To the Editor:
Autologous bone marrow (BM) and peripheral blood (PB) stem cell transplantation (SCT) is widely used in a variety of hematological malignancies and solid tumors.1 After high-dose conditioning therapy,2 the recovery of hematopoiesis is dependent on stem cell self-renewal and differentiation into lineage-committed progenitors, which undergo differentiation and maturation to morphologically recognizable precursors and terminal cells circulating in PB.
SCT is associated with absolute leukopenia before hematopoietic recovery.2 Administration of recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF ) and G-CSF accelerates hematopoietic recovery.3 A number of investigators have detected elevated plasma G-CSF,4-9 GM-CSF,4 interleukin-3 (IL-3),10 M-CSF,7,11 IL-6,4,5,11 and IL-8 following myeloablation and marrow or PB progenitor cell transplantation. Time-course studies have demonstrated kinetic profiles that imply a relationship between cytokine levels and engraftment. In this context, we have previously shown that (1) G-CSF, IL-8, and IL-6 levels peak at the time of neutrophil nadir8,9; (2) G-CSF, IL-8, and IL-6 peak levels directly correlate with maximum neutrophil counts. Hence, it was proposed that these three cytokines are coordinately secreted after SCT and mediate the production and activation of neutrophils.8,9 Finally, recent studies11,12 have provided evidence that accessory cells do not contribute to the production of endogenous cytokines released after SCT; in fact, patients transplanted with purified CD34+ cells release cytokines, including G-CSF, IL-6, IL-8, and IL-3, at levels similar to those observed in patients transplanted with total PBMCs.11 12
Ovarian Cancer Patients: Main Cliical Features, Infused Cells, and PBMC Recovery
| Patient No. . | Age . | Infused PBMCs . | PBMC Recovery . | |||
|---|---|---|---|---|---|---|
| . | . | MNC . | CFU-GM (× 104/kg) . | WBC . | PMNC . | Platelets >50 × 109/L days . |
| . | . | (× 108/kg) . | . | >1 × 109/L days . | >0.5 × 109/L days . | . |
| 1 | 35 | 11.7 | 40.3 | 10 | 10 | 11 |
| 2 | 36 | 7.3 | 59.2 | 11 | 12 | 10 |
| 3 | 38 | 13.0 | 67.2 | 9 | 10 | 10 |
| 4 | 35 | 9.2 | 19.4 | 10 | 10 | 12 |
| 5 | 47 | 8.9 | 19.2 | 11 | 11 | 11 |
| 6 | 44 | 5.8 | 46.1 | 11 | 11 | 11 |
| 7 | 43 | 7.8 | 130.1 | 7 | 7 | 11 |
| 8 | 57 | 6.1 | 36.0 | 8 | 8 | 10 |
| 9 | 47 | 7.9 | 51.9 | 8 | 8 | 9 |
| 10 | 54 | 6.9 | 18.6 | 9 | 9 | 11 |
| 11 | 36 | 7.7 | 22.3 | 10 | 10 | 14 |
| 12 | 46 | 8.4 | 35.5 | 10 | 10 | 10 |
| 13 | 54 | 5.4 | 12.5 | 8 | 8 | 13 |
| 14 | 44 | 4.5 | 44.0 | 9 | 9 | 10 |
| 15 | 44 | 7.0 | 46.2 | 8 | 7 | 12 |
| 16 | 56 | 1.7 | 33.2 | 9 | 9 | 11 |
| 17 | 40 | 6.7 | 45.1 | 9 | 8 | 10 |
| 18 | 55 | 5.0 | N.V. | 10 | 10 | 11 |
| 19 | 51 | 3.3 | 11.9 | 9 | 9 | 13 |
| 20 | 60 | 5.8 | N.V. | 10 | 9 | 12 |
| 21 | 57 | 5.1 | N.V. | 9 | 9 | 10 |
| Patients 1 to 6 PBSCT | Patients 7 to 15 PBSCT + G-CSF/Epo | Patients 16 to 21 PBSCT + G-CSF/Epo | ||||
| Patient No. . | Age . | Infused PBMCs . | PBMC Recovery . | |||
|---|---|---|---|---|---|---|
| . | . | MNC . | CFU-GM (× 104/kg) . | WBC . | PMNC . | Platelets >50 × 109/L days . |
| . | . | (× 108/kg) . | . | >1 × 109/L days . | >0.5 × 109/L days . | . |
| 1 | 35 | 11.7 | 40.3 | 10 | 10 | 11 |
| 2 | 36 | 7.3 | 59.2 | 11 | 12 | 10 |
| 3 | 38 | 13.0 | 67.2 | 9 | 10 | 10 |
| 4 | 35 | 9.2 | 19.4 | 10 | 10 | 12 |
| 5 | 47 | 8.9 | 19.2 | 11 | 11 | 11 |
| 6 | 44 | 5.8 | 46.1 | 11 | 11 | 11 |
| 7 | 43 | 7.8 | 130.1 | 7 | 7 | 11 |
| 8 | 57 | 6.1 | 36.0 | 8 | 8 | 10 |
| 9 | 47 | 7.9 | 51.9 | 8 | 8 | 9 |
| 10 | 54 | 6.9 | 18.6 | 9 | 9 | 11 |
| 11 | 36 | 7.7 | 22.3 | 10 | 10 | 14 |
| 12 | 46 | 8.4 | 35.5 | 10 | 10 | 10 |
| 13 | 54 | 5.4 | 12.5 | 8 | 8 | 13 |
| 14 | 44 | 4.5 | 44.0 | 9 | 9 | 10 |
| 15 | 44 | 7.0 | 46.2 | 8 | 7 | 12 |
| 16 | 56 | 1.7 | 33.2 | 9 | 9 | 11 |
| 17 | 40 | 6.7 | 45.1 | 9 | 8 | 10 |
| 18 | 55 | 5.0 | N.V. | 10 | 10 | 11 |
| 19 | 51 | 3.3 | 11.9 | 9 | 9 | 13 |
| 20 | 60 | 5.8 | N.V. | 10 | 9 | 12 |
| 21 | 57 | 5.1 | N.V. | 9 | 9 | 10 |
| Patients 1 to 6 PBSCT | Patients 7 to 15 PBSCT + G-CSF/Epo | Patients 16 to 21 PBSCT + G-CSF/Epo | ||||
In the present study, the PBSCT model has been analyzed to investigate (1) the influence of exogenous G-CSF/erythropoietin (Epo) and GM-CSF/Epo on the production of IL-3, GM-CSF, G-CSF, M-CSF, IL-8, and IL-6; (2) the relationship between IL-5 release and eosinophil response, and the effect of exogenous GM-CSF and G-CSF on these parameters; and (3) the relationship of lactoferrin (Lf) plasma levels with the initial G-CSF peak and the subsequent neutrophil rescue. Modifications of plasma cytokine levels may be due to changes in production, release, consumption, or clearance. This clinical approach allows to establish temporal correlations between hematopoietic growth factor (HGF) administration and fluctuations of endogenous serum cytokines. However, the underlying cause/effect mechanisms remain largely hypothetical. Furthermore, because exogenous GM-CSF or G-CSF was combined with Epo, the specific role of each cytokine cannot be determined. Despite these limitations intrinsic to the clinical setting, the results provide novel information on the interaction between exogenous HGFs and the SCT-related cytokine release network.
We have also monitored the plasma concentrations of HGFs (ie, IL-3, GM-CSF, G-CSF, Epo, IL-8, IL-6, and IL-5) and granule proteins (Lf and myeloperoxidase [Mpo]) in 21 ovarian cancer patients undergoing autologous PB SCT (Table 1).
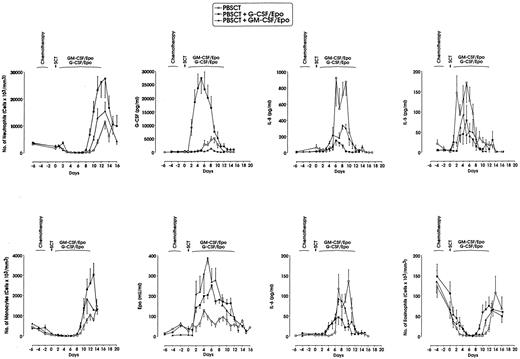
G-CSF levels were markedly higher after exogenous G-CSF/Epo, as expected; furthermore, they were significantly lower following GM-CSF/Epo than after SCT alone; GM-CSF levels, moderately elevated after exogenous GM-CSF/Epo, were similar in the G-CSF/Epo and control groups. Finally, patients treated with either G-CSF/Epo or GM-CSF/Epo showed higher Epo plasma concentrations than the group receiving no exogenous HGFs (Fig 1 and data not shown).
Neutrophil, monocyte, and eosinophil recovery, and G-CSF, Epo, IL-6, IL-8, and IL-5 plasma levels in 21 ovarian cancer patients (6 treated with no exogenous HGFs, 9 with G-CSF/Epo, and 6 with GM-CSF/Epo) undergoing SCT after high-dose chemotherapy. Chemotherapy days were −5 to −3 and SCT was performed on day 0. Mean ± SEM values are presented.
Neutrophil, monocyte, and eosinophil recovery, and G-CSF, Epo, IL-6, IL-8, and IL-5 plasma levels in 21 ovarian cancer patients (6 treated with no exogenous HGFs, 9 with G-CSF/Epo, and 6 with GM-CSF/Epo) undergoing SCT after high-dose chemotherapy. Chemotherapy days were −5 to −3 and SCT was performed on day 0. Mean ± SEM values are presented.
When compared with SCT alone, (1) G-CSF/Epo treatment moderately increased the IL-3 level peaking at day +10, and did not significantly modify M-CSF level (data not shown), whereas it almost completely suppressed IL-8 and moderately decreased IL-6 and IL-5 levels (Fig 1); (2) GM-CSF/Epo treatment increased M-CSF, associated with a moderate increase of IL-3 levels peaking at day +10, moderately decreased both IL-5 and IL-8 concentration and did not modify IL-6 (Fig 1 and data not shown).
These observations indicate that exogenous G-CSF treatment induces a marked decrease of IL-8 levels and moderately decreased IL-6 concentrations, whereas exogenous GM-CSF markedly reduces G-CSF and IL-8 concentrations and increases M-CSF levels. The increase of IL-3 and the decrease of IL-5 levels were similarly observed in patients treated with G-CSF/Epo or GM-CSF/Epo.
Altogether, these results indicate that exogenous HGFs modulate endogenous cytokine levels; consideration of these aspects may contribute to optimize HGF treatment protocols in the clinical SCT setting.
We also evaluated the relationship between these phenomena and Lf release: (1) in all three groups the Lf concentration is strictly and directly correlated with the neutrophil response with respect to time-response patterns and plasma levels. (2) In patients subjected to SCT alone, the G-CSF decrease after the G-CSF peak coincides with initiation of the Lf response; the inverse correlation between these two parameters is highly significant (data not shown). Altogether, these temporal correlations after SCT provide circumstantial evidence that, after the G-CSF peak, the generated granulocytes release Lf, which could negatively feedback on the release of G-CSF, thus in line with the experimental model proposed by Broxmeyer.13
ACKNOWLEDGMENT
We thank M. Fontana and C. Mastropietro for typing the manuscript, D. Marinelli for editorial assistance, and M. Teragnoli for graphics. This study was supported in part by CNR (ACRO Project, No. 95.00525.39, No. 95.00545.39), Rome and AIRC, Milan, Italy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal