All animals received intraperitoneal injections of tetracycline at 10 and 3 days before sacrifice. The percentage of labeled surface covering the cancellous bone surface was then determined as described in Materials and Methods.
P < .005 when compared with control values.
DISCUSSION
This study shows that although both heparin and LMWH decrease the process of bone formation, only heparin increases bone resorption by increasing both osteoclast number and activity. Because heparin increases bone resorption, while reducing bone formation, heparin produces more cancellous bone loss than does LMWH. These findings are likely to be valid because: (1) the differences in bone loss produced by the two agents are statistically significant and substantial; (2) the histomorphologic assessments were made by a single investigator who was blinded to treatment allocation, thereby preventing bias; and (3) the histomorphometric findings are supported by the results of biochemical markers of bone turnover, which also indicate that heparin increases bone resorption, while LMWH does not.
Both heparin and LMWH decreased the process of bone formation, as evidenced by a significant reduction in both the levels of serum ALP (Table 1), as well as the percentage of cancellous bone surface covered by osteoid (Fig 3B). Both agents also caused a significant decrease in the number of osteoblasts lining the cancellous bone surface. Although heparin and LMWH had similar effects on osteoblast number, heparin was found to increase both osteoclast number and activity, whereas LMWH had no effect (Fig 4A and B). Supporting this morphological distinction between the two agents, heparin also caused a transient increase in osteoclastic activity, as evidenced by an elevation in the urinary levels of PYD, whereas LMWH had no effect on PYD levels (Fig 1).
Our findings are consistent with some of the previous reports on the effects of heparin and LMWH on bone metabolism, but not with others.22-25,29-31 Thus, while heparin has been reported to stimulate collagen synthesis by cultured osteoblasts,30 both heparin and LMWH have been reported to reduce collagen synthesis in cultured fetal rat calvariae.29 Other investigators have reported that heparin has either no effect on bone resorption23,24 or that it inhibits bone resorption at high concentrations.31 In contrast, using fetal rat calvariae, we found that heparin increases bone resorption in a concentration-dependent fashion, and that LMWHs produce significantly less calcium loss than unfractionated heparin.22
Animal models comparing the effects of LMWH and heparin on bone have also produced conflicting results. Thus, when Monreal et al32 compared the effect of heparin with Fragmin, they found that although both agents decreased bone mineral density, Fragmin produced less of a decrease than heparin. In contrast, when Logiparin and heparin were compared in another study, both agents were reported to decrease bone density to a similar extent.33 The reasons for these discrepant findings are unclear. They are unlikely to reflect differences in molecular weight distribution of the two LMWH preparations, because in our studies, Fragmin and Logiparin had similar effects on 45Ca release from prelabeled fetal rat calvariae.22
The mechanisms responsible for our observation that only heparin increases osteoclastic activity and number, although both heparin and LMWH decrease osteoblast number and activity, are unclear. There are two possible explanations. The first is that both heparin and LMWH are able to bind to osteoblasts and to suppress osteoblastic proliferation and activity, whereas only heparin can bind to osteoclasts (or their precursors), thereby increasing osteoclast formation and/or activity. The second possibility is that the osteoblast controls both the formation and the activity of the osteoclast through the release of unknown stimulatory factors,34-36 and that the interaction of heparin and LMWH with osteoblasts is either quantitatively or qualitatively different. As a result, heparin, but not LMWH, causes an increase in the production and release of these stimulatory factors. Support for this latter possibility is provided by the transient nature by which heparin elevates urinary PYD levels. Thus, while heparin, but not LMWH, caused an initial increase in the urinary levels of PYD, this effect was transient, possibly because heparin causes a simultaneous decrease in the number of osteoblasts. As osteoblast numbers decrease, the levels of stimulatory factors released from the osteoblast in response to heparin would no longer be sufficient to sustain the increased osteoclast activity, resulting in a subsequent decrease in urinary PYD levels.
Given the young age of the rats used in this study, it is difficult to distinguish changes in bone volume caused by altered growth from changes due to bone remodeling. In the young and growing rat, much of the cancellous bone being sampled originates as a result of growth processes in which bone formation is favored over bone resorption.37 38 In contrast, in older rats, cancellous bone volume is maintained after linear bone growth has ceased by balancing bone formation with bone resorption. Because bone turnover is fundamentally different in growing animals compared with adults, the effects of heparin and LMWH on cancellous bone volume might be quantitatively, but not necessarily qualitatively, different in the older animal. Indeed, one might expect that the difference between heparin and LMWH-induced cancellous bone loss would be more pronounced in older animals, as the increase in bone resorption observed only in heparin-treated animals, would be partially offset by the higher levels of bone formation that are found in the young and/or growing animal. We are currently exploring this possibility.
It is well known that unfractionated heparin can cause spontaneous fractures of the rib or vertebrae.7,9,14 Our findings suggest that the risk of heparin-induced osteoporosis may be reduced with the use of LMWH. This concept is supported by a recent clinical report. Thus, in the only randomized trial addressing the relative effects of heparin and a LMWH on clinically-detected osteoporosis, the incidence of fracture was significantly higher in patients given heparin than in those treated with LMWH.13
In summary, we used biochemical markers of bone turnover and histomorphometric analysis to compare the effects of heparin and LMWH on bone. While both heparin and LMWH decrease bone formation, only heparin increased bone resorption. As a result, heparin produced significantly more cancellous bone loss than LMWH. These findings are consistent with the limited clinical studies, which also suggest that the risk of heparin-induced osteoporosis is lower with LMWH than with heparin.
Supported by the Heart and Stroke Foundation of Ontario, Canada. M.A. and J.I.W. are Career Investigators of the Heart and Stroke Foundation of Ontario.
Address reprint requests to Stephen G. Shaughnessy, PhD, Hamilton Civic Hospitals Research Centre, 711 Concession St, Hamilton, Ontario, Canada, L8V 1C3.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal