Abstract
The feasibility of transplantation of HLA-matched hematopoietic progenitor cells from the blood of normal donors given granulocyte colony-stimulating factor (G-CSF ) has been reported recently. In the current study, the changes in T-cell subsets as well as CD34+ cells were determined in one blood volume leukapheresis products of six normal individuals given G-CSF. Examination of the T-cell subsets in the leukapheresis products showed three different patterns: one in which a discrete population of CD4−CD8−αβ T cells was found in addition to the typical CD4+ and CD8+ T cells in the unfractionated as well as in high- and low-density cells; a second in which the discrete population of CD4−CD8−αβ T cells was predominant only in the low-density fractions; and a third in which a discrete population of CD4−CD8− T cells was not observed. The median yield of CD4−CD8− T cells was about fourfold to fivefold higher than the calculated number present in one blood volume (5L) from normal individuals. The ratios of CD34+ cells to CD4+ and CD8+ T cells, and of CD4−CD8− T cells to CD4+ and CD8+ T cells, were highest in the low-density fractions. These fractions suppressed the mixed leukocyte, and may ameliorate graft-versus-host disease as compared with unfractionated cells.
PERIPHERAL BLOOD hematopoietic progenitor cell (PBPC) transplantation has been used as an alternative to bone marrow transplantation (BMT) in both autologous and allogeneic hosts.1-6 In the case of autologous transplantation, hematopoietic progenitor cells have been mobilized into the blood by the administration of granulocyte colony-stimulating factor (G-CSF ) or G-CSF in combination with chemotherapy.1-3 In the case of allogeneic transplantation, progenitor cells have been mobilized into the blood of normal donors by G-CSF alone.4-6 In general, the rate of engraftment of neutrophils and platelets is more rapid with PBPC than with BMT.1-6 This may be related to the larger dose of CD34+ progenitor cells present in the mobilized leukapheresis products as compared with marrow.7 8
The levels of T cells infused into allogeneic hosts have been reported to be about tenfold higher in PBPC as compared with BMT.4,9 Surprisingly, the incidence of acute graft-versus-host disease (GVHD) has been reported to be no higher than in marrow transplantation.4-6 However, the incidence of chronic GVHD may be increased.10 A possible explanation of the lack of increased acute GVHD is that G-CSF alters the functional activities of CD4+ and CD8+ T cells which are known to induce GVHD. Evidence for such alterations has been obtained in mice.11 The CD4+ and CD8+ T-cell content of splenocytes from G-CSF–treated mice was similar to normal controls, but the cytokine secretion pattern was shifted from a Th1 to a Th2 pattern in which interleukin-4 (IL-4) secretion was increased and interferon-γ (IFN-γ) secretion was decreased.11
In addition to alterations in CD4+ and CD8+ T-cell function, G-CSF may alter the balance of T-cell subsets in the PB. Previous studies in mice and humans have shown that CD4− CD8−αβ T cells in the marrow can suppress the mixed leukocyte reaction (MLR) and acute lethal GVHD.12-14 The current study was performed to determine whether there is a change in the balance of CD4+ and CD8+ T cells as compared with CD4−CD8− T cells in the blood mononuclear cells in leukapheresis products of normal individuals administered G-CSF. The results show that CD4−CD8−αβ T cells are markedly enriched in the leukapheresis products of G-CSF–treated normal donors along with CD34+ hematopoietic progenitor cells. The ratio of CD4−CD8− to CD4+ and CD8+ T cells is increased as compared with the ratio in blood mononuclear cells from untreated donors. The ratios of CD4−CD8− T cells and CD34+ cells to CD4+ and CD8+ T cells were increased further in the low-density fractions of mobilized blood mononuclear cells separated on discontinuous Percoll density gradients. The low-density fractions suppressed the MLR and may contain a balance of T-cell subsets which may ameliorate GVHD.
MATERIALS AND METHODS
Normal donors.Six normal donors who were more than 18 years old volunteered to enter the study, and signed an informed consent form under protocols approved by the Stanford University Hospital Institutional Review Board for the Protection of Human Subjects.
G-CSF administration and leukapheresis.Normal donors were treated with G-CSF (Neupogen; Amgen, Thousand Oaks, CA) administered by subcutaneous (SC) injection of approximately 10 μg/kg/d. Leukapheresis was initiated 96 hours after start of G-CSF, because the white blood cell (WBC) count reached a plateau between day 3 and day 4. Leukapheresis was performed using a COBE Spectra Cell Separator (Lakewood, CO) at a rate of 80 mL/min for a total of one blood volume. This cell separator enriches for mononuclear cells by centrifugation of the apheresed blood. Leukapheresis was begun within 4 hours after the last injection of G-CSF due to logistic considerations.
Percoll gradient centrifugation.Percoll gradients were prepared using 2.5% steps ranging from 40% to 50% of stock solution of Percoll (Pharmacia LKB Biotechnology, Uppsala, Sweden). Stock solutions were adjusted for pH (7.35 to 7.45) and osmolarity (275 to 285 mOsm/L) using phosphate-buffered saline (PBS). Refractive indices ranged from 1.3410 ± 0.0001 (40% solution) to 1.3435 ± 0.0001 (50% solution) using a refractometer (Zeiss, Oberkochen, Germany). Discontinuous gradients were layered using 6.5 mL of each of the five solutions in 50-mL tubes.7 Between 1 and 2 × 109 nucleated cells were carefully layered on each gradient. The 50-mL tubes were centrifuged at 1,700 rpm (550g ) for 30 minutes at room temperature. Cell fractions from the interfaces between 40.0% to 42.5% (C1), 42.5% to 45.0% (C2), 45.0% to 47.5% (C3) and 47.5% to 50.0% (C4) Percoll solutions, as well as from the pellet (C5) were collected and washed once.
Preparation of PB lymphocytes (PBL).PB from normal leukapheresis donors and from other normal individuals were separated on a Ficoll-Hypaque gradient (Pharmacia), and the mononuclear cells were obtained as described previously.7 The latter cells were referred to as PBL and used in studies of the mixed leukocyte reaction.
Immunofluorescent staining.Samples of leukapheresis products, as well as fractions C1-C5, were stained with monoclonal antibodies (MoAbs) conjugated with phycoerythrin (PE) directed to CD34 (HPCA-2), CD4 and CD8, or with MoAbs conjugated with fluorescein isothiocyanate (FITC) directed to CD3 or αβTCR. Conjugated isotype-matched antibodies were used for background staining (PE-IgG1 , FITC-IgG1 ). All antibodies were purchased from Becton Dickinson Laboratories (San Jose, CA), except the anti-TCRαβ antibody (T Cell Sciences, Cambridge, MA). Cell staining was performed as previously described.7 14 Cells were incubated in the presence of unconjugated anti-CD16 MoAbs (G022; Becton Dickinson), or anti-CD32 MoAbs (C1KM5; Caltag, Inc, South San Francisco, CA) at saturation to block nonspecific binding to FcγR III A/FcγR III B receptors on monocytes and granulocytes or FcγR II receptors on B cells, monocytes, and granulocytes, respectively. Anti-CD16 antibodies (3G8) conjugated to CY-5/PE (tricolor) were purchased from Caltag, Inc. Stained cells were washed and analyzed using a fluorescence-activated cell sorter (FACS III; Becton Dickinson) linked to a micro VAX computer (Digital Electronic Corp, Santa Clara, CA) equipped with FACS-Desk software (Becton Dickinson). Measurement of CD34+ cells in the blood of normal donors during the time points before leukapheresis was done by immunoflourescent staining of PB cells after lysis with ammonium chloride. The “mononuclear/lymphocyte” gate was used for this measurement.
MLR and suppressor cell assay.Responder and irradiated stimulator cells were cultured at a concentration of 1 × 105 cells each in U-bottomed microculture plates (Costar, Cambridge, MA). Cells were incubated for 120 hours at 37°C with 5% CO2 as described before.14 Stimulator cells were irradiated with 3,000 cGy from a 137Cs source (Mark I model 125 irradiator; J.L. Shepherd and Associates, Glendale, CA). 3H-thymidine (3H-TdR) incorporation was assayed by the addition of 1 μCi of 3H-TdR (specific activity 6.7 Ci/mmol/L; New England Nuclear Corp, Boston, MA) to each well during the final 18 hours of the incubation period. Triplicate wells were obtained and counted in a scintillation counter. Data are the means ± SE of the triplicate values.
In the suppressor cell assays, 1 × 105 irradiated (1,500 cGy) putative suppressor cells were added to 96-well, U-bottomed microtiter plates containing 1 × 105 responder and 1 × 105 irradiated (3,000 cGy) stimulator cells/well in a final volume of 200 μL. Controls included the addition of 1 × 105 irradiated (1,500 cGy) responder cells, or no addition of cells to the responder/stimulator mixtures. Cultures were incubated at 37°C in 5% CO2 . After 120 hours, plates were pulsed with 1 μCi/well 3H-TdR as described above. The percent suppression was calculated as follows:
RESULTS
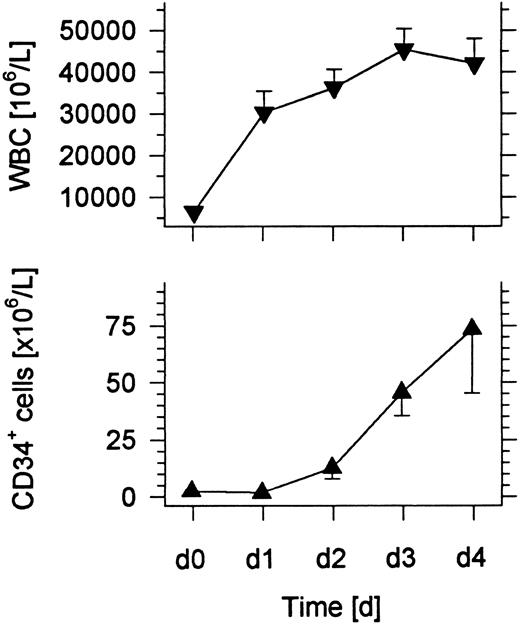
Changes in the absolute numbers of WBCs and CD34+ cells in the blood after administration of G-CSF.Six normal individuals were administered 10 μg/kg/d G-CSF subcutaneously for 4 days, and blood samples were collected immediately before the first injection and subsequent injections on days 1, 2, 3, and 4. A 1 blood volume leukapheresis was performed within 4 hours after the injection of G-CSF on the fourth day. Figure 1 shows that the mean WBC count of the six individuals increased about sevenfold over the 4-day interval. The most rapid increase was observed within the first day. The mean number of CD34+ hematopoietic progenitor cells rose at least 75-fold and reached about 75 × 106 cells/L on day 4.
Changes in the mean WBC count and concentration of CD34+ cells in the blood of six normal donors given subcutaneous injections of G-CSF daily for 4 days. Bars show standard errors.
Changes in the mean WBC count and concentration of CD34+ cells in the blood of six normal donors given subcutaneous injections of G-CSF daily for 4 days. Bars show standard errors.
Density and light scatter of CD34+ cell subsets in the day 4 leukapheresis.The CD34+ hematopoietic progenitor cell and T-cell content of leukapheresis samples from G-CSF–treated normal donors was examined after density gradient fractionation in the present study. Cells obtained from the leukapheresis samples were separated on a discontinuous Percoll gradient using 2.5% steps from 40% to 50% of the Percoll stock solution. Five fractions were obtained from the interfaces or pellet as follows: C1, 40.0% to 42.5%; C2, 42.5% to 45.0%; C3, 45.0% to 47.5%; C4, 47.5% to 50.0%; C5, cell pellet.
The median yield of unfractionated cells (U) was about 13 × 109 cells after a leukapheresis of 1 blood volume. Table 1 shows the median recoveries of nucleated cells in each fraction per 1 × 109 cells placed on each gradient. About two thirds of the cells were recovered in the highest density fraction (C5), and the remaining fractions showed decreasing recoveries as the density decreased. Less than 2% of nucleated cells were recovered in fractions C1 or C2. About 82% of cells were recovered from all fractions.
Recovery of Cells in Day 4 Leukapheresis Samples
| Fraction . | Absolute No. of Cells (×106)* . | Percentage of Cells* . | Ratio CD34+ Cells: . | Ratio CD4−CD8−: . | |||||
|---|---|---|---|---|---|---|---|---|---|
| . | Nucleated Cells . | CD34+ . | CD4+/CD8+ . | CD4−CD8− . | CD34+ . | CD4+/CD8+ . | CD4−CD8− . | CD4+/CD8+ T Cells . | CD4+/CD8+ T Cells . |
| U | 1,000 | 5.0 | 188 | 37 | 0.5 | 18.8 | 3.7 | 1:38 | 1:5 |
| C1 | 18 | 0.4 | 2.1 | 1.1 | 2.1 | 11.8 | 6.0 | 1:6 | 1:2 |
| C2 | 16 | 0.2 | 1.2 | 0.7 | 1.0 | 7.2 | 4.2 | 1:7 | 1:2 |
| C3 | 32 | 0.5 | 5.3 | 2.0 | 1.5 | 16.5 | 6.2 | 1:11 | 1:3 |
| C4 | 73 | 1.2 | 14.4 | 4.7 | 1.6 | 19.8 | 6.5 | 1:12 | 1:3 |
| C5 | 678 | 1.4 | 159.3 | 27.1 | 0.2 | 23.5 | 4.0 | 1:118 | 1:6 |
| C1-C5† | 817 | 3.7 | 182.3 | 35.6 | |||||
| C1-C4† | 139 | 2.3 | 23.0 | 8.5 | |||||
| Fraction . | Absolute No. of Cells (×106)* . | Percentage of Cells* . | Ratio CD34+ Cells: . | Ratio CD4−CD8−: . | |||||
|---|---|---|---|---|---|---|---|---|---|
| . | Nucleated Cells . | CD34+ . | CD4+/CD8+ . | CD4−CD8− . | CD34+ . | CD4+/CD8+ . | CD4−CD8− . | CD4+/CD8+ T Cells . | CD4+/CD8+ T Cells . |
| U | 1,000 | 5.0 | 188 | 37 | 0.5 | 18.8 | 3.7 | 1:38 | 1:5 |
| C1 | 18 | 0.4 | 2.1 | 1.1 | 2.1 | 11.8 | 6.0 | 1:6 | 1:2 |
| C2 | 16 | 0.2 | 1.2 | 0.7 | 1.0 | 7.2 | 4.2 | 1:7 | 1:2 |
| C3 | 32 | 0.5 | 5.3 | 2.0 | 1.5 | 16.5 | 6.2 | 1:11 | 1:3 |
| C4 | 73 | 1.2 | 14.4 | 4.7 | 1.6 | 19.8 | 6.5 | 1:12 | 1:3 |
| C5 | 678 | 1.4 | 159.3 | 27.1 | 0.2 | 23.5 | 4.0 | 1:118 | 1:6 |
| C1-C5† | 817 | 3.7 | 182.3 | 35.6 | |||||
| C1-C4† | 139 | 2.3 | 23.0 | 8.5 | |||||
Median (n = 6).
Sum of recoveries of CD1-C4 or C1-C5.
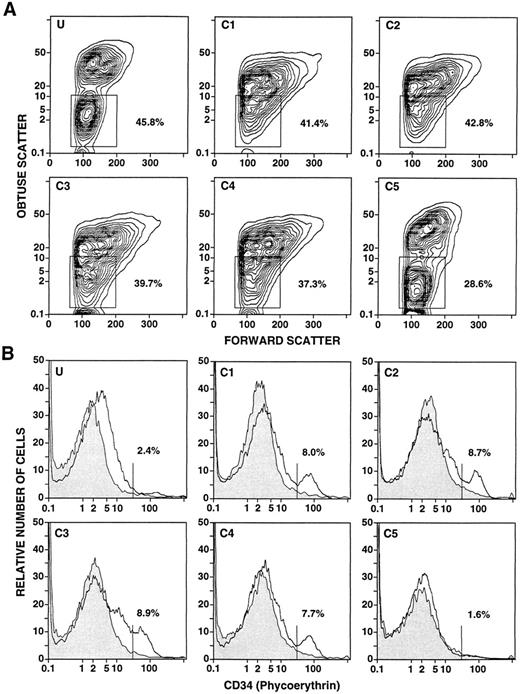
The light scatter characteristics of the unfractionated and fractionated cells from a representative leukapheresis sample are shown in Fig 2A. The boxes in panels U and C5 enclose the typical “mononuclear/lymphocyte” gate, and concentric contours of the cells are clearly recognized. The percentages of cells within the boxes are shown. However, a discrete population of cells within the “mononuclear/lymphocyte” gate is not observed in fractions C1-C4. About 37% to 42% of cells were contained within the boxes. Figure 2B shows the one-color flow cytometric analysis after immunofluorescent staining for CD34+ cells in fractions C1-C5. In view of the light scatter patterns in Fig 2A, a “mononuclear/lymphocyte” gate was not used, and only erythrocytes were excluded by gating. Staining with the anti-CD34+ MoAbs is shown in the open area, and the background control with irrelevant MoAbs is shown in the shaded area. A clear peak of staining was observed in fractions C1, C2, and C4. A threshold (vertical line) was placed immediately to the left of the peak in C1 to determine the percentage of CD34+ cells within the peak. A clear peak was not observed in the unfractionated cells (U), or in C3 or C5. Quantitation of CD34+ cells in these fractions was based on the threshold determined in C1. Cells stained above background but below the threshold (U and C3) were considered CD34− cells for purposes of quantitation. As shown in Table 1, the median percentage of CD34+ cells in the unfractionated leukapheresis samples (0.5%) was enriched about threefold to fourfold in the low-density fractions, and depleted in the high-density fraction (C5). However, quantitation of CD34+ cells in the latter fraction was inaccurate compared with the low-density fractions, because there was no clear peak of CD34+ cell staining and the percentage of CD34+ cells was below 1%.
Flow cytometric analysis of unfractionated and density fractionated cells from a day 4 leukapheresis sample from a normal donor given daily G-CSF injections. Panels U and C1-C5 in (A) show the obtuse versus forward light scatter profiles of unfractionated (U) and density fractionated cells (C1-C5). Boxes enclose the “mononuclear/lymphocyte” gate, and the percentages of cells enclosed in the boxes are shown. Panels U and C1-C5 in (B) show the one-color analysis of immunofluorescent staining with PE-conjugated anti-CD34 monoclonal antibodies (open area) compared with background control staining (shaded area). The vertical lines show the staining threshold established from C1, and the percentages of cells staining with anti-CD34 antibodies above the threshold are shown.
Flow cytometric analysis of unfractionated and density fractionated cells from a day 4 leukapheresis sample from a normal donor given daily G-CSF injections. Panels U and C1-C5 in (A) show the obtuse versus forward light scatter profiles of unfractionated (U) and density fractionated cells (C1-C5). Boxes enclose the “mononuclear/lymphocyte” gate, and the percentages of cells enclosed in the boxes are shown. Panels U and C1-C5 in (B) show the one-color analysis of immunofluorescent staining with PE-conjugated anti-CD34 monoclonal antibodies (open area) compared with background control staining (shaded area). The vertical lines show the staining threshold established from C1, and the percentages of cells staining with anti-CD34 antibodies above the threshold are shown.
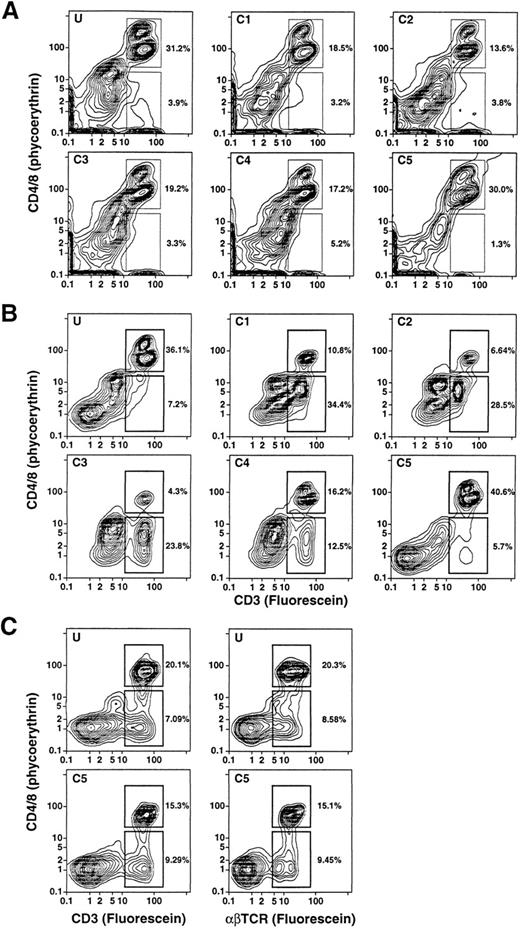
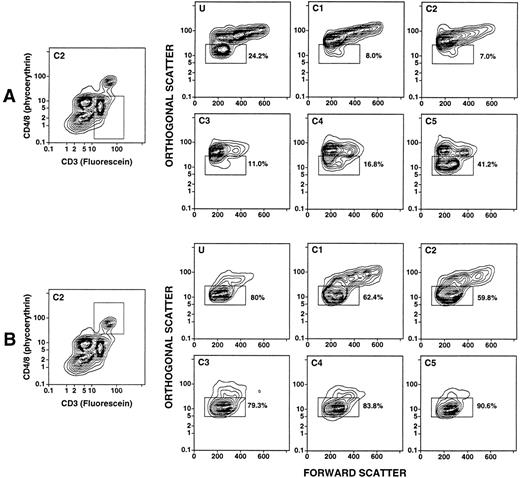
Density and light scatter characteristics of T-cell subsets in the day 4 leukapheresis.The day 4 leukapheresis samples were stained for T cells expressing CD4, CD8, CD3, and TCRαβ markers. Unconjugated anti-CD16 antibodies were used to block nonspecific binding to Fcγ receptors. Figure 3A and B show the two-color staining patterns of cells incubated with a combination of PE-conjugated anti-CD4 and anti-CD8 MoAbs and counterstained with FITC-conjugated anti-CD3 antibodies. CD4+CD3+ and CD8+CD3+ T cells are enclosed in the upper boxes. The median percentage of CD4+ and CD8+ T cells among the unfractionated cells in the day 4 leukapheresis samples from the six donors was reduced about threefold to fourfold (19%) (Table 1), compared with the median of 68% observed in the unfractionated blood mononuclear cell of six normal individuals. Donors with three different characteristics of T-cell subsets were observed. One type of donor (2/6) showed a discrete population of CD4+CD3+ and CD8+CD3+ T cells in the unfractionated and fractionated cells with no discrete population of CD4−CD8−CD3+ T cells (Fig 3A). Another type of donor (3/6) showed discrete populations of both CD4+ and CD8+ T cells, as well as CD4−CD8− T cells (upper and lower boxes, respectively), which are seen most clearly in fractions C1-C4 (Fig 3B). In some instances, the percentages of CD4−CD8− T cells were threefold to fourfold higher than that of the CD4+ and CD8+ T cells (fractions C1-C3, Fig 3B). The CD4−CD8− T cells were less clearly discerned in the unfractionated and high-density (C5) fraction in these cases. In the third type, the CD4−CD8−CD3+ T cells were clearly observed in both the unfractionated and high-density fractions (Fig 3C), and were not enriched in the low-density fractions (data not shown).
Two-color analysis of immunofluorescent staining of T cells in the unfractionated and density fractionated cells from three different donors (A, B, and C). Cells were stained with PE-conjugated anti-CD4 and anti-CD8 monoclonal antibodies, and counterstained with FITC-conjugated anti-CD3 or anti-TCRαβ MoAbs. The upper boxes in (A) and (B) enclose CD4+CD3+ and CD8+CD3+ T cells. The lower boxes enclose CD4−CD8−CD3+ T cells. In (C), the staining with anti-CD3 antibodies was compared to that with anti-TCRαβ antibodies for unfractionated cells and cells in fraction C5.
Two-color analysis of immunofluorescent staining of T cells in the unfractionated and density fractionated cells from three different donors (A, B, and C). Cells were stained with PE-conjugated anti-CD4 and anti-CD8 monoclonal antibodies, and counterstained with FITC-conjugated anti-CD3 or anti-TCRαβ MoAbs. The upper boxes in (A) and (B) enclose CD4+CD3+ and CD8+CD3+ T cells. The lower boxes enclose CD4−CD8−CD3+ T cells. In (C), the staining with anti-CD3 antibodies was compared to that with anti-TCRαβ antibodies for unfractionated cells and cells in fraction C5.
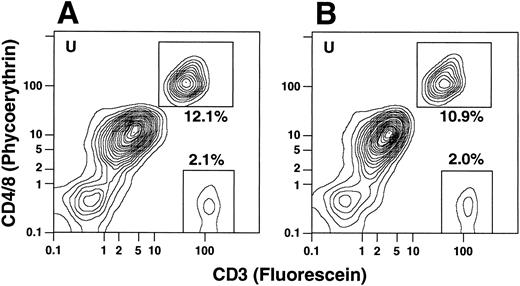
To determine whether the CD4−CD8−CD3+ T cells coexpressed the TCRαβ marker, cells from the same individuals were stained with PE-conjugated anti-CD4 and anti-CD8 antibodies as before and counterstained with FITC-conjugated anti-CD3 or anti-TCRαβ antibodies. In all instances the percentages of CD3+ T cells and TCRαβ+ T cells were similar regardless of whether the T cells belonged to the CD4+ and CD8+ or CD4−CD8− subsets (Fig 3C). In several experiments, cells were stained for the T-cell markers CD4, CD8, or CD3 and counterstained with CY-5Y/PE-conjugated anti-CD16 antibodies to determine whether the anti-T cell marker reagents were binding nonspecifically to macrophages or granulocytes. In the latter experiments, unconjugated anti-CD16 antibodies were not added to the staining mixture. Less than 1% of the CD4+, CD8+, or CD3+ cells stained above background with the anti-CD16 antibodies (data not shown). Because the anti-T cell marker reagents can bind nonspecifically to FcγR II receptors (CD32) on B cells, monocytes, and granulocytes,15 additional experiments were performed using two additional donors (not included in Table 1), using unconjugated anti-CD32 for blocking instead of anti-CD16 MoAbs. In both cases, the percentages of CD4+ and CD8+ or CD4−CD8− T cells were essentially unchanged in the unfractionated G-CSF–mobilized mononuclear cells. The flow cytometry patterns for one of these samples with and without anti-CD32 antibodies is shown in Fig 4. CD4+ and CD8+ T cells in the upper boxes were 12.1% and 10.9%, and CD4−CD8− T cells in the lower boxes were 2.1% and 2.0% in the absence or presence of the blocking antibodies, respectively.
Two-color analysis of immunofluorescent staining of T cells in the unfractionated cells from an additional donor in the presence or absence of unconjugated anti-CD32 MoAbs. In (A), cells were stained with PE-conjugated anti-CD4 and anti-CD8 MoAbs, and counter-stained with FITC-conjugated anti-CD3 antibodies in the absence of anti-CD32 antibodies. In (B), cells were stained in the presence of a saturating concentration of anti-CD32 antibodies (100 μg/mL).
Two-color analysis of immunofluorescent staining of T cells in the unfractionated cells from an additional donor in the presence or absence of unconjugated anti-CD32 MoAbs. In (A), cells were stained with PE-conjugated anti-CD4 and anti-CD8 MoAbs, and counter-stained with FITC-conjugated anti-CD3 antibodies in the absence of anti-CD32 antibodies. In (B), cells were stained in the presence of a saturating concentration of anti-CD32 antibodies (100 μg/mL).
A discrete population of CD4−CD8−CD3+ T cells was not detected in the unfractionated blood mononuclear cells of any of the donors in the current study before the administration of G-CSF, and the median percentage of CD4−CD8−CD3+ T cells was about 1% (data not shown). Thus, the populations of CD4−CD8−CD3+ T cells contained in the lower boxes of the panels in Fig 3B and C appear to be mobilized into the blood as a consequence of the administration of G-CSF.
To determine whether the light scatter characteristics of the discrete population of CD4−CD8−CD3+ T cells in the day 4 leukapheresis were different from that of the CD4+ and CD8+ T cells, the subsets of T cells were studied as shown in Fig 5. Figures 5A and B show examples of the boxes used for gating of the T-cell subsets in fraction C2 of one donor. The panels in Fig 5A show the light scatter characteristics of the gated CD4−CD8− T cells in the unfractionated (U) and fractionated (C1-C5) samples. Between 7% and 41% of the cells were within the “mononuclear/lymphocyte” gate of all cell samples (Fig 5B). The differences in light scatter characteristics of the CD4−CD8−, and CD4+ and CD8+ T cells were most striking in fractions C1 and C2, since the percentages of the two subsets in the “mononuclear/lymphocyte” gate varied by about eightfold (7% to 8% v 60% to 62%). The “mononuclear/lymphocyte” gate used for quantitation of T-cell subsets in the unfractionated samples (U) included 80% of CD4+ and CD8+ T cells, but excluded about 75% of CD4−CD8− T cells.
Light scatter analysis of unfractionated and density fractionated leukapheresis cells after gating on T-cell subsets. The two panels on the left show the boxes used to gate on CD4−CD8− T cells (A) or CD4+/CD8+ T cells (B). The remaining panels show the light scatter analysis of gated CD4+/CD8+ T cells or CD4−CD8− T cells in unfractionated or density fractionated cells from the same donor. Boxes in the panels labeled U and C1-C5 enclose the “mononuclear/lymphocyte” gate, and the percentages of cells enclosed in the boxes are shown.
Light scatter analysis of unfractionated and density fractionated leukapheresis cells after gating on T-cell subsets. The two panels on the left show the boxes used to gate on CD4−CD8− T cells (A) or CD4+/CD8+ T cells (B). The remaining panels show the light scatter analysis of gated CD4+/CD8+ T cells or CD4−CD8− T cells in unfractionated or density fractionated cells from the same donor. Boxes in the panels labeled U and C1-C5 enclose the “mononuclear/lymphocyte” gate, and the percentages of cells enclosed in the boxes are shown.
Recovery of CD34+ and T cells in day 4 leukapheresis samples.Table 1 compares the median recoveries of nucleated cells, CD34+ cells, CD4+ and CD8+ T cells, and CD4−CD8− T cells in the unfractionated and fractionated samples from the six donors. The highest density fraction (C5) contained a median of 68% of the nucleated cells, 85% of the CD4+ and CD8+ T cells and 73% of the CD4−CD8− T cells applied to the gradients. In contrast, C5 contained only 28% of the CD34+ cells. A comparison of the cell recoveries in the combined lower density fractions (C1-C4) as compared with all fractions (C1-C5) showed that only 13% (23 × 106) of the CD4+ and CD8+ T cells were recovered in the lower density fractions as compared with 62% (2.3 × 106) of the CD34+ cells. The ratio of CD34+ cells to CD4+ and CD8+ T cells was lowest in C5 (1:118), and increased to 1:6 in C1. In addition, the recovery of the CD4−CD8− T cells in combined C1-C4 was at least twice that of the CD4+ and CD8+ T cells. This was reflected in the higher ratios of CD4−CD8− to CD4+ and CD8+ T cells in C1-C4 compared with C5. The day 4 leukapheresis samples with a median of 13 × 109 nucleated cells contained a median of 64 × 106 CD34+ cells, 2.5 × 109 CD4+ and CD8+ T cells, and 486 × 106 CD4−CD8− T cells.
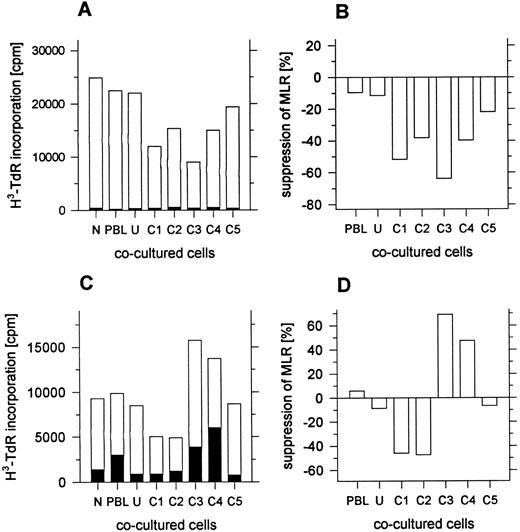
Suppression of the MLR by day 4 leukapheresis cells.Previous studies have shown that enriched or purified CD4−CD8− T cells from the marrow of mice and humans suppress the MLR.12 14 Since some of the day 4 leukapheresis samples contained a discrete population of CD4−CD8− T cells that were enriched in the low-density fractions, fractions from the density gradients were added to the MLR. Unfractionated cells from day 4 leukapheresis samples failed to respond in the MLR to irradiated allogeneic blood mononuclear cells from normal individuals (data not shown), and responder cells from other sources were used instead. Figure 6A shows the results of an MLR in which the responder cells (1 × 105) were obtained from the peripheral blood of the donor before administration of G-CSF. These donor mononuclear cells were cryopreserved, and then thawed at the time the MLR was initiated. Fresh allogeneic stimulator cells were obtained from a normal individual, and cocultured cell fractions were obtained from the day 4 donor leukapheresis sample. The open bars show the mean cpm of triplicate samples using the allogeneic irradiated stimulator cells (1 × 105) and the closed bars show the background cpm using autologous stimulator cells (1 × 105). The MLR without cocultured cells (N) and the control MLR (PBL) with cocultured irradiated responder cells (1 × 105) was about 25 × 103 cpm. Little change was observed by substituting the unfractionated leukapheresis cells or fraction C5 for the responder cells as cocultured cells. However, the MLR was suppressed about 40% to 70% when fractions C1-C4 were substituted (Fig 6A and B). A similar pattern was observed in four of four experiments.
Suppression of the MLR by unfractionated or fractionated cells from day 4 leukapheresis samples. In (A) and (B), responder cells were cryopreserved PBL from the leukapheresis donor obtained before administration of G-CSF. Open bars show the mean 3H-TdR incorporation of triplicate wells with allogeneic stimulator cells, and closed bars show the incorporation with autologous stimulator cells. In (C) and (D), responder cells were fresh PBL from an unrelated normal individual. N, no cocultured cells added to the MLR; PBL, irradiated responder cells added to the MLR; U and C1-C5, unfractionated or fractionated irradiated day 4 leukapheresis cells added to the MLR. Standard errors were less than 10% of the mean values shown.
Suppression of the MLR by unfractionated or fractionated cells from day 4 leukapheresis samples. In (A) and (B), responder cells were cryopreserved PBL from the leukapheresis donor obtained before administration of G-CSF. Open bars show the mean 3H-TdR incorporation of triplicate wells with allogeneic stimulator cells, and closed bars show the incorporation with autologous stimulator cells. In (C) and (D), responder cells were fresh PBL from an unrelated normal individual. N, no cocultured cells added to the MLR; PBL, irradiated responder cells added to the MLR; U and C1-C5, unfractionated or fractionated irradiated day 4 leukapheresis cells added to the MLR. Standard errors were less than 10% of the mean values shown.
Figure 6C shows an MLR assay using another donor in which responder cells were the fresh blood mononuclear cells from a normal individual unrelated to the donor, and allogeneic-irradiated stimulator cells were obtained from another normal individual. Irradiated cocultured cells from the day 4 leukapheresis sample were added to the MLR. The mean cpm of the MLR without cocultured cells was about 9 × 103. Addition of cocultured irradiated responder cells or unfractionated leukapheresis cells had little effect on the assay. However, when fractions C1 or C2 were added to the MLR, the response was suppressed about 40% (Fig 6C and D). In contrast, the addition of fractions C3 or C4 augmented the response by 40% to 60%. Purified CD4−CD8−CD3+ T cells from leukapheresis samples were not evaluated for their suppressive activity in this study.
DISCUSSION
Studies of autologous mobilized peripheral blood stem cell transplantation have shown that a single leukapheresis product can provide sufficient hematopoietic progenitors for rapid durable engraftment.16 The rapidity of engraftment of neutrophils and platelets is related to the dose of CD34+ cells per kilogram of the recipient's body weight infused after the myeloablative regimen. Optimum engraftment times require the infusion of at least 2 × 106 CD34+ cells/kg in autologous hosts,16 and perhaps a higher dose may be required in allogeneic hosts. In general, engraftment is more rapid using mobilized PBPCs instead of BM cells for transplantation.1-6,17 The difference is likely due to the dose of CD34+ cells infused, because the threshold dose of 2 × 106 CD34+ cells/kg is achieved in most recipients of blood cell but not of marrow cell transplants.7
Recent reports have indicated that engraftment of allogeneic-mobilized blood progenitor cell transplants is more rapid than with marrow cell transplants in HLA-identical siblings.4-6 It is surprising that the incidence of acute severe GVHD appears to be similar in blood progenitor cell as compared with marrow cell transplantation, because the dose of T cells per kilogram is at least 10-fold higher using the progenitor cell transplants.4-6 9 This may be due to changes in blood T-cell subsets and/or function induced by G-CSF, or to a plateau of GVHD beyond a threshold number of infused T cells.
The current study was performed to further characterize the CD34+ cells and T-cell subsets in the blood of normal individuals given G-CSF with regard to surface markers, density, and light scatter. Previous studies in humans have shown that low-density fractions of normal BM cells or mobilized peripheral blood mononuclear cells from lymphoma patients given cyclophosphamide and G-CSF are enriched for hematopoietic precursors and depleted of CD4+ and CD8+ T cells.7,14 Studies in mice have shown that low-density fractions of marrow and spleen cell mixtures have a markedly reduced capacity to induce severe GVHD, but still mediate graft-versus-leukemia activity.18
In the current study, six normal individuals were given G-CSF daily for 4 days, and a leukapheresis of 1 blood volume was performed on the fourth day. During the 4-day interval, the mean WBC count increased to about 50 × 106/L, and the concentration of CD34+ cells increased from undetectable levels to about 75 × 106/L. The median CD34+ cell content of the day 4 leukapheresis was 64 × 106, and a median of 13 × 109 nucleated cells was collected. By extrapolation, a 4 blood volume leukapheresis would be expected to contain about 256 × 106 CD34+ cells, and would provide sufficient CD34+ cells to exceed 2 × 106/kg of recipient body weight.
Fractionation of the leukapheresis samples on discontinuous Percoll density gradients (40% to 50% Percoll stock solution) showed that about two thirds of the nucleated cells were recovered in the highest density fraction (C5), and progressively fewer cells were recovered as the density decreased. As in previous studies, the CD34+ cells were enriched in the low-density fractions C1, C3, and C4 about threefold to fourfold.
Immunofluorescent staining of T-cell subsets in the different density fractions of the day 4 leukapheresis sample showed that the percentage of CD4+ and CD8+ T cells was highest in the high-density fraction which contained a median of about 85% of these T cells placed on the gradients. The total number of CD4+ and CD8+ T cells contained in the day 4 leukapheresis sample was 2.5 × 109 cells. Thus, the ratio of CD4+ and CD8+ T cells to CD34+ cells in the unfractionated sample was about 40:1. This ratio is about 10-fold higher than that (4:1) reported for BM cells depleted of red blood cells after separation on hetastarch, and about sevenfold higher than that (6:1) of marrow mononuclear cells after separation on Ficoll-Hypaque density gradients.7 However, the ratio (8:1) of CD4+ and CD8+ T cells to CD34+ cells in the combined low-density fractions (C1 to C4) of the day 4 leukapheresis sample was similar to that of the marrow mononuclear cells.
Evaluation of CD4−CD8−CD3+ T cells in the leukapheresis samples showed three patterns. One had a discrete population of cells observed by two-color flow cytometric analysis in the unfractionated cells as well as in all density fractions without enrichment in the low-density fractions. A second had a small discrete population in the unfractionated cells with marked enrichment in the lowest density fractions (C1-C3). The third showed no discrete population in the unfractionated or fractionated cells. The median percentage of CD4+ and CD8+ T cells was about fivefold to sixfold higher than that of CD4−CD8− T cells in the unfractionated and high-density cells, and about twofold to threefold higher in the low-density cells. The light scatter characteristics of CD4−CD8− T cells were markedly different from those of the CD4+ and CD8+ T cells. As much as 90% of the CD4−CD8− T cells were outside the “mononuclear/lymphocyte” gate, but the majority of the CD4+ and CD8+ T cells in all fractions were within this gate.
Staining of leukapheresis samples with both anti-CD3 and anti-TCRαβ MoAbs showed that almost all of the CD4−CD8−CD3+ T cells expressed the αβTCR. The ratio of CD4+ and CD8+αβ T cells to CD4−CD8−αβ T cells in the blood mononuclear cells of normal donors before G-CSF administration was about 70:1. The balance of these subsets in the leukapheresis samples from donors given G-CSF was altered markedly. This change was in part due to a mobilization of CD4−CD8− T cells in the blood in the six donors, because a median of 486 × 106 CD4−CD8− T cells was present in a median of 13 × 109 nucleated cells in the leukapheresis samples. This number of nucleated cells represented only about 7% of the WBCs present in a single blood volume of about 5 L (WBC count of 40 × 109/L × 5 L = 200 × 109) on the day of leukapheresis. In contrast, 1 blood volume from a normal individual contains about 25 × 109 WBCs, of which less than 100 × 106 are CD4−CD8− T cells (≤1% × 2 × 109 lymphocytes/L × 5 L). Thus, the day 4 leukapheresis samples from donors given G-CSF contained about four to five times the total number of CD4−CD8− T cells in 1 blood volume of normal individuals. The leukapheresis samples contained a median of 2.5 × 109 CD4+ and CD8+ T cells. This represents only about 37% of the calculated number of CD4+ and CD8+ T cells in 1 blood volume from a normal individual (68% × 2 × 109 lymphocytes/L × 5 L = 6.8 × 109). The sources of the CD4−CD8− T cells in the leukapheresis samples is unknown, and their mobilization may be caused by the administration of G-CSF, as well as the leukapheresis procedure itself. The marrow is a likely source of both hematopoietic progenitors, as well as CD4−CD8− T cells, since both types of cells are generated at a high rate in the mouse BM.19
Because CD4−CD8−αβ+ T (“natural suppressor”) cells from the marrow of mice and humans are able to suppress the MLR,12 14 leukapheresis cells from the different density fractions were assayed for their capacity to suppress the MLR in the current study. Cryopreserved blood mononuclear cells obtained from the donors before G-CSF administration and fresh blood mononuclear cells from normal individuals were used as responder cells. Allogeneic irradiated stimulator cells were obtained from normal individuals unrelated to the responder cell donors. Leukapheresis cells were irradiated also so that they would not respond to the MLR. Addition of the leukapheresis cells in the low-density fractions suppressed the MLR using responder cells from both sources by about 40% to 70%. Little suppression was observed with the unfractionated cells. This was not surprising, since about 85% of the unfractionated cells are high-density cells that did not suppress significantly. The isolated CD4−CD8− T cells obtained from the low-density fractions were not assayed for suppression in the present study.
In view of the altered balance of T-cell subsets favoring CD4−CD8− T cells in the leukapheresis samples, and studies in mice which show that fresh and cloned CD4−CD8− T cells suppress lethal GVHD,12 20 it is possible that the presence of these cells in G-CSF–mobilized blood ameliorates GVHD. The low-density fractions may further reduce the likelihood of GVHD, because these fractions were able to suppress the MLR.
Supported in part by US Public Health Service Grants (PO1-CA-49605 and R01-CA-55793) awarded by the National Cancer Institute, Department of Health and Human Services. R.S.N. is supported in part by a grant from the Fundacion Internacional Jose Carreras para la Lucha Contra La Leucemia (FIJC-92/INT-2). C.R.K.-G. was supported by a grant from the Dr Mildred Scheel Foundation for Cancer Research and by a gift from Activated Cell Therapy, Inc, Mountain View, CA.
Address reprint requests to Samuel Strober, MD, Division of Immunology and Rheumatology, Stanford University Medical Center, 300 Pasteur Dr, Room S105B, Stanford, CA 94305-5111.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal