Abstract
It is vital to develop effective therapy for children with acute lymphoblastic leukemia (ALL), in whom no remission occurs or who suffer relapse with current protocols. Cellular drug resistance is thought to be an important cause of induction failure and relapse. We performed in vitro tests of bone marrow samples in 196 children with newly diagnosed ALL with a 4-day culture and a methyl-thiazol-tetrazolium assay. We tested 16 drugs and calculated the 70% lethal dose (LD70) for 14 drugs and the leukemic cell survival (LCS) rate for dexamethasone and prednisolone. For each single drug, patients were classified into two groups, sensitive or resistant, by median concentration of LD70 or LCS. When patients were classified into three groups by sensitivity to four drugs of DPAV (dexamethasone, prednisolone, L-asparaginase, and vincristine), 3-year event-free survival (EFS; 95% confidence intervals) of the super sensitive group (SS; sensitive to all 4 drugs) was 0.833 (0.690 to 0.976), that of the intermediate sensitive group (IS; sensitive to 2 or 3 drugs) was 0.735 (0.609 to 0.863), and that of the relatively resistant group (RR; sensitive to no drugs or to 1 drug) was 0.541 (0.411 to 0.670; P = .0008). We then investigated the relationship between the above four-drug sensitivity and the time of relapse. The SS and IS patients tended to maintain continuous complete remission, and RR patients tended to undergo induction failure and early and late relapse (P = .004). Initial white blood cell count, immunologic classification, and age were also predictive factors, but the patient numbers showed no statistical correlation between these factors and the four-drug sensitivity groups (SS, IS, and RR). When we took three groups SS/IS/RR and investigated the EFS for various clinical groups, DPAV sensitivity strongly influenced EFS in the standard-risk ALL (P = .016). In vitro drug sensitivity testing provides additional prognostic information about childhood ALL, and early detection of drug resistance at the time chemotherapy commences may provide a successful strategy for individualizing treatment, as the results indicate de novo resistance to front-line drugs and suggest alternative, second-line drugs.
THE PROGNOSIS FOR children with acute lymphoblastic leukemia (ALL) has improved in the past few decades with the use of combination chemotherapy. However, even with a modern treatment protocol, about one third of patients still suffer relapse. Pui and Crist1 suggested that the identification of features that accurately predict a patient's response to therapy has been a continuing goal of leukemia specialists. Advance prognostic information can stimulate clinical trials to reduce toxic effects or can indicate the need for more intensive treatment. Age (<1 year or >9 years) and hyperleukocytosis (>50,000/μL) have been identified as poor prognostic factors.1,2 Some studies have indicated that expression of myeloid-associated antigens predicts a poor outcome.3 It is vital to investigate the mechanism of induction failure and relapse in ALL patients. Although it is assumed that cellular drug-resistance plays an important role in induction failure and early relapse, less is known about the relationship between drug resistance and clinical outcome in childhood ALL.4-6
There has been an increase in reports of a chemosensitivity assay that uses methyl-thiazol-tetrazolium (MTT) dyes.6-11 With some modification, this assay can be applied clinically to select effective drugs, as it can be performed in a 96-well plate, and the results can be analyzed using a scanning multiwell spectrophotometer. Thus, many samples and many drugs can be analyzed simply and rapidly. We previously reported that the MTT assay serves as a reliable tool for the selection of effective chemotherapy in patients with acute leukemia.12
In this report, we studied the relationship between in vitro sensitivity to 16 drugs, measured by MTT assay, and clinical outcomes in 196 children with newly diagnosed ALL. We explored how sensitivity to the four drug combination (dexamethasone, prednisolone, L-asparaginase, and vincristine [DPAV]) predicted prognostic information, especially induction failure and early relapse (IF/e.rel). We also explored whether the sensitivity of leukemic cells in IF/e.rel patients was lower than the sensitivity of leukemic cells in continuous complete remission (CCR) patients.
PATIENTS AND METHODS
Patients and samples.Children (0 to 16 years of age), newly diagnosed between 1989 and 1995, were eligible. Bone marrow samples were sent for in vitro drug testing within 24 hours of sampling. We tested only when samples contained more than 70% leukemic cells that were isolated by density gradient centrifugation with Ficoll-Paque (Pharmacia, Uppsala, Sweden). After isolation, the cells were washed twice in medium RPMI-1640. Informed consent was obtained from all patients or their parents before sampling.
There were 143 samples of common ALL (cALL), 21 of T-cell type ALL (T-ALL), 27 of mixed lineage ALL (mix ALL; defined as precursor-B antigen positive or T-lineage antigen positive, and two or more positive of following myeloid antigens: CD13, 14, 15, 33), and 5 of undifferentiated ALL (uALL; defined as HLA-DR antigen positive and/or CD7+ or CD19+). The leukemic cells of these 196 samples were negative to peroxidase staining.
All patients were treated with the standard-risk or high-risk ALL protocol, consisting of prednisolone (Pred) or dexamethasone (Dex), L-asparaginase (L-ASP), vincristine (VCR), and/or cyclophosphamide (CPA) and daunorubicin (DNR) according to the initial white blood cell count (WBC; 10,000 to 50,000/μL) for standard-risk ALL (1 to 9 years old and <50,000/μL initial WBC without central nervous system involvement), and Pred, L-ASP, VCR, CPA, and DNR or pirarubicin (THP-ADR) for high-risk ALL and T-ALL as induction therapy. We had no special protocol for the patients with mix ALL and uALL. Standard-risk and high-risk patients were treated according to three protocols: Tokai Pediatric Oncology Study Group (Tokai-POSG; n = 140), Tokyo Children's Cancer Study Group (TCCSG; n = 27), and Children's Cancer and Leukemia Study Group (CCLSG; n = 29).
From prognostic outcomes on December 20, 1995, patients were divided into three categories: CCR, IF/e.rel, and late relapse (late rel). Two patients were excluded from prognostic analysis because they died of infection within 4 weeks of diagnosis (at 23 days and 25 days). The CCR category included the patients who were in complete remission until observation time (n = 148; median follow-up time, 24.1 months). IF/e.rel included patients who did not reach complete remission within 6 weeks or who relapsed within 180 days of diagnosis (n = 17; median remission duration, 3.1 months; median survival time, 13.2 months). Late relapse included patients who relapsed 181 or more days after diagnosis (n = 29; median remission duration, 18.8 months; median survival time, 32.1 months). All other data of sex, age at onset, initial WBC count, immunologic markers, treatment protocol, clinical response, and clinical events were obtained from participating hospitals within 2 months of diagnosis and then again on December 20, 1995.
In vitro sensitivity assay.In vitro testing was performed with a 4-day culture and the MTT assay.12 Briefly, we began cultivation using 96-well U microplates (Nunc, Roskilde, Denmark) containing 2 to 3 × 105 cells/well with five concentrations of each drug. The 16 drugs, their abbreviated names, and the range of concentrations that were tested are listed in Table 1. The highest in vitro concentration of each drug was calculated from the maximal serum concentration when the standard dose or high dose is used clinically. Untreated control cells were set up in four wells. After incubation in 5% carbon dioxide for 4 days at 37°C, the supernatant was aspirated. Then, 100 μL RPMI 1640 medium with 10 μL MTT dye (5 mg/mL) was added to each well and the plate was incubated for 6 hours. The tetrazolium salt was reduced to a colored formazan by the living cells. The formazan crystals were dissolved with 100 μL acid isopropanol. The optical density (OD) of each well was measured with a microplate reader at 540 nm.
Tested Range and Median Concentration of LD70 of Each Drug
| Abbreviation (drug name) . | Tested Range (μg/mL) Maximum-Minimum . | Median . |
|---|---|---|
| . | . | Concentration (μg/mL) of LD70 . |
| DNR (daunorubicin) | 0.8-0.00128 | 0.087 |
| ACR (aclarubicin) | 0.8-0.00128 | 0.08 |
| VP16 (etoposide) | 20-0.032 | 1.05 |
| L-ASP (L-asparaginase) | 10-0.016* | 1.925* |
| THP-ADR (pirarubicin) | 0.8-0.00128 | 0.135 |
| MIT (mitoxantrone) | 0.4-0.00064 | 0.08 |
| L-PAM (melphalan) | 20-0.032 | 5.75 |
| 4HC (4-hydroperoxy-CPA)† | 40-0.064 | 5.2 |
| AraC (cytarabine) | 40-0.064 | 1 |
| MTX (methotrexate) | 500-0.8 | 500 |
| BLM (bleomycine) | 40-0.064 | 40 |
| VCR (vincristine) | 1-0.0016 | 1 |
| VLB (vinblastine) | 10-0.016 | 2.8 |
| MMC (mitomycin C) | 2-0.0032 | 0.3 |
| Pred (prednisolone) | 100-0.16 | 36‡ |
| Dex (dexamethasone) | 4-0.0064 | 35‡ |
| Abbreviation (drug name) . | Tested Range (μg/mL) Maximum-Minimum . | Median . |
|---|---|---|
| . | . | Concentration (μg/mL) of LD70 . |
| DNR (daunorubicin) | 0.8-0.00128 | 0.087 |
| ACR (aclarubicin) | 0.8-0.00128 | 0.08 |
| VP16 (etoposide) | 20-0.032 | 1.05 |
| L-ASP (L-asparaginase) | 10-0.016* | 1.925* |
| THP-ADR (pirarubicin) | 0.8-0.00128 | 0.135 |
| MIT (mitoxantrone) | 0.4-0.00064 | 0.08 |
| L-PAM (melphalan) | 20-0.032 | 5.75 |
| 4HC (4-hydroperoxy-CPA)† | 40-0.064 | 5.2 |
| AraC (cytarabine) | 40-0.064 | 1 |
| MTX (methotrexate) | 500-0.8 | 500 |
| BLM (bleomycine) | 40-0.064 | 40 |
| VCR (vincristine) | 1-0.0016 | 1 |
| VLB (vinblastine) | 10-0.016 | 2.8 |
| MMC (mitomycin C) | 2-0.0032 | 0.3 |
| Pred (prednisolone) | 100-0.16 | 36‡ |
| Dex (dexamethasone) | 4-0.0064 | 35‡ |
Values are units per milliliter.
Cyclophosphamide.
Percentage of leukemic cell survival.
We tested 16 drugs and calculated the 70% lethal dose in vitro (LD70) from the dose-response curve for 14 drugs and the percentage of leukemic cell survival (LCS) for Dex and Pred because of their lack of dose-response cytotoxicity in the tested range. The LCS was calculated as follows: LCS = (OD Drug Exposed Well/Mean OD Control Wells) × 100 (%). When LD70 values were higher than the maximum or lower than the minimum concentration tested, the highest or lowest concentration was used as LD70 for statistical analysis.
For each single drug, patients were classified into two groups, either as S (lower than median LD70 or LCS) or R (median or higher than median concentration). Median values for the 16 drugs are listed in Table 1. Patients were also classified into three categories (SS, IS, and RR) by sensitivity to the combination of four drugs (DPAV; Dex, Pred, L-ASP, and VCR). For each of these four drugs, patients were classified as either S or R according to the definitions given above, and for the DPAV combinations, SS (super sensitivity) was defined as S to all four drugs, IS (intermediate sensitivity) as S to two or three drugs, and RR (relative resistance) as S to no drugs or to one drug.
Statistics.Event-free survival (EFS) is defined as the time from diagnosis to the first relapse or to disease-related death. In the case of induction failure, EFS is taken as 30 days.
The Kaplan-Meier method for estimation of EFS, log-rank test, post hoc test (Fisher's Protected Least Significant Difference), and contingency table analysis for multivariate comparison were conducted using StatView-J 4.5 and Survival Tools (Abacus Concepts, Berkeley, CA). The null hypothesis in contingency table analysis is that the distribution of data is independent of row and column position.
RESULTS
During our survey, we received 223 samples of initial ALL, but could not obtain results for 27 samples (25 of cALL, 2 of T-ALL) because of an insufficient number of cells for testing four or more drugs (14 samples), low absorbance in control wells (6 samples), no dose-response curve for calculating LD70 (4 samples), or less than 70% leukemic cells (3 samples). The technical success rate was 94% (196 of 209 samples). We excluded the clinical data of these 27 patients from the following analysis.
Median follow-up time for the 196 patients was 21 months (ranging from 1 to 84 months). Three-year EFS (95% confidence intervals) of patients with cALL, T-ALL, mix ALL, and uALL were 0.722 (0.632 to 0.811), 0.501 (0.237 to 0.766), 0.575 (0.285 to 0.865), and 0.600 (0.171 to 1.0), respectively. Patients with cALL had longer EFS than patients with T-ALL and mix ALL (P < .05, respectively). Initial WBC count and age were also strong prognostic factors in 196 patients (P < .0001, respectively).
Drug sensitivity testing and prognosis.We first investigated the relationship between drug sensitivity and prognosis. Patients sensitive to Pred, VCR, BLM, VP16, and MIT had superior EFS compared with patients whose blast cells were resistant to these drugs (P < .05). For the other 11 drugs, EFS of S group patients was not superior to that of R group patients (Table 2).
Three-year EFS for Each Single Drug for S and R Sensitivity Groups
| Drug . | 3-yr EFS . | Log-Rank Test P Value . | |
|---|---|---|---|
| . | S Group . | R Group . | . |
| DNR | 0.728 | 0.624 | .212 |
| ACR | 0.680 | 0.690 | .792 |
| VP16 | 0.726 | 0.592 | .028 |
| L-ASP | 0.721 | 0.631 | .086 |
| THP-ADR | 0.728 | 0.636 | .159 |
| MIT | 0.781 | 0.567 | .009 |
| L-PAM | 0.737 | 0.584 | .074 |
| 4HC | 0.676 | 0.626 | .080 |
| AraC | 0.700 | 0.647 | .471 |
| MTX | 0.757 | 0.665 | .494 |
| BLM | 0.865 | 0.635 | .049 |
| VCR | 0.821 | 0.596 | .002 |
| VLB | 0.706 | 0.667 | .582 |
| MMC | 0.801 | 0.609 | .185 |
| Pred | 0.755 | 0.595 | .028 |
| Dex | 0.762 | 0.606 | .158 |
| Drug . | 3-yr EFS . | Log-Rank Test P Value . | |
|---|---|---|---|
| . | S Group . | R Group . | . |
| DNR | 0.728 | 0.624 | .212 |
| ACR | 0.680 | 0.690 | .792 |
| VP16 | 0.726 | 0.592 | .028 |
| L-ASP | 0.721 | 0.631 | .086 |
| THP-ADR | 0.728 | 0.636 | .159 |
| MIT | 0.781 | 0.567 | .009 |
| L-PAM | 0.737 | 0.584 | .074 |
| 4HC | 0.676 | 0.626 | .080 |
| AraC | 0.700 | 0.647 | .471 |
| MTX | 0.757 | 0.665 | .494 |
| BLM | 0.865 | 0.635 | .049 |
| VCR | 0.821 | 0.596 | .002 |
| VLB | 0.706 | 0.667 | .582 |
| MMC | 0.801 | 0.609 | .185 |
| Pred | 0.755 | 0.595 | .028 |
| Dex | 0.762 | 0.606 | .158 |
S group patients have lower sensitivity than median concentration for each drug. R group patients have median or higher sensitivity than median concentration for each drug.
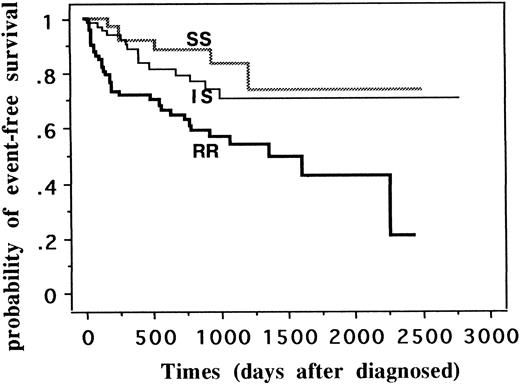
When we classified all patients into three categories (SS, IS, and RR) by sensitivity to DPAV combination, 3-year EFS (95% confidence intervals) of SS group (n = 42) was 0.833 (0.690 to 0.976), that of IS (n = 80) was 0.735 (0.608 to 0.863), and that of RR (n = 74) was 0.541 (0.411 to 0.670) (P = .0008; Fig 1). For DPAV sensitivity, there was a significant worsening of the prognosis from the extremely sensitive patients through an intermediate sensitive group to the most resistant group. From the cumulative hazard function curve, leukemia in RR patients relapsed earlier than in SS and IS patients (data not shown).
EFS of 196 patients classified into SS, IS, and RR groups by sensitivity to four-drug combination. Patients were classified into three categories (SS, IS, and RR) by sensitivity to the combination of four drugs (dexamethasone, prednisolone, asparaginase, and vincristine). SS was defined as sensitive to all four drugs, IS as sensitive to two or three drugs, and RR as sensitive to no drugs or to one drug. Three-year EFS (95% confidence intervals) of SS group (n = 42) was 0.833 (0.690 to 0.976), that of IS (n = 80) was 0.735 (0.608 to 0.863), and that of RR (n = 74) was 0.541 (0.411 to 0.670) (P = .0008).
EFS of 196 patients classified into SS, IS, and RR groups by sensitivity to four-drug combination. Patients were classified into three categories (SS, IS, and RR) by sensitivity to the combination of four drugs (dexamethasone, prednisolone, asparaginase, and vincristine). SS was defined as sensitive to all four drugs, IS as sensitive to two or three drugs, and RR as sensitive to no drugs or to one drug. Three-year EFS (95% confidence intervals) of SS group (n = 42) was 0.833 (0.690 to 0.976), that of IS (n = 80) was 0.735 (0.608 to 0.863), and that of RR (n = 74) was 0.541 (0.411 to 0.670) (P = .0008).
We then investigated whether drug sensitivity was related to the three prognostic groups (CCR, IF/e.rel, and late rel) using contingency table analysis. When we used SS, IS, and RR groupings as DPAV sensitivity categories, SS and IS group patients tended to maintain CCR and RR group patients tended to undergo IF/e.rel and late relapse (P = .004). There was only 1 IF/e.rel patient of 42 SS group patients, 4 IF/e.rel patients of 79 in the IS group, and 12 IF/e.rel of 73 in the RR group (Table 3). More induction failure and relapse occurred in the RR group.
The Relationship Between DPAV Sensitivity and the Three Prognostic Groups by Contingency Table Analysis
| DPAV Sensitivity . | No. of Patients . | Total . | χ2P Value . | ||
|---|---|---|---|---|---|
| . | CCR . | IF/e.rel . | Late rel . | . | . |
| SS | 36 | 1 | 5 | 42 | |
| IS | 67 | 4 | 8 | 79 | |
| RR | 45 | 12 | 16 | 73 | |
| Total | 148 | 17 | 29 | 1943-150 | .004 |
| DPAV Sensitivity . | No. of Patients . | Total . | χ2P Value . | ||
|---|---|---|---|---|---|
| . | CCR . | IF/e.rel . | Late rel . | . | . |
| SS | 36 | 1 | 5 | 42 | |
| IS | 67 | 4 | 8 | 79 | |
| RR | 45 | 12 | 16 | 73 | |
| Total | 148 | 17 | 29 | 1943-150 | .004 |
Abbreviations: SS (super sensitivity), sensitive to all four drugs (Dex, Pred, ASP, VCR); IS (intermediate sensitivity), sensitive to two or three of the four drugs; RR (relative resistance), sensitive to one or none of the four drugs; CCR, continuous complete remission; IF/e.rel, induction failure or relapse within 180 days of diagnosis; Late rel, relapse 181 or more days after diagnosis.
Two patients excluded from this analysis because they died of infection within 4 weeks of diagnosis.
Decreased sensitivity in IF/e.rel.Moreover, we investigated how the decreased sensitivity of leukemic cells in IF/e.rel and in late relapse patients compared with that in CCR patients. For each drug, the mean and median values of LD70 and LCS for IF/e.rel patients (n = 17) and late relapse patients (n = 29) were higher than those for CCR patients (n = 148). For the drugs DNR, L-ASP, THP-ADR, MIT, 4HC, AraC, BLM, VCR, VLB, MMC, and Pred, LD70 and LCS of the IF/e.rel group were significantly higher than those of the CCR group, according to Fisher's PLSD test (Table 4). Age distribution and mean blasts percentage of bone marrow did not differ between the three prognostic groups, but mean absorbance per 1 × 105 cells of IF/e.rel group was significantly higher than that of CCR group. Initial WBC count of IF/e.rel group was also significantly higher than that of CCR and late rel groups.
Mean LD70 and LCS for Each Drug for Each of the Three Prognostic Groups
| Drugs and Factors . | Mean LD70 (μg/mL) . | Post Hoc . | ||
|---|---|---|---|---|
| . | or Mean Value . | Test (P Value) . | ||
| . | CCR . | IF/e.rel . | Late rel . | . |
| DNR | 0.1194-150 | 0.3144-150 | 0.19 | .001 |
| ACR | 0.168 | 0.211 | 0.164 | .827 |
| VP16 | 3.12 | 5.77 | 2.94 | .19 |
| L-ASP | 3.424-150 | 5.884-150 | 4.28 | .046 |
| THP-ADR | 0.2254-150 | 0.3954-150 | 0.241 | .044 |
| MIT | 0.1274-150 | 0.249*† | 0.1214-151 | .009*, .0184-151 |
| L-PAM | 15.4 | 25.7 | 13.6 | .337 |
| 4HC | 7.854-150 | 15.574-150 | 9.54 | .012 |
| AraC | 6.734-150 | 15.054-150 | 8.33 | .025 |
| MTX | 449.8 | 482.9 | 455.9 | .641 |
| BLM | 26.94-150 | 40.04-150 | 31.7 | .012 |
| VCR | 0.5904-150 | 0.8364-150 | 0.742 | .039 |
| VLB | 4.154-150 | 6.64-150 | 4.67 | .033 |
| MMC | 0.344-150 | 0.664-150 | 0.46 | .025 |
| Pred (%) | 33.14-150 | 50.44-150 | 37.2 | .011 |
| Dex (%) | 36.6 | 46.8 | 41.6 | .319 |
| Age (yr) | 6.9 | 8.2 | 6.8 | .501 |
| Bone marrow blasts (%) | 89.3 | 90.4 | 87.5 | .681 |
| Absorbance (1 × 105/w) | 0.1364-150 | 0.224-150 | 0.156 | .005 |
| Initial WBC (median, ×103/μL) | 28.1*‡ | 95.3*† | 53.3†‡ | .0001*, .0244-151 |
| .037‡ | ||||
| Drugs and Factors . | Mean LD70 (μg/mL) . | Post Hoc . | ||
|---|---|---|---|---|
| . | or Mean Value . | Test (P Value) . | ||
| . | CCR . | IF/e.rel . | Late rel . | . |
| DNR | 0.1194-150 | 0.3144-150 | 0.19 | .001 |
| ACR | 0.168 | 0.211 | 0.164 | .827 |
| VP16 | 3.12 | 5.77 | 2.94 | .19 |
| L-ASP | 3.424-150 | 5.884-150 | 4.28 | .046 |
| THP-ADR | 0.2254-150 | 0.3954-150 | 0.241 | .044 |
| MIT | 0.1274-150 | 0.249*† | 0.1214-151 | .009*, .0184-151 |
| L-PAM | 15.4 | 25.7 | 13.6 | .337 |
| 4HC | 7.854-150 | 15.574-150 | 9.54 | .012 |
| AraC | 6.734-150 | 15.054-150 | 8.33 | .025 |
| MTX | 449.8 | 482.9 | 455.9 | .641 |
| BLM | 26.94-150 | 40.04-150 | 31.7 | .012 |
| VCR | 0.5904-150 | 0.8364-150 | 0.742 | .039 |
| VLB | 4.154-150 | 6.64-150 | 4.67 | .033 |
| MMC | 0.344-150 | 0.664-150 | 0.46 | .025 |
| Pred (%) | 33.14-150 | 50.44-150 | 37.2 | .011 |
| Dex (%) | 36.6 | 46.8 | 41.6 | .319 |
| Age (yr) | 6.9 | 8.2 | 6.8 | .501 |
| Bone marrow blasts (%) | 89.3 | 90.4 | 87.5 | .681 |
| Absorbance (1 × 105/w) | 0.1364-150 | 0.224-150 | 0.156 | .005 |
| Initial WBC (median, ×103/μL) | 28.1*‡ | 95.3*† | 53.3†‡ | .0001*, .0244-151 |
| .037‡ | ||||
Abbreviations: CCR, continuous complete remission; IF/e.rel, induction failure or relapse within 180 days of diagnosis; Late rel, relapse 181 or more days after diagnosis.
Statistically significant for two categories: CCR v IF/e.rel.
Statistically significant for two categories: IF/e.rel v late rel.
Statistically significant for two categories: CCR v late rel.
Initial WBC count (>50,000/μL) was strongly related to poor EFS (P < .0001) and also related to IF/e.rel rate (P = .0001), but the patient numbers showed no statistical correlation between initial WBC count and DPAV sensitivity (P = .239). There was no relation between immunologic classification and DPAV sensitivity (P = .391). There was a marginal relation between T-ALL and DPAV resistance (RR), when compared DPAV sensitivity between cALL and T-ALL (P = .066) only. There was also a marginal relation between older age (>9 years old) and DPAV resistance (RR) and between intermediate age and IS sensitivity (P = .062; Table 5).
The Relationship Between DPAV Sensitivity and Three Prognostic Factors
| Three Prognostic Factors . | 3-yr EFS (95% confidence intervals) . | No. of Patients for DPAV Sensitivity . | Contingency Table Analysis . | ||
|---|---|---|---|---|---|
| . | . | SS . | IS . | RR . | . |
| Immunologic classification | |||||
| cALL (n = 143) | 0.722 (0.632-0.811) | 34 | 62 | 47 | |
| T-ALL (n = 21) | 0.501 (0.237-0.766) | 3 | 6 | 12 | |
| mix ALL (n = 27) | 0.575 (0.285-0.865) | 4 | 10 | 13 | |
| uALL (n = 5) | 0.600 (0.171-1.000) | 1 | 2 | 2 | P = .391 |
| Age | |||||
| Infant (<1 yr old, n = 10) | 0.111 (0-0.316)5-150 | 2 | 2 | 6 | |
| 1-9 yr old (n = 127) | 0.772 (0.678-0.866) | 28 | 60 | 39 | |
| >9 yr old (n = 59) | 0.607 (0.461-0.753) | 12 | 18 | 29 | P = .062 |
| Initial WBC count (/μL) | |||||
| <10,000 (n = 88) | 0.747 (0.636-0.857) | 14 | 34 | 40 | |
| 10,000-50,000 (n = 66) | 0.738 (0.594-0.882) | 18 | 29 | 19 | |
| >50,000 (n = 42) | 0.421 (0.244-0.598) | 10 | 17 | 15 | P = .239 |
| Three Prognostic Factors . | 3-yr EFS (95% confidence intervals) . | No. of Patients for DPAV Sensitivity . | Contingency Table Analysis . | ||
|---|---|---|---|---|---|
| . | . | SS . | IS . | RR . | . |
| Immunologic classification | |||||
| cALL (n = 143) | 0.722 (0.632-0.811) | 34 | 62 | 47 | |
| T-ALL (n = 21) | 0.501 (0.237-0.766) | 3 | 6 | 12 | |
| mix ALL (n = 27) | 0.575 (0.285-0.865) | 4 | 10 | 13 | |
| uALL (n = 5) | 0.600 (0.171-1.000) | 1 | 2 | 2 | P = .391 |
| Age | |||||
| Infant (<1 yr old, n = 10) | 0.111 (0-0.316)5-150 | 2 | 2 | 6 | |
| 1-9 yr old (n = 127) | 0.772 (0.678-0.866) | 28 | 60 | 39 | |
| >9 yr old (n = 59) | 0.607 (0.461-0.753) | 12 | 18 | 29 | P = .062 |
| Initial WBC count (/μL) | |||||
| <10,000 (n = 88) | 0.747 (0.636-0.857) | 14 | 34 | 40 | |
| 10,000-50,000 (n = 66) | 0.738 (0.594-0.882) | 18 | 29 | 19 | |
| >50,000 (n = 42) | 0.421 (0.244-0.598) | 10 | 17 | 15 | P = .239 |
Abbreviations: SS (super sensitivity), sensitive to all four drugs (Dex, Pred, ASP, VCR); IS (intermediate sensitivity), sensitive to two or three of the four drugs; RR (relative resistance), sensitive to one or none of the four drugs.
Two-year EFS.
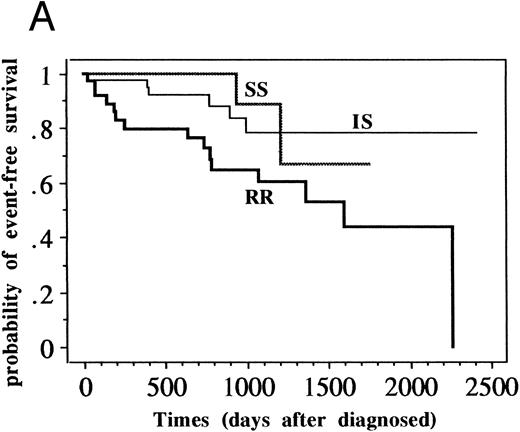
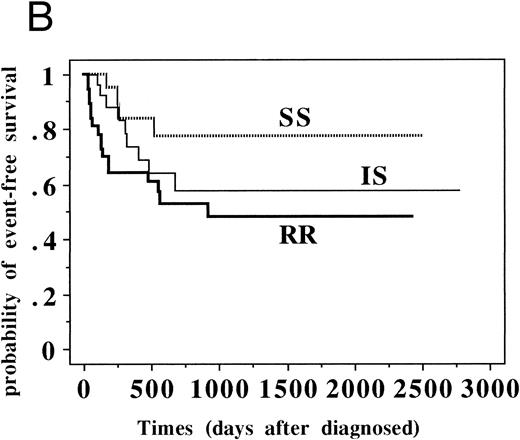
Although the patient numbers in Table 5 show no relationship between DPAV sensitivity and prognostic factors, it became obvious that EFS of RR patients was significantly worse for the standard-risk ALL group (n = 109, P = .016) but not significantly worse for the high-risk ALL group (n = 87, P = .109) when they were analyzed based on various clinical groups (Fig 2 and Table 6).
EFS of patients with standard-risk and high-risk ALL classified into SS, IS, and RR groups. (A) Patients with standard-risk ALL (1 to 9 years old and less than 50,000/μL initial WBC without central nervous system involvement) were classified into three categories (SS, IS, and RR). Three-year EFS (95% confidence intervals) of SS group (n = 21) was 0.889 (0.784 to 0.994), that of IS (n = 51) was 0.788 (0.706 to 0.870), and that of RR (n = 37) was 0.602 (0.509 to 0.695) (P = .016). (B) Patients with high-risk ALL (all patients except standard-risk ALL) were classified into SS/IS/RR group. Three-year EFS of SS group (n = 21) was 0.774 (0.673 to 0.875), that of IS (n = 29) was 0.576 (0.465 to 0.687), and that of RR (n = 37) was 0.484 (0.392 to 0.576). (P = .109).
EFS of patients with standard-risk and high-risk ALL classified into SS, IS, and RR groups. (A) Patients with standard-risk ALL (1 to 9 years old and less than 50,000/μL initial WBC without central nervous system involvement) were classified into three categories (SS, IS, and RR). Three-year EFS (95% confidence intervals) of SS group (n = 21) was 0.889 (0.784 to 0.994), that of IS (n = 51) was 0.788 (0.706 to 0.870), and that of RR (n = 37) was 0.602 (0.509 to 0.695) (P = .016). (B) Patients with high-risk ALL (all patients except standard-risk ALL) were classified into SS/IS/RR group. Three-year EFS of SS group (n = 21) was 0.774 (0.673 to 0.875), that of IS (n = 29) was 0.576 (0.465 to 0.687), and that of RR (n = 37) was 0.484 (0.392 to 0.576). (P = .109).
Three-Year EFS for Each Factor for Each of the DPAV Sensitivity
| Prognostic Factors . | 3-yr EFS of Patients for DPAV Sensitivity . | Log-Rank Test P Value . | ||
|---|---|---|---|---|
| . | SS . | IS . | RR . | . |
| Clinical group | ||||
| Standard-risk ALL (n = 109) | 0.889 | 0.788 | 0.602 | .016 |
| High-risk ALL (n = 87) | 0.774 | 0.576 | 0.484 | .109 |
| Immunologic classification | ||||
| cALL (n = 143) | 0.834 | 0.713 | 0.62 | .087 |
| non-cALL (n = 53) | 0.857 | 0.791 | 0.39 | .052 |
| Age | ||||
| Infant (<1 yr old, n = 10) | 0 | 0 | 0 | .734 |
| 1-9 yr old (n = 127) | 0.873 | 0.777 | 0.656 | .059 |
| >9 yr old (n = 59) | 0.9 | 0.559 | 0.517 | .159 |
| Initial WBC count (/μL) | ||||
| <10,000 (n = 88) | 0.791 | 0.758 | 0.673 | .201 |
| 10,000-50,000 (n = 66) | 0.75 | 0.85 | 0.472 | .002 |
| >50,000 (n = 42) | 0.656 | 0.363 | 0.327 | .175 |
| Prognostic Factors . | 3-yr EFS of Patients for DPAV Sensitivity . | Log-Rank Test P Value . | ||
|---|---|---|---|---|
| . | SS . | IS . | RR . | . |
| Clinical group | ||||
| Standard-risk ALL (n = 109) | 0.889 | 0.788 | 0.602 | .016 |
| High-risk ALL (n = 87) | 0.774 | 0.576 | 0.484 | .109 |
| Immunologic classification | ||||
| cALL (n = 143) | 0.834 | 0.713 | 0.62 | .087 |
| non-cALL (n = 53) | 0.857 | 0.791 | 0.39 | .052 |
| Age | ||||
| Infant (<1 yr old, n = 10) | 0 | 0 | 0 | .734 |
| 1-9 yr old (n = 127) | 0.873 | 0.777 | 0.656 | .059 |
| >9 yr old (n = 59) | 0.9 | 0.559 | 0.517 | .159 |
| Initial WBC count (/μL) | ||||
| <10,000 (n = 88) | 0.791 | 0.758 | 0.673 | .201 |
| 10,000-50,000 (n = 66) | 0.75 | 0.85 | 0.472 | .002 |
| >50,000 (n = 42) | 0.656 | 0.363 | 0.327 | .175 |
Abbreviations: standard-risk ALL, 1 to 9 years old and less than 50,000/μL initial WBC without CNS involvement; high-risk ALL, all patients except standard-risk ALL.
DISCUSSION
We previously reported the usefulness of the MTT assay for screening anticancer drugs for patients with leukemia.12,13 This report is a study concerned with initial ALL. Knowledge about drug resistance in childhood ALL was limited because of the lack of a suitable in vitro drug sensitivity assay, which is due to low viability of ALL cells in vitro. Pieters et al14 reported in 1990 an highly efficient MTT assay for testing cells from ALL patients with improved culture conditions, adding insulin, transferrin, and selenite. We adopted their method. The technical success rate was high (196 of 209 samples [94%]) and it was possible to analyze the drug resistance and clinical outcome on a large scale.
The major challenge in the clinical management of ALL is the development of effective therapy for the 30% of children in whom no remission occurs or who suffer relapse with current protocols.1,2 We thought that drug resistance of leukemic cells was responsible for induction failure and early relapse (and/or late relapse). There have been studies relating leukemic cellular drug sensitivity to DNA hyperdiploidy,15 to immunologic markers, and to age.16,17 Pieters et al6 authored the first study that related initial chemosensitivity to long-term clinical outcome. From the results for 42 new ALL patients, the probability of CCR was significantly lower in patients with resistant cells than those with sensitive cells for thioguanine (P < .01), DNR (P < .02) and Pred (P < .05). Interestingly, the prognosis worsened with decreasing sensitivity of the cells to Pred. They concluded that cellular resistance to Pred is a very important factor in the failure to chemotherapy in childhood ALL. We investigated glucocorticoid (Dex and Pred) sensitivity, too, and found a relationship between not only Pred sensitivity and prognosis (P = .028), but also VP16, BLM, MIT, and VCR sensitivity and prognosis (P < 0.5), as shown in Table 2. Moreover, we found by contingency table analysis that low EFS of R group patients to these five drugs was related to IF/e.rel (data not shown).
Although Pieters et al6 could not detect a relation between in vitro drug sensitivity to VCR and L-ASP and clinical outcome, we were able to when we considered the combination of four drugs (DPAV; Dex, Pred, L-ASP, and VCR) that is widely used for the treatment of induction therapy. DPAV resistance is clearly related to induction failure and early relapse in childhood ALL, as shown in the Fig 1 and in Table 3. This is the first report stating that the above-mentioned four-drug sensitivity is strongly related to clinical outcome.
In another study, Pieters et al16 also reported the correlation between immunophenotype (and age) and in vitro sensitivity to eight drugs in 84 children with initial ALL. They showed that T-ALL cells were significantly more resistant than pre-B ALL cells to Pred, DNR, L-ASP, AraC, and 6-thioguanine; that cells from children younger than 18 months were more resistant to Pred and DNR; and that cells from children older than 10 years were more resistant to Pred than those in the intermediate age group. Myeloid-antigen positive ALL samples were more resistant to glucocorticoids than myeloid-antigen negative ALL samples.17 They suggested from their data that cellular drug-resistant patterns might partly explain the prognostic value of immunophenotype and age in childhood ALL. Our results suggested that cellular drug-resistance to the four drugs DPAV could not clearly explain the prognostic value of immunophenotype, age, and initial WBC count (Table 5). DPAV sensitivity was itself of prognostic value. This means that treatment drugs themselves are important prognostic factors. Children with DPAV sensitive leukemia may have good clinical outcomes, whereas children with DPAV-resistant leukemia may undergo induction failure or early relapse when treated with the same drug combination.
When we took three groups (SS/IS/RR) and investigated the EFS for various clinical groups, DPAV sensitivity strongly influenced EFS in the standard-risk ALL group, but not in the high-risk group (Table 6). DPAV sensitivity was one of the prognostic factors for standard-risk ALL patients. From the data in Table 6, it may be possible to select the good prognostic patients from the high WBC group and high-risk ALL patients and to select the poor prognostic patients from the intermediate age group and non-cALL group using DPAV sensitivity testing.
We previously reported the differing sensitivity of lymphoblasts of 21 initial ALL patients and those of 31 relapsed patients and the difference between good clinical responders and non-responders in relapsed ALL.13 Klumper et al18 reported recently that, compared with those of 141 children with initial ALL, cells from 137 children with relapsed ALL were significantly more resistant to glucocorticoids, L-ASP, DNR, doxorubicin (DOX), 6-thioguanine, and 6-mercaptopurine, but not to vinca-alkaloids, AraC, ifosfamide, and epipodophyllotoxins. Moreover, they showed that, in relapsed/refractory childhood ALL, in vitro drug resistance was related to the clinical response to chemotherapy. Cells from children who failed to achieve a second CR were more resistant to Dex, Pred, DNR, and DOX compared with ALL cells from children who achieved a second CR. As in initial ALL, treatment drugs themselves were important prognostic factors in relapsed ALL.
MTT assay is based on measuring the total cell kill of both proliferating and nonproliferating cells and measures the end effect of the actual resistance mechanism. MTT assay determines in vitro resistance of dominant clones and is not capable of detecting small resistant subclones. The examination of initial ALL samples shows the dominant clone state at the initial stage. From the results of Table 4, IF/e.rel leukemic cells were more viable (absorbance was 0.22/105 cells v 0.136/105 cells of CCR group leukemic cells, P = .005) and more resistant to various drugs, even at the initial stage. Late relapsed leukemic cells had the same absorbance and same mean LD70 as CCR group cells. The higher mean LD70 value of IF/e.rel, compared with that of CCR, indicates that children who are at risk of induction failure and early relapse are already drug resistant at the initial stage. This shows that IF/e.rel may be related to de novo resistance and that late relapse may be related to acquired resistance.
There have been several studies on the drug-resistance mechanism in childhood ALL, for example, on the enzyme activities of ecto-5′-nucleotidase and adenosine deaminase to 6-thioguanine19,20 and topoisomerase II α gene expression to anthracycline,21 but none of these has yielded positive results. Despite intensive studies on the drug-resistance mechanism, we have not yet obtained the clinical methodology to overcome de novo or acquired resistance. If patients could be identified as de novo resistant to front-line drugs by MTT assay, it would then be possible to select second-line drugs, such as VP16, AraC, or in vitro sensitive drugs.
The patients in this study were treated according to three different protocols, although almost the same drug combination was used for induction therapy. Retrospective analysis of the study allowed us to analyze the prognostic value of sensitivity using the MTT assay. A prospective study of the MTT assay and the clinical outcome in patients treated with a single protocol is needed. When it is possible to use DPAV sensitivity, the number of IF/e.rel patients might be reduced by transferring them to an alternative treatment program, instead of carrying on with a probably unsuccessful treatment.
The present study clearly shows that in vitro drug sensitivity testing provides significant prognostic information in childhood ALL at the time chemotherapy commences and that early detection of drug resistance may provide a successful strategy for individualizing treatment.
ACKNOWLEDGMENT
The authors thank Dr H. Kawasaki (Mie University School of Medicine, Mie, Japan), Dr K. Horibe (Nagoya University, Nagoya, Japan), Dr M. Yazaki (Nagoya City University School of Medicine, Nagoya, Japan), Dr Y. Nishimura (Toyohashi Municipal Hospital, Toyohashi, Japan), Dr H. Kito (Seirei Hamamatsu General Hospital, Hamamatsu, Japan), Dr T. Shimizu (Tenri Hospital, Tenri, Japan), Dr M. Ishii (Japanese Red Cross Nagoya Second Hospital, Nagoya, Japan), Dr S. Kojima (Children's Medical Center, Japanese Red Cross Nagoya First Hospital, Nagoya, Japan), Dr K. Yamada (Gifu University, Gifu, Japan), and Dr T. Kawakami (Tottori University, Tottori, Japan) for providing the leukemic samples and data collection. We gratefully thank Dr Y. Sakakura (Hamamatsu University School of Medicine, Hamamatsu, Japan) for technical help with data analysis and P. Robertson for language advice. We also thank Prof Y. Igarashi (Vice President, Hamamatsu University School of Medicine, Hamamatsu, Japan) for encouragement and general support.
Supported by Grant-in-Aid for Scientific Research of The Japanese Ministry of Education, Science and Culture.
Address reprint requests to Teruaki Hongo, MD, Department of Pediatrics, Hamamatsu University School of Medicine, Handa-cho 3600, Hamamatsu City 431-31, Japan.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal