Abstract
The purpose of this report is to describe the results of stem cell transplantation as initial treatment for secondary acute myeloid leukemia (AML). Forty-six patients (median age 42 years) with secondary AML (17 therapy-related, 29 myelodysplasia-related) who had not received remission induction chemotherapy underwent allogeneic (n = 43) or syngeneic (n = 3) transplantation. The 5-year actuarial disease-free survival was 24.4%, and the cumulative incidences of relapse and nonrelapse mortality were 31.3% and 44.3%, respectively. Lower peripheral blood blast count was associated with a lower risk of relapse (P = .05) and shorter time from AML diagnosis to transplant was associated with a lower risk of nonrelapse mortality (P = .02) and improved disease-free survival (P = .026). Patients with therapy-related secondary AML tended to have lower disease-free survival (P = .16) and a higher relapse rate (P = .16) than patients whose leukemia was not therapy-related. The results of these 46 previously untreated patients were compared to 20 patients (median age 36 years, 12 therapy-related, 8 myelodysplasia-related) transplanted with chemotherapy-sensitive disease after induction chemotherapy (first complete remission [n = 6], second complete remission [n = 3], first untreated relapse [n = 11]). We found no statistically significant difference in outcome between these 2 groups of patients. These results suggest that prompt transplantation should be considered after diagnosis of secondary AML or, if possible, high-risk myelodysplasia, particularly in patients with low peripheral blast counts. Innovative transplant strategies are needed to reduce the high risks of relapse and nonrelapse mortality seen in this patient population.
SECONDARY acute myeloid leukemia (AML) can be defined as AML that develops following exposure to cytotoxic agents or as a subsequent event in another hematologic disorder, usually myelodysplasia (MDS). Therapy-related secondary AML is increasing in incidence and is frequently associated with an initial MDS phase and cytogenetic abnormalities associated with poor outcomes, including −7/del7q, −5/del5q, or complex abnormalities.1,2 MDS-related secondary AML occurs in about 20% to 30% of patients with MDS, in particular, patients with MDS with excess blasts. The reported complete remission rates for therapy-related and MDS-related secondary AML using standard remission induction chemotherapy vary from 3% to 73%,1,3-5 with an average of 28% for therapy-related AML3 and 36% for MDS-related AML.4 The lowest remission rates generally occur in patients with poor prognostic karyotypes or a long history of MDS.2,3,6 Remission duration in patients with secondary AML is usually short and few patients are likely to be cured with conventional chemotherapy.4,7,8 Therefore, patients with secondary AML are often referred for allogeneic stem cell transplantation, if age, donor availability, and medical condition permit.2,3 9
Although patients with newly diagnosed de novo AML routinely undergo remission induction chemotherapy before being considered for allogeneic stem cell transplantation, the use of such pretransplant cytoreduction following diagnosis of secondary AML is controversial. Some investigators suggest that patients with secondary AML should undergo remission induction chemotherapy before transplantation and that those who achieve a complete remission have a longer disease-free survival after transplantation than patients transplanted without attempt at remission induction.10 Other investigators conclude the opposite, that there is no demonstrated benefit to administering induction chemotherapy before transplantation for secondary AML.11-13
There are few published reports on outcome of allogeneic stem cell transplantation for secondary AML.10-13 Most of these reports included both patients who had MDS without leukemic transformation and who had secondary AML. Furthermore, most of these patients had received varying intensities of pretransplant treatments, with few patients undergoing transplantation as initial therapy. Therefore, the main purpose of this retrospective analysis was to determine the outcome of transplantation as front line therapy for 46 patients with secondary AML and to determine the predictive variables associated with outcome. An additional purpose of this analysis was to help determine the potential benefit of pretransplant cytoreductive therapy by comparing the outcome of transplantation for the previously untreated patients to 20 patients with “chemotherapy-sensitive” secondary AML who were transplanted following cytoreductive therapy.
PATIENTS AND METHODS
Medical records were reviewed retrospectively on all patients with AML who had a history of prior chemotherapy or radiation exposure or a diagnosis of MDS and underwent allogeneic stem cell transplantation at Fred Hutchinson Cancer Research Center (Seattle, WA) through September 1994. AML was defined as a bone marrow exam with ≥30% myeloblasts (by morphology or cell surface antigen expression) or, in one case, a marrow with ≥20% myeloblasts associated with ≥30% peripheral blood (PB) myeloblasts. In addition, one patient with MDS with 15% marrow blasts and 7% PB blasts 1 month before transplantation who progressed to 40% PB blasts at time of transplantation was included, although a repeat bone marrow exam was not performed. Therapy-related secondary AML was defined as AML developing at any time following therapeutic chemotherapy or radiation treatment or accidental radiation exposure. Patients with therapy-related disease who had a myelodysplastic phase before progression into AML were categorized as having therapy-related AML. MDS-related secondary AML was defined as AML developing 2 or more months after a diagnosis of MDS based on marrow morphology.14 Patients with either therapy-related or MDS-related AML were included in this analysis if either (1) they received no induction chemotherapy after the AML diagnosis (n = 46) or (2) were in a first or second complete remission or first untreated relapse following induction chemotherapy after the AML diagnosis (n = 20). Complete remission was defined by usual morphologic and PB criteria.15 Patients were also included in this analysis only if the stem cell donor was a HLA matched or 1 HLA-A, B, or DR mismatched related donor or a HLA-A, B, DRB1 matched or 1-antigen minor mismatched16 unrelated donor. Patients were enrolled in preparative regimen and graft-versus-host disease (GVHD) prophylaxis studies that were ongoing at the time of the transplant. Informed consent was obtained from all patients or their guardian before initiating therapy. Tables 1 and 2 outline the clinical and transplantation characteristics of the 66 patients. The 46 patients whose transplant was the initial therapy for secondary AML and the 20 patients whose transplant followed remission induction chemotherapy for secondary AML are presented and analyzed separately.
Clinical Characteristics of Patients With Secondary AML Based on Pretransplant Therapy for AML
| . | Preceding Induction Chemotherapy* . | ||
|---|---|---|---|
| . | No . | Yes . | P . |
| No. of patients | 46 | 20 | |
| Median age, yrs (range) | 41.9 (6-61) | 35.8 (12-53) | .202 |
| Gender | 29 M, 17 F | 10 M, 10 F | .322 |
| Median time from MDS or AML diagnosis to SCT, mo (range) | 9 (1-57) | 11 (4-58) | .304 |
| MDS-related AML, no. | 29 | 8 | .083 |
| MDS classification at diagnosis | |||
| Refractory anemia (RA), no. | 7 | 2 | |
| RA with excess blasts (RAEB), no. | 12 | 2 | |
| RAEB in transformation, no. | 6 | 1 | |
| Chronic myelomonocytic leukemia, no. | 0 | 1 | |
| Not known† | 4 | 2 | |
| Median time from MDS diagnosis to SCT, mo. (range) | 11 (2-57) | 15 (10-58) | .057 |
| Median time from MDS diagnosis to AML, mo. (range) | 9 (2-54) | 6 (2-33) | .327 |
| Median time from AML diagnosis to SCT, mo. (range) | 1 (<1-8) | 7.5 (5-55) | .0001 |
| Therapy-related AML, no. | 17 | 12 | .083 |
| Etiology of therapy-related AML | |||
| Hodgkin's disease | 12 | 3 | |
| Non-Hodgkin's lymphoma | 2 | 1 | |
| Breast cancer | 0 | 2 | |
| Acute lymphocytic leukemia | 1 | 1 | |
| Ovarian cancer | 0 | 2 | |
| Vasculitis | 0 | 2 | |
| Autoimmune hemolytic anemia | 1 | 0 | |
| Radiation accident | 1 | 0 | |
| Osteosarcoma | 0 | 1 | |
| Diagnosis of MDS before AML, no. | 13 | 3 | .006 |
| Median time from AML diagnosis to SCT | 1 (<1-4) | 5 (3-14) | .0001 |
| PB counts at time of SCT | |||
| Blasts as a % of total white cell count, median (range) | 25 (0-92) | 0 (0-98) | N/A |
| Median absolute blast count, 109 cells/L (range) | 1.85 (0-59.66) | 0 (0-79.09) | N/A |
| Median neutrophil count, 109 cells/L (range) | 0.66 (0-16.84) | 2.74 (0-8.19) | .354 |
| Median platelet count, 109 cells/L (range) | 37 (5-960) | 124 (10-438) | .012 |
| Median hematocrit, % (range) | 28 (16-37) | 34 (25-43) | .0002 |
| Phase of pretransplant treatment at time of SCT | |||
| No induction chemotherapy for secondary AML | 46 | N/A | N/A |
| First complete remission | N/A | 6 | N/A |
| Second complete remission | N/A | 3 | N/A |
| First untreated relapse | N/A | 11 | N/A |
| Cytogenetics before or at time of SCT | |||
| Not done/inadequate | 6 | 7 | .039 |
| Normal | 10 | 2 | .256 |
| Single abnormality | 14‡ | 6ρ | .972 |
| Two or more abnormalities | 161-155 | 51-154 | .433 |
| Patient cytomegalovirus serology, no. | |||
| Positive/negative | 25/19 (2 missing) | 13/6 (1 missing) | .316 |
| . | Preceding Induction Chemotherapy* . | ||
|---|---|---|---|
| . | No . | Yes . | P . |
| No. of patients | 46 | 20 | |
| Median age, yrs (range) | 41.9 (6-61) | 35.8 (12-53) | .202 |
| Gender | 29 M, 17 F | 10 M, 10 F | .322 |
| Median time from MDS or AML diagnosis to SCT, mo (range) | 9 (1-57) | 11 (4-58) | .304 |
| MDS-related AML, no. | 29 | 8 | .083 |
| MDS classification at diagnosis | |||
| Refractory anemia (RA), no. | 7 | 2 | |
| RA with excess blasts (RAEB), no. | 12 | 2 | |
| RAEB in transformation, no. | 6 | 1 | |
| Chronic myelomonocytic leukemia, no. | 0 | 1 | |
| Not known† | 4 | 2 | |
| Median time from MDS diagnosis to SCT, mo. (range) | 11 (2-57) | 15 (10-58) | .057 |
| Median time from MDS diagnosis to AML, mo. (range) | 9 (2-54) | 6 (2-33) | .327 |
| Median time from AML diagnosis to SCT, mo. (range) | 1 (<1-8) | 7.5 (5-55) | .0001 |
| Therapy-related AML, no. | 17 | 12 | .083 |
| Etiology of therapy-related AML | |||
| Hodgkin's disease | 12 | 3 | |
| Non-Hodgkin's lymphoma | 2 | 1 | |
| Breast cancer | 0 | 2 | |
| Acute lymphocytic leukemia | 1 | 1 | |
| Ovarian cancer | 0 | 2 | |
| Vasculitis | 0 | 2 | |
| Autoimmune hemolytic anemia | 1 | 0 | |
| Radiation accident | 1 | 0 | |
| Osteosarcoma | 0 | 1 | |
| Diagnosis of MDS before AML, no. | 13 | 3 | .006 |
| Median time from AML diagnosis to SCT | 1 (<1-4) | 5 (3-14) | .0001 |
| PB counts at time of SCT | |||
| Blasts as a % of total white cell count, median (range) | 25 (0-92) | 0 (0-98) | N/A |
| Median absolute blast count, 109 cells/L (range) | 1.85 (0-59.66) | 0 (0-79.09) | N/A |
| Median neutrophil count, 109 cells/L (range) | 0.66 (0-16.84) | 2.74 (0-8.19) | .354 |
| Median platelet count, 109 cells/L (range) | 37 (5-960) | 124 (10-438) | .012 |
| Median hematocrit, % (range) | 28 (16-37) | 34 (25-43) | .0002 |
| Phase of pretransplant treatment at time of SCT | |||
| No induction chemotherapy for secondary AML | 46 | N/A | N/A |
| First complete remission | N/A | 6 | N/A |
| Second complete remission | N/A | 3 | N/A |
| First untreated relapse | N/A | 11 | N/A |
| Cytogenetics before or at time of SCT | |||
| Not done/inadequate | 6 | 7 | .039 |
| Normal | 10 | 2 | .256 |
| Single abnormality | 14‡ | 6ρ | .972 |
| Two or more abnormalities | 161-155 | 51-154 | .433 |
| Patient cytomegalovirus serology, no. | |||
| Positive/negative | 25/19 (2 missing) | 13/6 (1 missing) | .316 |
Abbreviations: SCT, stem cell transplantation; N/A, not applicable.
Induction chemotherapy use as treatment for secondary AML before transplantation.
In 6 cases classification at diagnosis was not known because records regarding blast count were insufficient to distinguish between RA, RAEB, and RAEB in transformation.
5 cases of −7, 3 cases of −5/del5q−, 1 case of +8, 1 case involved chromosome 11q23, 4 cases involved other chromosome abnormalities.
ρ 3 cases involved chromosome 11q23; 2 cases of −7; and 1 case of +8.
6 cases included −7/del7q−, 5 cases included −5/del5q−, 4 cases included +8, 4 cases included other abnormalities only.
2 cases included −5/del5q; 3 cases included other abnormalities.
Transplantation Characteristics of Patients With Secondary AML Based on Pretransplant Therapy for AML
| . | Preceding Induction Chemotherapy* . | |
|---|---|---|
| . | No . | Yes . |
| Stem cell source† number | ||
| Identical twin | 3 | 0 |
| Genotypically HLA identical sibling | 27 | 14 |
| Phenotypically HLA-A, B, Dw/DRB1 matched family member | 2 | 0 |
| 1 HLA-A, B, DR mismatched family member‡ | 4 | 1 |
| HLA-A, B, Dw/DRB1 matched unrelated donor | 8 | 3 |
| 1 “minor” HLA locus mismatched unrelated donorρ | 2 | 2 |
| Preparative regimen | ||
| Chemotherapy only2-155 | 12 | 5 |
| Cyclophosphamide-TBI2-154 | 22 | 15 |
| Busulfan (or dimethylmyleran)-cyclophosphamide-TBI2-167 | 12 | 0 |
| GVHD prophylaxis | ||
| None | 3 | 0 |
| Methotrexate only | 7 | 2 |
| Cyclosporine only | 3 | 2 |
| Cyclosporine and methotrexate | 21 | 14 |
| Cyclosporine and corticosteroids | 7 | 1 |
| Cyclosporine and FK506 | 5 | 1 |
| . | Preceding Induction Chemotherapy* . | |
|---|---|---|
| . | No . | Yes . |
| Stem cell source† number | ||
| Identical twin | 3 | 0 |
| Genotypically HLA identical sibling | 27 | 14 |
| Phenotypically HLA-A, B, Dw/DRB1 matched family member | 2 | 0 |
| 1 HLA-A, B, DR mismatched family member‡ | 4 | 1 |
| HLA-A, B, Dw/DRB1 matched unrelated donor | 8 | 3 |
| 1 “minor” HLA locus mismatched unrelated donorρ | 2 | 2 |
| Preparative regimen | ||
| Chemotherapy only2-155 | 12 | 5 |
| Cyclophosphamide-TBI2-154 | 22 | 15 |
| Busulfan (or dimethylmyleran)-cyclophosphamide-TBI2-167 | 12 | 0 |
| GVHD prophylaxis | ||
| None | 3 | 0 |
| Methotrexate only | 7 | 2 |
| Cyclosporine only | 3 | 2 |
| Cyclosporine and methotrexate | 21 | 14 |
| Cyclosporine and corticosteroids | 7 | 1 |
| Cyclosporine and FK506 | 5 | 1 |
Abbreviation: TBI, total body irradiation.
Induction chemotherapy use as treatment for secondary AML before transplantation.
Non T-cell depleted marrow was used in 65 cases. Growth factor mobilized PB stem cells were used in 1 case.
Mismatched at A locus in 1 case, B locus in 1 case, and DR locus in 3 cases.
ρ “Minor” mismatch was defined as a single HLA mismatch for a pair of DRB1 alleles belonging to the same DR type (DR1-18) (3 cases) or for a pair of HLA-A or B antigens within a cross-reactive group (1 case).
Cyclophosphamide-busulfan (15 cases), cyclophosphamide only (1 case), carmustine-cyclophosphamide-etoposide (1 case).
10 Gy (1 case), 12 Gy (8 cases), 13.2 Gy (13 cases), 14.4 Gy (3 cases), 15.75 Gy (12 cases).
Busulfan (11 cases), dimethylmyleran (1 case).
Methods of time-to-event analysis were used with date of last contact before January 1996 for patients remaining at risk. Disease-free survival estimates and curves were calculated using the method of Kaplan and Meier.17 Relapse and nonrelapse mortality estimates and curves were expressed as cumulative incidences.18 Death without relapse was considered a competing risk for the endpoint of relapse, and relapse was considered a competing risk for the endpoint of nonrelapse mortality. Relapse was defined as either recurrence of a previously present cytogenetic abnormality or presence of ≥5% marrow blasts. For time-to-event analyses, the resulting univariable and multivariable p-values were derived from the Wald test.19 Multivariable analyses were performed using a stepwise proportional hazards Cox regression model.19 Univariable comparisons of patient characteristics were made using the Chi-square test for categorical variables and the Wilcoxon's rank sum test for continuous variables.19 All reported P-values were two-sided and no adjustments were made for multiple comparisons.
RESULTS
Outcome for patients with previously untreated secondary AML.Forty-six patients underwent transplantation as initial therapy after diagnosis of secondary AML (Tables 1 and 2). Treatment of the myeloid disorder (MDS or secondary AML) before transplantation included transfusions, hematopoietic growth factors, splenectomy, corticosteroids, androgens, and vitamins. In addition, 4 patients with MDS-related AML received cytotoxic agents (low dose chemotherapy [n = 2], pentostatin because of an erroneous diagnosis of hairy cell leukemia [n = 1], remission induction chemotherapy [n = 1]) during the MDS phase, but not after the AML diagnosis.
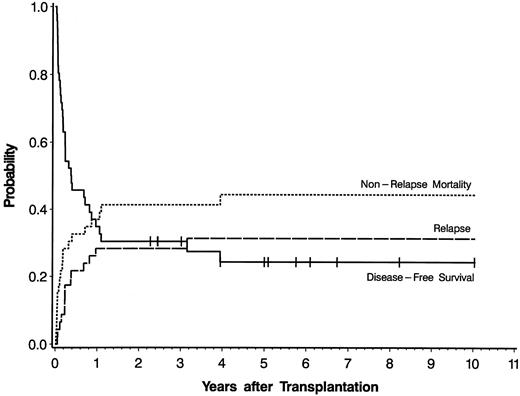
Twelve of the 46 patients survive disease-free between 2.3 and 10.0 (median 5.0) years after transplantation. Fourteen patients relapsed between 20 days and 3.2 years (median 2.8 months) after transplantation, and all subsequently died. The remaining 20 patients died of nonrelapse causes between 8 days and 3.9 years (median 53 days) after transplantation. The 5-year actuarial estimate of disease-free survival was 24.4% and the cumulative incidences of relapse and nonrelapse mortality were 31.3% and 44.3%, respectively (Fig 1). The current Karnofsky performance status of the 12 survivors ranges from 80% to 100 % (median 100%). Causes of death included GVHD with or without associated infection, respiratory, or cardiac failure (n = 8), infection without GVHD (n = 5), hemorrhage (n = 3), respiratory failure (n = 2), veno-occlusive disease of the liver (n = 1), and a secondary esophageal cancer (n = 1). No patient with therapy-related AML had evidence of progression or recurrence of the primary malignancy. Of the 3 syngeneic recipients, 1 survives disease-free, while 1 relapsed, and 1 died of pneumonitis.
Actuarial probability curve of disease-free survival and cumulative incidence curves of relapse and nonrelapse mortality for 46 patients with previously untreated secondary AML who underwent allogeneic stem cell transplantation. Tick marks represent patients alive in continuous complete remission.
Actuarial probability curve of disease-free survival and cumulative incidence curves of relapse and nonrelapse mortality for 46 patients with previously untreated secondary AML who underwent allogeneic stem cell transplantation. Tick marks represent patients alive in continuous complete remission.
Cytogenetic analyses of marrow cells were performed on the majority of patients either immediately before transplantation or before arriving at Fred Hutchinson Cancer Research Center (Table 1). Of the 12 disease-free survivors, pretransplant cytogenetic results were available on 11: 3 had all normal metaphases, 5 had a single abnormality (monosomy 7 [n = 3], trisomy 8 [n = 1], del5q [n = 1]), and 3 had complex abnormalities involving 3 or more chromosomes. All of these patients had normal donor karyotype demonstrated after transplantation.
Table 3 shows the 5-year actuarial estimates of disease-free survival and cumulative incidences of relapse and nonrelapse mortality and the univariate p values for patients based on clinical and transplant characteristics. A higher PB blast count immediately before the start of the transplant preparative regimen was associated with a statistically significantly higher incidence of relapse (Fig 2) and a trend towards lower actuarial disease-free survival. A shorter time from diagnosis of secondary AML to transplant was associated with statistically significantly lower nonrelapse mortality and higher disease-free survival rates. Six of 11 patients transplanted more than 1 month after AML diagnosis, compared to 14 of 35 patients transplanted 1 month or less after AML diagnosis died of nonrelapse causes, resulting in 5-year actuarial disease-free survival rates of 18% and 26%, respectively. Increasing patient age was associated with a trend towards greater risk of nonrelapse mortality and lower disease-free survival.
Five-Year Actuarial Estimate of Disease-Free Survival and Cumulative Incidences of Relapse and Nonrelapse Mortality and Univariate P for Patients With Previously Untreated Secondary AML
| Patient Category . | No. . | Disease-Free Survival . | Relapse . | Nonrelapse Mortality . | |||
|---|---|---|---|---|---|---|---|
| . | . | % . | P . | % . | P . | % . | P . |
| All untreated patients | 46 | 24.4 | 31.3 | 44.3 | |||
| Discrete variables: | |||||||
| PB blasts at SCT3-150 | |||||||
| 0-3.0 (×109 cells/L) | 16 | 31.3 | 18.8 | 50.0 | |||
| 3.1-5.5 (×109 cells/L) | 15 | 32.0 | .676 | 21.3 | .919 | 46.7 | .667 |
| >5.5 (×109 cells/L) | 15 | 20.0 | .224 | 53.3 | .034 | 46.7 | .702 |
| Disease etiology | |||||||
| MDS-related | 29 | 34.5 | 24.1 | 41.4 | |||
| Therapy-related | 17 | 7.8 | .159 | 43.1 | .160 | 49.0 | .516 |
| Patient age | |||||||
| <40 yrs | 22 | 31.8 | 36.4 | 31.8 | |||
| ≥40 yrs | 24 | 14.6 | .335 | 28.1 | .780 | 57.3 | .142 |
| Donor | |||||||
| Genotypic match | 30 | 27.5 | 31.3 | 41.3 | |||
| Nongenotypic match | 16 | 18.8 | .432 | 31.3 | .728 | 50.0 | .464 |
| Preparative regimen | |||||||
| Chemo only or Cy + ≤14.4 Gy TBI3-151 | 28 | 19.1 | 40.5 | 40.5 | |||
| 15.75 Gy TBI or Bu-Cy-TBI3-152 | 18 | 33.3 | .382 | 16.7 | .116 | 50.0 | .817 |
| Continuous variables: | |||||||
| Peripheral blood blasts at SCT | .111 | .050 | .677 | ||||
| Patient age | .152 | .802 | .096 | ||||
| Disease duration | .137 | .352 | .245 | ||||
| Time from MDS diagnosis to AML diagnosis | .212 | .374 | .371 | ||||
| Time from AML diagnosis to SCT | .026 | .659 | .020 | ||||
| Hematocrit at time of SCT | .415 | .525 | .593 | ||||
| Neutrophil count at time of SCT | .087 | .045 | .607 | ||||
| Platelet count at time of SCT | .104 | .320 | .198 | ||||
| Patient Category . | No. . | Disease-Free Survival . | Relapse . | Nonrelapse Mortality . | |||
|---|---|---|---|---|---|---|---|
| . | . | % . | P . | % . | P . | % . | P . |
| All untreated patients | 46 | 24.4 | 31.3 | 44.3 | |||
| Discrete variables: | |||||||
| PB blasts at SCT3-150 | |||||||
| 0-3.0 (×109 cells/L) | 16 | 31.3 | 18.8 | 50.0 | |||
| 3.1-5.5 (×109 cells/L) | 15 | 32.0 | .676 | 21.3 | .919 | 46.7 | .667 |
| >5.5 (×109 cells/L) | 15 | 20.0 | .224 | 53.3 | .034 | 46.7 | .702 |
| Disease etiology | |||||||
| MDS-related | 29 | 34.5 | 24.1 | 41.4 | |||
| Therapy-related | 17 | 7.8 | .159 | 43.1 | .160 | 49.0 | .516 |
| Patient age | |||||||
| <40 yrs | 22 | 31.8 | 36.4 | 31.8 | |||
| ≥40 yrs | 24 | 14.6 | .335 | 28.1 | .780 | 57.3 | .142 |
| Donor | |||||||
| Genotypic match | 30 | 27.5 | 31.3 | 41.3 | |||
| Nongenotypic match | 16 | 18.8 | .432 | 31.3 | .728 | 50.0 | .464 |
| Preparative regimen | |||||||
| Chemo only or Cy + ≤14.4 Gy TBI3-151 | 28 | 19.1 | 40.5 | 40.5 | |||
| 15.75 Gy TBI or Bu-Cy-TBI3-152 | 18 | 33.3 | .382 | 16.7 | .116 | 50.0 | .817 |
| Continuous variables: | |||||||
| Peripheral blood blasts at SCT | .111 | .050 | .677 | ||||
| Patient age | .152 | .802 | .096 | ||||
| Disease duration | .137 | .352 | .245 | ||||
| Time from MDS diagnosis to AML diagnosis | .212 | .374 | .371 | ||||
| Time from AML diagnosis to SCT | .026 | .659 | .020 | ||||
| Hematocrit at time of SCT | .415 | .525 | .593 | ||||
| Neutrophil count at time of SCT | .087 | .045 | .607 | ||||
| Platelet count at time of SCT | .104 | .320 | .198 | ||||
Cumulative incidence curves of relapse for 46 patients with previously untreated secondary AML based on PB blast count before the start of the transplant preparative regimen: (1) 0 to 3.0 × 109 cells/L (n = 16); (2) 3.1 to 5.5 × 109 cells/L (n = 15); (3) < 5.5 × 109 cells/L (n = 15).
Cumulative incidence curves of relapse for 46 patients with previously untreated secondary AML based on PB blast count before the start of the transplant preparative regimen: (1) 0 to 3.0 × 109 cells/L (n = 16); (2) 3.1 to 5.5 × 109 cells/L (n = 15); (3) < 5.5 × 109 cells/L (n = 15).
Patients with therapy-related secondary AML had a trend towards a worse outcome than patients with MDS-related disease, primarily because of a higher relapse rate: 7 of 17 patients with therapy-related versus 7 of 29 patients with MDS-related secondary AML relapsed. Patients receiving chemotherapy-only and preparative regimens with lower radiation exposure (see Table 3 for definitions) had higher relapse rates than patients receiving more intensive preparative regimens (11 of 28 versus 3 of 18 patients, respectively). However, these associations of relapse with disease etiology and preparative regimen are confounded because most of the patients with therapy-related AML (14 of 17) received a lower intensity preparative regimen compared to a more equal distribution among patients with MDS-related AML. Among patients with MDS-related AML the incidence of relapse was similar between the less intensive and more intensive preparative regimens (4 of 14 v 3 of 15, respectively).
Outcome for patients who received induction chemotherapy before transplantation and comparison to untreated patients.The decision to administer induction chemotherapy for treatment of secondary AML before transplantation was based on physician discretion and was mostly administered before arrival in Seattle. In general, patients who arrived in Seattle for the pretransplant evaluation with untreated AML were transplanted immediately without attempt at remission induction therapy. Twenty patients with secondary AML underwent transplantation while in first (1 MDS-related, 5 therapy-related) or second (2 MDS-related, 1 therapy-related) complete remission or in first untreated relapse (5 MDS-related, 6 therapy-related) following remission induction chemotherapy (Tables 1 and 2). The patients received a median of 1 cycle (range 1 to 4 cycles) of intensive chemotherapy (induction and consolidation type regimens, not maintenance chemotherapy). The regimens mostly consisted of standard-dose cytosine arabinoside and an anthracycline or high-dose cytosine arabinoside.
Three of the 20 patients survive disease-free (15% 5-year actuarial incidence), 5 relapsed (25% cumulative incidence), and 12 died of toxicity (60% cumulative incidence). One of 8 with MDS-related and 2 of 12 with therapy-related AML survive. Among the 9 patients who were transplanted while in first or second complete remission, 2 relapsed and 7 died of nonrelapse causes. Among the 11 patients who were transplanted while in untreated relapse following a first complete remission, 3 survive, 3 relapsed, and 5 died of nonrelapse causes.
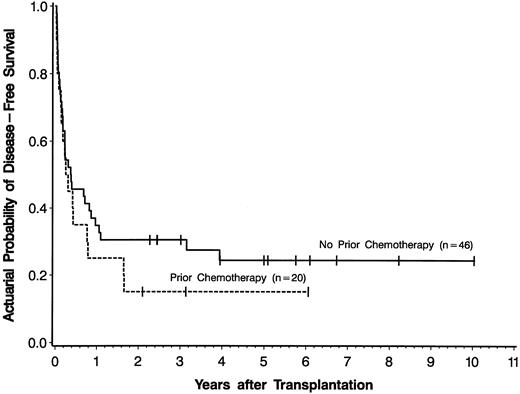
There was no statistically significant difference in outcome for the 20 patients who received induction chemotherapy before transplantation compared to the 46 patients who did not (univariate P = .328 for disease-free survival [Fig 3], P = .959 for relapse, and P = .238 for nonrelapse mortality). To determine whether other variables influenced this lack of association between induction chemotherapy use and outcome, we performed sequential multivariable analyses using the treatment group variable (ie, induction chemotherapy use) and adding in individually the other variables listed in Table 3. Even after adjusting for these individual variables, there remained no statistically significant association between use of induction chemotherapy before transplantation and disease-free survival, relapse, or nonrelapse mortality after transplantation (data not shown).
Actuarial probability curves of disease-free survival for patients with secondary AML who received induction chemotherapy before transplantation and were in first or second complete remission or first untreated relapse at time of transplantation (n = 20) compared to patients with secondary AML who received no induction chemotherapy before transplantation (n = 46). Tick marks represent patients alive in continuous complete remission.
Actuarial probability curves of disease-free survival for patients with secondary AML who received induction chemotherapy before transplantation and were in first or second complete remission or first untreated relapse at time of transplantation (n = 20) compared to patients with secondary AML who received no induction chemotherapy before transplantation (n = 46). Tick marks represent patients alive in continuous complete remission.
DISCUSSION
The primary purpose of this report was to evaluate the outcome for patients with therapy-related and MDS-related secondary AML who underwent allogeneic or syngeneic stem cell transplantation as front line therapy for AML. An additional purpose was to compare results of these patients to patients who were transplanted following remission induction chemotherapy. However, because patients were not prospectively identified and the decision to give induction chemotherapy was physician, not protocol dependent, the results of the comparison between the 2 groups of patients must be interpreted and applied cautiously.
We report a 5-year actuarial estimate of 24.4% for disease-free survival and cumulative probabilities of 31.3% and 44.3%, respectively, for relapse and nonrelapse mortality for the 46 patients transplanted without prior therapy. This report is the largest detailed analysis with the longest follow-up of such a group of patients. Other publications included smaller numbers of patients and included patients who had MDS without leukemic transformation.10-13 Sutton et al12 reported a 48% 15-month disease-free survival for 18 patients with previously untreated secondary AML or refractory anemia with excess blasts in transformation (RAEB-T), whereas DeWitte et al10 reported an 18% 2-year disease-free survival for 13 patients with secondary AML. The superior outcome in the former report12 compared to ours may be due to the inclusion of patients with RAEB-T, the shorter follow-up, or other factors. Bandini et al,11 in a review of published cases of therapy-related secondary AML, identified 4 disease-free survivors among 9 patients transplanted without prior remission induction chemotherapy. Overall, our results are consistent with these other reports and, with a median follow-up of 5 years, suggest that long-term cure can be obtained in a fraction of patients with an otherwise lethal disease.
In the univariable analysis of our study, three factors that were associated with improved outcome included younger age and shorter time from AML diagnosis to transplant, both of which were associated with a decreased nonrelapse mortality rate, and having fewer PB blasts, which was associated with a lower relapse rate. These features are consistent with our findings in transplantation for MDS,20-22 and suggest that outcome after transplantation for secondary AML may be improved by transplanting as soon as possible after diagnosis (in attempt to reduce toxicity) and before increase in disease burden (in attempt to reduce risk of relapse). Furthermore, for patients who present with a myelodysplastic phase, the relapse rate may be decreased further by transplantation before progression to AML.
The outcome for patients in our study with therapy-related secondary AML was worse than for patients with MDS-related AML, primarily due to a higher incidence of relapse. The increased incidence of relapse may be related to inadequate tumor ablation by the preparative regimen since these patients were more likely to receive a less intensive preparative regimen due to contraindications to the use of total body irradiation. However, even among patients receiving a chemotherapy-only preparative regimen there appeared to be a higher incidence of relapse among patients with therapy-related AML (6 of 12) versus patients with MDS-related AML (1 of 6). Thus, it is more likely that the patients in this study with therapy-related AML had intrinsically more resistant myeloid disease than patients with MDS-related AML. Clearly, the number of patients in any of these categories is too small to draw definitive conclusions regarding an association between the incidence of relapse and disease etiology or preparative regimen.
The use of remission induction chemotherapy before transplantation for secondary AML is not well defined. The complete remission rate is low, particularly in patients with poor risk cytogenetic abnormalities,2,6,8 the risk of death during induction chemotherapy ranges from 15% to 64%,3-5 and whether being in remission improves the outcome of transplantation is not clear.11-13 We found that the 20 patients transplanted with demonstrated “chemotherapy-sensitive” secondary AML (ie, patients in first or second complete remission or in first untreated relapse) had no difference in relapse, nonrelapse mortality, or disease-free survival compared to the 46 previously untreated patients. We specifically excluded from our analysis patients with demonstrated chemorefractory disease or those with an indeterminate response to chemotherapy because their outcome was expected to be poor (only 1 disease-free survivor among 28 patients transplanted, data not shown) and, therefore, to provide no more information than has already been published.10 12 However, despite such a selection bias of the treated patients and correction for other confounding variables in a multivariable model, we were unable to show a superior outcome after transplantation for patients with chemoresponsive secondary AML over patients who had no attempt at induction chemotherapy. Moreover, patients who die during an attempt at induction chemotherapy are excluded from the potential benefit of transplantation. Therefore, we conclude that currently available data do not support the routine use of induction chemotherapy before transplantation.
Overall, the long-term disease-free survival for patients with untreated or chemotherapy-sensitive secondary AML undergoing stem cell transplantation is poor, with both high relapse and high nonrelapse mortality rates. However, the apparent plateau in the disease-free survival curve, with a median patient follow-up of 5 years and excellent performance status of the survivors, suggests that some patients are truly cured, and have a superior outcome to that seen with conventional chemotherapy.7,8 Because of the increasing risk of relapse with increasing blast count, we propose that patients with de novo or therapy-related MDS be considered for transplantation before further increase in blast count. If secondary AML develops, our results suggest that outcome may be improved by transplanting patients as soon as possible and that the routine use of pretransplant cytoreductive therapy in attempt to obtain a remission is not warranted for all patients. Because occurrence of therapy-related MDS and AML appears to be increasing with increasing use of intensive therapy for more common malignancies,23 strategies to prevent or detect early such lethal complications are needed, as are innovative transplant strategies to reduce the relapse and nonrelapse mortality rates.
Supported by Grants CA18029, CA18221, HL36444, from the National Cancer Institute and the National Heart, Lung, and Blood Institute, National Institutes of Health. J.E.A. is a recipient of an American Cancer Society Clinical Oncology Career Development Award.
Address reprint requests to Jeanne E. Anderson, MD, Fred Hutchinson Cancer Research Center, 1124 Columbia St, M-385, Seattle, WA 98104.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal