Abstract
Retinoic acid receptors (RARs) α, β, and γ contain retinoic acid response elements (RAREs) in their promoter regions and respond to their own activation, thus forming an autoregulatory loop. We generated transgenic mice that expressed an antisense construct of the RARα. Homozygous transgenic mice demonstrated 30% to 80% reduction in RARα protein expression in various tissues. Unlike RARα null mice generated by knockout, our antisense mice demonstrated significant compensatory increases in the expression of RARβ and RARγ proteins. Coarse fur, male sterility, and low body weight were other abnormalities observed in these mice. Most importantly, lymphoma developed in 44% of our homozygous transgenic mice at an early stage of life. These data suggest that RARα is necessary for appropriate response of the RARβ and RARγ genes to physiologic changes and deregulation of the RARα in transgenic mice, which resulted in upregulation of RARβ and RARγ, can be associated with lymphomagenesis. Thus, the data support the hypothesis that a balance among the RARs is necessary for appropriate response to various homeostatic needs.
THE VITAMIN A–DERIVED hormones and their natural and synthetic analogues (retinoids) affect a large number of biological processes, including growth, differentiation, morphogenesis, metamorphosis, development, vision, immunity, and fertility.1-6 Retinoids have also shown promise in the treatment and prevention of various tumors.1,3 4
The effects of retinoids are believed to be mediated by two families of receptors, retinoic acid receptors (RARs) and retinoid X receptors (RXRs).3 The RAR family includes RARα, RARβ, and RARγ, which bind all-trans–retinoic acid and to 9-cis-retinoic acid with high affinity.3,7 The RXR family includes RXRα, RXRβ, and RXRγ, which specifically binds to 9-cis–retinoic.3,8 These two families belong to the steroid/thyroid hormone receptor superfamily, which is a family of ligand-induced transcription-regulation factors. RARs form homodimers or heterodimers with RXRs to bind specifically to DNA sequences.9
Each of the RARs is expressed in distinct patterns throughout development and in the mature organism.3,10 Furthermore, genes encoding these receptors show greater conservation between species than between each other.4 11 These observations suggest that various RARs mediate different functions.
The targets of the retinoid-activated receptors are highly regulated genes. These genes contain specific DNA sequences called retinoic acid response elements (RAREs) that contain direct repeats (AGGTCA) separated by 3, 4, or 5 nucleotides.3,4,10,12,13 Interestingly, RARs themselves contain RAREs in their promoters and respond to RAR-mediated activation forming an autoregulatory loop. The effects of the retinoids may be amplified through the upregulation of RARs in this autoregulatory loop, so that small increases in retinoids may result in disproportionately greater biologic changes. It is believed that the remarkable difference in differentiation seen in the developing limb bud upon minimal difference in the concentration of retinoic acid is the result of this functional amplification.3,4 14
Complete abolition of the expression of the RARα gene in mice by homologous recombination and “knockout” leads to changes similar to those seen in mice fed a vitamin A-deficient diet.11 Approximately 90% of RARα null mice die before the age of 2 months. This indicates that RARα plays a role in the maintenance of some homeostatic processes. These RARα null mice do not demonstrate compensatory increases in the expression of RARβ or RARγ.11 Both RARβ and RARγ contain RARE in their promoter regions, and the complete absence of RARα protein in these null mice may alter their ability to respond to physiologic alterations.
To investigate the effects of a significant change in (but not the complete absence of ) RARα expression on various homeostatic processes, we generated transgenic mice expressing an antisense RARα gene. Expression of antisense RARα in these transgenic mice leads to 30% to 80% reduction in the level of RARα protein in various tissues. Interestingly, these antisense transgenic mice showed compensatory increases in the RARβ and RARγ proteins. These mice were characterized by coarse fur, low body weight, and male sterility, and lymphoma developed in 44% of these mice. These data expand our understanding of the regulatory function of RARα and suggest that RARα deregulation may be involved in lymphomagenesis.
MATERIALS AND METHODS
Generation of transgenic mice.We inserted the human RARα cDNA in the sense and antisense orientation at the Sal I site of the expression vector pMAMneo (CLONTECH, Palo Alto, CA) using an appropriate linker. This expression vector contains the RSV/MMTV-LTR (Rous sarcoma virus/mouse mammary tumor virus-long terminal repeat) promoter/enhancer and SV40 splicing/polyadenylation sites. The inserted human RARα cDNA is 3 kb in length and is highly homologous to the mouse RARα cDNA. The DNA construct was isolated from the bacterial sequence by digestion with EcoRI and Pvu I and microinjected into fertilized mouse eggs as previously described.15 Briefly, DNA was microinjected into the male pronucleus of fertilized F1 hybrid eggs from SWRxSJL mice. The injected eggs were incubated in Whittens medium at 37°C and transferred into the oviduct of a CD-1 pseudopregnant foster mother. Generated mice were first screened using the polymerase chain reaction (PCR), then confirmed by Southern blot analysis. Six independent transgenic lines were generated carrying the antisense construct and four lines were generated carrying the sense RARα construct. These mice were intercrossed and intracrossed to generate homozygous mice.
DNA isolation and analysis.Genomic DNA was prepared from the tails and lymphoma tissues of the transgenic mice. Transgenic mice were screened by PCR analysis. The 3′ primer (5′-GATGCTGCGGCGGAAGAAGCCCTT-3′) corresponds to RARα and the 5′ primer (5′-ACTCACCATAGGGACCAAGCT-3′) corresponds to MMTV. The amplified fragment was approximately 469 bp and was resolved on denaturing polyacrylamide gel as previously described.15 PCR-positive transgenic mice were confirmed by Southern blot analysis. When the intensity of the transgene band was compared with that of the mouse α-globin band seen by PCR, we estimated the transgene copies to be 2, 4, and 8 in three lines that were further studied. DNA was digested with BamHI and Sal I, electrophoresed through a 0.8% agarose gel, blotted onto a nitrocellulose membrane, and probed with a 32P-labeled 0.5 Kb Pst I fragment of the human RARα cDNA. Southern blotting was performed as previously described.16 As expected, we detected a 2.1-kb fragment upon digestion with BamHI and Sal I. Digestion with BamHI showed a relatively strong 3.5-kb fragment that is expected to span two adjacent fragments of the transgene, which suggests that the transgenes are integrated in a tandem repeat (head to tail) fashion. Considering the low copy number of transgenes 2, 4, and 8, it is most likely that the integration of transgenes was in a single site. Homozygous mice were determined based on the intensity of the transgene bands using densitometric analyses. Genomic DNA from lymphoma samples was digested with EcoRI, BamHI, and HindIII and analyzed by Southern blot analysis using the 1-kb Xba I-EcoRI mouse Ig JH3-4 heavy-chain genomic probe.17
RNA isolation and analysis.Total RNA was extracted from various tissues of transgenic mice by the guanidine hydrochloride method as previously described.18 RARα antisense mRNA expression was detected using reverse transcription (RT)-PCR using the following two primers: 5′-TGTGTCGGCCCATCTACTGTC-3′ and 5′-GAGTTTTCCCAGACCTGGCTC-3′. Expression of the RARα transgene was detected using the following two primers: 5′-TGAAGCCCACCAGAGCCCCCT-3′ and 5′-GATGCTGCGGCGGAAGAAGCC-3′. The 3′ primer of this set is a unique primer that amplifies only the human mRNA transgene. Expression of the endogenous mouse RARα gene was detected using the following primers: 5′-CAAGACAAATCATCCGGCTAC-3′ for the 5′ primer and 5′-GTACTTGCCCAGCTGGCAGAG-3′ for the 3′ primer in the PCR and RT. The amplification product was 338 bp. The following primers were used for RT/PCR of the mouse β-actin: 5′-CCATCCTGCGTCTGGACCTGGCTG-3′ and 5′-GCTCGTTGCCAATAGTGATGACCT-3′. These primers amplify a 240-bp fragment of β-actin mRNA. RT was performed using the 3′ primers. PCR was performed using the following cycling conditions: denaturation at 95°C for 5 minutes, followed by 30 cycles of 58°C for 30 seconds, 74°C for 2 minutes, and 94°C for 1 minute. In each PCR reaction, one of the two primers of each set was end-labeled with [γ-32P] adenosine triphosphate (ATP) in the presence of T4 polynucleotide kinase. The amplification products were resolved on denaturing polyacrylamide gels as previously described.15 Coamplification of β-actin and RARα was used as a semiquantitative assay to compare the expression of RARα in hemizygous mice with that in homozygous transgenic mice. The coamplification was always performed side by side with the amplification of RARα alone and β-actin alone and the coamplification was accepted when the intensity of the bands in the coamplification tube was comparable to that seen in the tubes with RARα alone and β-actin alone. The linearity of such an assay in the condition described here was previously reported.15
Protein isolation and analysis.Fresh tissue from various organs of normal and transgenic mice was homogenized in ice-cold buffer (20 mmol/L Tris/HCL, PH 7.2, 1 mmol/L EDTA, 1 mmol/L EGTA, 0.1 mol/L NaCl, 1 mmol/L phenylmethyl sulfonyl fluoride [PMSF ], 0.1% aprotinin, 0.1% leupeptin, 0.1% pepstatin, and 0.1% Triton 100 X). After solubilization on ice for 1 hour, the lysate was centrifuged at 35,000 rpm for 45 minutes, and the supernatant was collected. The protein concentration was estimated by BioRad (Hercules, CA) standard assay, and 200 mg of each extract was run on a 9.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel and stained with Coomassie blue R-250 to check the protein profile. After necessary adjustment for protein concentration was made, 200 mg of protein extract was electrophoresed on a 9.5% SDS-PAGE gel and transferred to nitrocellulose membrane. The nitrocellulose membrane was blocked with 5% nonfat milk in phosphate-buffered saline (PBS) solution containing 0.1% Tween 20 for 6 hours at room temperature. The blot was then incubated with 1 μg/mL of mouse monoclonal anti-RARα antibody (IgG1, MA1-810), RARβ antibody (IgG2a, MA1-811) (both from Affinity Bioreagents, Neshanic Station, NJ), and RARγ (a generous gift from Dr P. Chambon, Strasbourg Cedex, France), in 5% bovine serum albumin overnight at 4°C. After washing with PBS containing 0.1% Tween 20, the membrane was incubated with sheep antimouse-horseradish peroxidase (HRP)–IgG conjugate for 2 hours at room temperature. Immunoblots were then washed, and immunoreactive bands were visualized with an enhanced chemilluminescence detection system according to the manufacturer's instructions (Amersham, Arlington Heights, IL). The autoradiographs were scanned, and bands were quantified using Scan Analysis software from Biosoft (Cambridge, UK) and a Macintosh-based scanner and computer (Cupertino, CA). Some membranes were stripped and reprobed with another antibody using the procedures recommended by the manufacturer (Amersham, Arlington Heights, IL). To block nonspecific binding of the secondary antibody (sheep antimouse IgG), membrane was first blocked with nonfat milk then incubated with sheep antimouse IgG for 2 hours at room temperature before incubation with the RARα and RARβ antibodies. This step was adequate to block the nonspecific high molecular bands seen on some blots.
Tissue sections.Tissues for light microscopy were fixed in 10% neutral buffered formalin and processed for paraffin embedding. The sections were stained with hematoxylin and eosin.
RESULTS
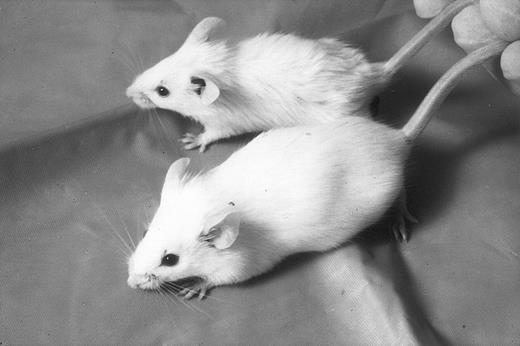
Generation of transgenic mice expressing an RARα sense and antisense gene.In one set of experiments, we microinjected into mouse oocytes a human sense RARα DNA construct and in a second set of experiments, we injected the antisense RARα DNA construct. The human 3-kb full-length RARα cDNA was inserted in sense and antisense orientation downstream of the RSV/MMTV-LTR promoter/enhancer. Four founder transgenic lines expressing sense RARα were generated from the first set of experiments, and expression of sense RARα mRNA was detected using RT/PCR (data not shown). No phenotypic abnormality was observed in hemizygous or homozygous sense RARα mice. Six founder transgenic lines carrying the antisense construct were generated from the second set of experiments. Five of the six founders expressed RARα antisense mRNA as detected by RT/PCR (data not shown). Only three of the five lines designated here as lines 1, 2, and 3, were analyzed further. None of the hemizygous antisense RARα mice displayed phenotypical abnormality despite a variation in the copy number of the transgene (2, 4, and 8 copies). However, homozygous antisense transgenic mice of the three lines displayed a distinct phenotype (Fig 1). All homozygous mice demonstrated coarse fur and low body weight. We compared 20 randomly selected transgenic mice with 20 littermates and found that homozygous transgenic mice weighed an average of 15% to 20% less than their normal or transgenic heterozygous littermates at 4 weeks of age. The transgenic mice remained smaller throughout their lives and demonstrated coarse fur (Fig 1). Homozygous mice obtained by intercrossing line 1 and line 2 showed a similar phenotype. None of the transgenic homozygous male mice was fertile because, although they were constantly caged with females, they failed to sire litters.
Appearance of RARα antisense mice. Homozygous RARα antisense transgenic mice are smaller and have coarse fur; back, transgenic mouse; front, normal littermate.
Appearance of RARα antisense mice. Homozygous RARα antisense transgenic mice are smaller and have coarse fur; back, transgenic mouse; front, normal littermate.
Reduction of expression of RARα mRNA and protein in antisense transgenic mice.Total RNAs from various tissues from normal littermate controls, transgenic hemizygous mice, and homozygous mice from three transgenic lines were analyzed for the level of expression of RARα mRNA by RT/PCR. The level of RARα mRNA was compared with β-actin mRNA in three transgenic lines. In each amplification, one of the primers of each set was 32P-end–labeled so that the cDNA products could be directly visualized by autoradiography. As shown in Fig 2, significant reduction in the RARα amplification product as compared with the β-actin amplification product is noted in hemizygous and homozygous transgenic mice from the three lines.
Comparison of RARα and β-actin mRNA levels in normal (−/−), hemizygous transgenic (T/−), and homozygous transgenic (T/T) mice in three transgenic lines. Representative RT-PCR coamplification of RARα and β-actin mRNAs in kidneys.
Comparison of RARα and β-actin mRNA levels in normal (−/−), hemizygous transgenic (T/−), and homozygous transgenic (T/T) mice in three transgenic lines. Representative RT-PCR coamplification of RARα and β-actin mRNAs in kidneys.
Although RT/PCR assay is not quantitative, more reduction in RARα mRNA is seen in homozygous mice as compared with hemizygous mice. Figure 2 shows the level of RARα mRNA reduction in kidney tissue samples. Similar levels of reduction were noted in most tissue samples that were examined.
The level of expression of RARα protein in homozygous antisense transgenic mice was examined using immunoblot analysis. In Fig 3, we compared the RARα protein levels in the skin, lung, testis, spleen, liver, and kidney of homozygous and hemizygous transgenic mice with those in the same tissues of normal littermate mice resulting from intercrossing line 1 hemizygous mice. The Western blot analysis demonstrated striking reductions in the levels of RARα protein in the various tissues of the homozygous transgenic mice and a lesser degree in those of hemizygous mice (Fig 3). Figure 4A is representative Western blot analysis showing changes in RARα protein expression in homozygous transgenic mice generated from intercrossing line 2 hemizygous mice. The most striking reduction (almost complete absence or 40% to 85% reduction, as determined by densitometer) in RARα protein level was observed in lung, testis, liver, and kidney tissues. No significant variation was observed between line 2 and line 3. Equal loading of protein from transgenic and normal mice was confirmed by staining filters with amido black stain. A similar pattern of reduction of expression was detected in homozygous transgenic mice resulting from interline crossing.
Comparison of RARα protein in normal (−/−), hemizygous transgenic (T/−), and homozygous transgenic (T/T) mice generated from line 1 in various tissues. The Western blot analysis shows marked reduction in RARα in homozygous mice and less reduction in RARα in hemizygous mice.
Comparison of RARα protein in normal (−/−), hemizygous transgenic (T/−), and homozygous transgenic (T/T) mice generated from line 1 in various tissues. The Western blot analysis shows marked reduction in RARα in homozygous mice and less reduction in RARα in hemizygous mice.
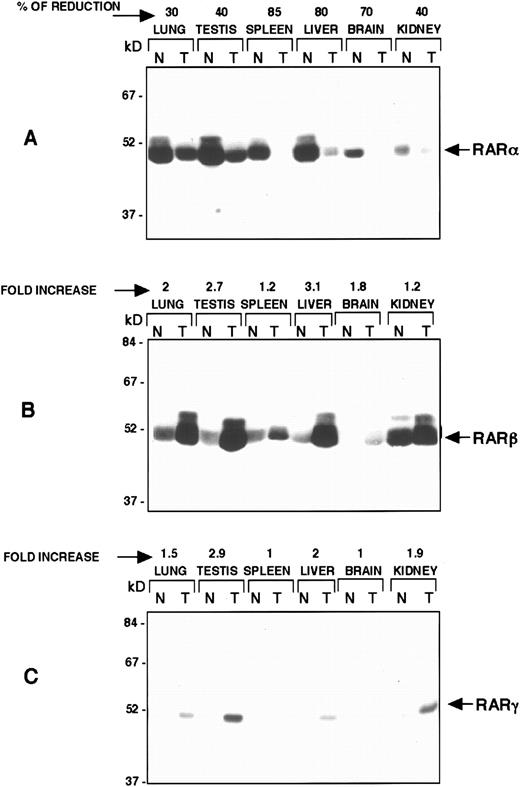
Reduction of RARα protein by the RARα antisense construct and compensatory increase in the RARβ and RARγ proteins. Western blot analysis of protein isolated from normal control (N) mice and transgenic (T) mice (line 2) from the indicated organs. Equal amounts of protein were resolved on SDS-PAGE, transferred to nitrocellulose membrane, and probed with specific RARα (A), RARβ (B), and RARγ (C) antisera as indicated. The percentage of RARα reduction in each tissue is indicated in (A) and the levels of increase in RARβ and RARγ proteins are shown in (B and C).
Reduction of RARα protein by the RARα antisense construct and compensatory increase in the RARβ and RARγ proteins. Western blot analysis of protein isolated from normal control (N) mice and transgenic (T) mice (line 2) from the indicated organs. Equal amounts of protein were resolved on SDS-PAGE, transferred to nitrocellulose membrane, and probed with specific RARα (A), RARβ (B), and RARγ (C) antisera as indicated. The percentage of RARα reduction in each tissue is indicated in (A) and the levels of increase in RARβ and RARγ proteins are shown in (B and C).
Compensatory increase in the RARβ and RARγ proteins.To test whether there was a compensatory increase in the expression of RARβ or RARγ in the homozygous mice, we compared the levels of expression of RARβ and RARγ in various tissues of the transgenic mice and normal control mice (Fig 4). We used Western blot analysis and monoclonal antibodies to study RARγ and RARβ levels. There was significant compensatory (threefold in some tissues) increase in the levels of RARβ and RARγ in most tissues examined (lung, testis, spleen, liver, brain, and kidney). Figure 4 shows representative Western blots showing tissues from line 2. Using a densitometer, we detected a twofold increase in the RARβ in lung and a 3.1-fold increase in liver (Fig 4). We detected similar levels of increase in RARγ in lung, testis, and kidney (Fig 4). Equal loading of protein from transgenic and normal mice was confirmed by staining filters with amido black stain. In some experiments, the Western blot filters were stripped and reprobed with antibodies for RARα, and the inverse relationship between RARα protein levels and RARβ and RARγ (data not shown) protein levels was confirmed. This contrasts with the previously reported lack of compensatory increase in RARβ mRNA in RARα null mice.
Glandular atrophy and squamous metaplasia.The transgenic mice had coarse fur (see Fig 1). Microscopic examination of skin sections showed significant atrophy in sebaceous glands and hair follicles (Fig 5A and B). Empty hair follicles and no inflammatory response in the dermis represented the most striking histologic abnormality in the skin. In addition to the atrophy in the hair follicles and the surrounding sebaceous gland, a thick, fibrous sheath was noted in the dermis.
Histologic changes in the RARα antisense mice as compared with normal littermates. Sections of skin of normal (A) and transgenic (B) mice showing significant atrophy and degeneration in pilosebaceous units (arrowhead) in transgenic mice (B) as compared with normal mice (A). (C) and (D) are sections from normal and transgenic mice, respectively. Poor order of spermatogenesis (arrow) and few spermatozoa are seen in the seminiferous tubules of transgenic mice as compared with those of normal mice. Sections of epididymides of normal (E) and transgenic (F ) mice show marked squamous metaplasia (arrow) in transgenic mice, but pseudostratified epithelium in normal mice (arrow).
Histologic changes in the RARα antisense mice as compared with normal littermates. Sections of skin of normal (A) and transgenic (B) mice showing significant atrophy and degeneration in pilosebaceous units (arrowhead) in transgenic mice (B) as compared with normal mice (A). (C) and (D) are sections from normal and transgenic mice, respectively. Poor order of spermatogenesis (arrow) and few spermatozoa are seen in the seminiferous tubules of transgenic mice as compared with those of normal mice. Sections of epididymides of normal (E) and transgenic (F ) mice show marked squamous metaplasia (arrow) in transgenic mice, but pseudostratified epithelium in normal mice (arrow).
Microscopic and gross examination of the vas deferens of infertile homozygous male mice showed no obvious obstruction. Histologic examination of the testes of homozygous transgenic mice showed some atrophy in some of the seminiferous tubules (Fig 5D). Some seminiferous tubules demonstrated morphologic changes similar to those described in incomplete spermatocytic arrest syndrome.19 Normal maturation of spermatogonia into spermatozoa seems to be progressing in poor order as shown in Fig 5D. Spermatogenic cells in normal mice differentiate progressively from the basal region of the tubule to the lumen (Fig 5C). In contrast, in transgenic mice, immature germ cells can be seen in the lumen and more differential cells (small with abundant cytoplasm) are seen in the basal area (Fig 5D). The tubules were slightly reduced in diameter. The majority of the tubules contained sloughed spermatogenic cells. The sloughed cells consisted primarily of spermatocytes admixed with mature elements. Based on examining seminiferous tubules of 4 different transgenic mice (1 mouse from line 1, 2 from line 2, and 1 from line 3), it appears that more than 50% of seminiferous tubules were affected. Rare tubules were surrounded by mild fibrosis (one or two in each histologic section). Sertoli and Leydig cells appeared normal. Focal squamous metaplasia was observed in the epididymides (Fig 5F ) and the prostate and bulbourethral glands of some male mice.
Lymphoma development in transgenic mice.The most important abnormality we observed in these antisense homozygous transgenic mice was the development of tumors in a significant number of mice at an early stage of life. As shown in Table 1, most tumors were lymphomas. Lymphomas developed in 15 (44%) of the 34 surviving homozygous mice, and sweat gland tumors developed in two mice. Most of the lymphomas formed in the abdomen (Fig 6A). However, one lymphoma developed in an orbital cavity, and three were found in the thymus (Fig 6B). One lymphoma developed in the axilla. Histologically, most lymphomas appeared to be of low-grade and of small cell type (Fig 6C and F ). Some lymphomas (thymus, orbital, and axillary) showed aggressive high-grade morphology with larger cells and more open chromatin (Fig 6D). Most lymphomas were of B-cell lineage as determined by restriction of immunoglobulin genes (Fig 7). Furthermore, IgG gene rearrangement study demonstrates clonality of the tumors and confirms that these tumors are malignant and not a benign lymphoid hyperplasia. One case of lymphoma involving the thymus was Thy-1.2 and CD-3 positive and showed no evidence of immunoglobulin gene rearrangement (Fig 7). Lymphoma developed in two heterozygous founders of the transgenic mice at a late stage of life.
Tumor Formation in RAR α Antisense Mice
| Case . | Line . | Sex . | Genotype . | Age (mo) . | Tumor (lineage) . | Site . |
|---|---|---|---|---|---|---|
| 1 | 1 | M | Homozygous | 2 | Lymphoma (ND) | Thymus |
| 2 | 1 | F | Homozygous | 3 | Lymphoma (T cell) | Thymus |
| 3 | 3 | M | Homozygous | 6 | Lymphoma (ND) | Eye |
| 4 | 2 | M | Homozygous | 7 | Lymphoma (B cell) | Abdomen |
| 5 | 1 | F | Homozygous | 5 | Lymphoma (T cell) | Thymus |
| 6 | 3 | M | Homozygous | 7 | Lymphoma (ND) | Abdomen |
| 7 | 2 | F | Homozygous | 9 | Lymphoma (B cell) | Abdomen |
| 8 | 1 | F | Homozygous | 8 | Lymphoma (ND) | Abdomen |
| 9 | 1 | M | Homozygous | 6 | Lymphoma (ND) | Axillary |
| 10 | 2 | F | Homozygous | 10 | Lymphoma (B cell) | Abdomen |
| 11 | 2 | F | Homozygous | 4 | Lymphoma (ND) | Abdomen |
| Carcinoid | Lung | |||||
| 12 | 2 | M | Homozygous | 4 | Lymphoma (ND) | Abdomen |
| 13 | 1 × 2 | F | Homozygous | 4 | Lymphoma (B cell) | Abdomen |
| 14 | 1 | F | Homozygous | 10 | Sweat gland | Axillary |
| 15 | 1 × 2 | F | Homozygous | 4 | Lymphoma (B cell) | Abdomen |
| 16 | 3 | M | Homozygous | 12 | Lymphoma (ND) | Abdomen |
| 17 | 1 | F | Homozygous | 16 | Sweat gland | Axillary |
| 18 | 1 | F | Hemizygous* | 18 | Lymphoma (B cell) | Abdomen |
| 19 | 2 | M | Hemizygous* | 18 | Lymphoma (ND) | Abdomen |
| Case . | Line . | Sex . | Genotype . | Age (mo) . | Tumor (lineage) . | Site . |
|---|---|---|---|---|---|---|
| 1 | 1 | M | Homozygous | 2 | Lymphoma (ND) | Thymus |
| 2 | 1 | F | Homozygous | 3 | Lymphoma (T cell) | Thymus |
| 3 | 3 | M | Homozygous | 6 | Lymphoma (ND) | Eye |
| 4 | 2 | M | Homozygous | 7 | Lymphoma (B cell) | Abdomen |
| 5 | 1 | F | Homozygous | 5 | Lymphoma (T cell) | Thymus |
| 6 | 3 | M | Homozygous | 7 | Lymphoma (ND) | Abdomen |
| 7 | 2 | F | Homozygous | 9 | Lymphoma (B cell) | Abdomen |
| 8 | 1 | F | Homozygous | 8 | Lymphoma (ND) | Abdomen |
| 9 | 1 | M | Homozygous | 6 | Lymphoma (ND) | Axillary |
| 10 | 2 | F | Homozygous | 10 | Lymphoma (B cell) | Abdomen |
| 11 | 2 | F | Homozygous | 4 | Lymphoma (ND) | Abdomen |
| Carcinoid | Lung | |||||
| 12 | 2 | M | Homozygous | 4 | Lymphoma (ND) | Abdomen |
| 13 | 1 × 2 | F | Homozygous | 4 | Lymphoma (B cell) | Abdomen |
| 14 | 1 | F | Homozygous | 10 | Sweat gland | Axillary |
| 15 | 1 × 2 | F | Homozygous | 4 | Lymphoma (B cell) | Abdomen |
| 16 | 3 | M | Homozygous | 12 | Lymphoma (ND) | Abdomen |
| 17 | 1 | F | Homozygous | 16 | Sweat gland | Axillary |
| 18 | 1 | F | Hemizygous* | 18 | Lymphoma (B cell) | Abdomen |
| 19 | 2 | M | Hemizygous* | 18 | Lymphoma (ND) | Abdomen |
We generated 42 homozygous (17, line 1; 13, line 2; 8, line 3, and 4, crossing 1 × 2) transgenic mice. Eight died at 2 to 4 months of age (4, line 1; 2, line 2; 1 line 3, and 1, crossing 1 × 2). Of the remaining 34, 15 (44%) developed lymphomas and two developed sweat gland tumors.
Abbreviation: ND, not determined.
Only 2 of 86 hemizygous mice developed lymphomas.
Development of lymphoma in transgenic mice. (A) and (B) depict the gross appearance of malignant lymphomas developed in transgenic mice. In (A), the tumor (T) was abdominal and compressed, but did not invade the intestines (I). In (B), the tumor (T) involved the thymus and invaded lungs (L) and heart (H). (C) and (D) show representative histologic sections of the tumors shown in (A) and (B), respectively. Diffuse small lymphocytic (low-grade) lymphoma is seen in the abdominal tumor, and a more aggressive lymphoma with open chromatin (lymphoblastic-like) is seen in the thymus tumor. (E) Shows lymphoma invading skeletal muscles. (F ) Shows another pattern of lymphoma observed in abdominal tumors with small cells and condensed chromatin.
Development of lymphoma in transgenic mice. (A) and (B) depict the gross appearance of malignant lymphomas developed in transgenic mice. In (A), the tumor (T) was abdominal and compressed, but did not invade the intestines (I). In (B), the tumor (T) involved the thymus and invaded lungs (L) and heart (H). (C) and (D) show representative histologic sections of the tumors shown in (A) and (B), respectively. Diffuse small lymphocytic (low-grade) lymphoma is seen in the abdominal tumor, and a more aggressive lymphoma with open chromatin (lymphoblastic-like) is seen in the thymus tumor. (E) Shows lymphoma invading skeletal muscles. (F ) Shows another pattern of lymphoma observed in abdominal tumors with small cells and condensed chromatin.
Clonality of the lymphoid tumors as demonstrated by immunoglobulin gene rearrangement studies. Southern blot analysis of some lymphoid tumors showing immunoglobulin gene rearrangement. Three different lymphomas (labeled 1-3) were digested with EcoRI and HindIII as indicated. C, control liver DNA from one of the mice; T, DNA from a T-cell lymphoma showing no rearrangement for the immunoglobulin gene. Germline band is indicated by a dash on the side.
Clonality of the lymphoid tumors as demonstrated by immunoglobulin gene rearrangement studies. Southern blot analysis of some lymphoid tumors showing immunoglobulin gene rearrangement. Three different lymphomas (labeled 1-3) were digested with EcoRI and HindIII as indicated. C, control liver DNA from one of the mice; T, DNA from a T-cell lymphoma showing no rearrangement for the immunoglobulin gene. Germline band is indicated by a dash on the side.
DISCUSSION
There are few examples of the use of antisense DNA constructs in transgenic animals.20-24 Pepin et al23 used an antisense DNA construct to partially knock out the expression of the type II glucocorticoid receptor in transgenic mice and demonstrated significant reduction (50% to 70%) in the expression of the receptor mRNA. The guanosine triphosphate-binding regulatory protein (Gai2 ) was partially knocked out (<5% Gai2 protein in some tissues) in transgenic mice using a construct containing only 39 bp of the Gai2 gene upstream of the phosphoenolpyruvate carboxykinase (PEPCK) gene.25 In the study reported here, we focused on producing transgenic mice that expressed antisense RARα mRNA. We found that the expression of this antisense RARα mRNA led to partial knockout of RARα mRNA and protein expression in hemizygous and homozygous transgenic mice (Figs 2 and 3). Hemizygous mice did not demonstrate any phenotypic abnormality despite the reduction in RARα expression. In contrast, mice that contain a similar construct, but express the sense RARα, do not show any abnormality.
Based on Western blot analysis (Figs 3 and 4), we demonstrated significant reduction in the level of RARα protein (30% to 80%) in various tissues in homozygous mice and to a lesser degree in hemizygous mice. Although the level of the RARα protein was low, it could still be detected in all tissues. Unlike the complete knockout of the expression of RARα gene in RARα null mice,11 the low level of expression of the RARα led to a compensatory increase in the expression of RARβ and RARγ proteins (Fig 4). The mechanism of this compensatory increase in RARβ and γ is not clear. RARs are involved in regulating numerous genes and they are regulated by several genes. However, it is possible that the autoregulatory function of the RARs is contributing to this compensatory increase of RARβ and RARγ proteins. It is possible that the remaining RARα protein binds along with other factors to the RARE in the promoters of the RARs and leads to this compensatory increase in RARβ and RARγ, and this may explain the lack of any compensatory increase in RARβ and RARγ in the complete RARα knockout mice. Therefore, it seems that a low level of RARα is necessary for the appropriate response of RARβ and RARγ. This raises the possibility that in RARα null mice, the function of the RARβ and RARγ is actually impaired because of the complete lack of RARα expression.
Despite variation in the copy number (approximately 2, 4, and 8) of the antisense transgene in various lines, the levels of RARα downregulation were similar; this may reflect that the expression of the transgene is not copy number-dependent. This is consistent with previous reports by several investigators indicating that transgenes are expressed in transgenic mice in copy number-independent fashion unless they are linked to a locus control region, which provides open chromatin structure and confers copy number-dependent expression.26 It is speculated that this is because usually all copies of the transgene are integrated in a head-to-tail fashion in a single site in the genome, and not all copies are expressed. Significant increase of expression in homozygous mice usually is because the expression in homozygous is generated from two independent sites on two chromosomes.
Major characteristics of the homozygous transgenic mice were their coarse fur and low body weight (Fig 1). All homozygous transgenic mice weighed approximately 15% to 20% less at 4 weeks of age than their normal littermates and remained smaller throughout their lives. Histologic examination of sections of the skin showed some focal atrophy in sebaceous and sweat glands (Fig 5B). The males were infertile. The testicles were normal in size, and normal-appearing spermatozoa were seen in the lumina of the seminiferous tubules. However, in the homozygous mice most of the seminiferous tubules were smaller in diameter than those in the normal mice and showed some irregularity in the maturation of the spermatozoa and sloughing of cells (Fig 5D). Sperm was seen in the vas deferens. Focal squamous metaplasia was observed in the epididymides and prostates of some mice (Fig 5). Abnormal maturation of spermatozoa and disorganized order of maturation has been reported to be a cause for infertility in humans.19 In our antisense transgenic mice, it is possible that this abnormal maturation combined with some degeneration in the glandular tissue of the genital system (prostate, bulbouretheral gland, etc) leads to infertility. RARα null mice show more severe degeneration in the germinal epithelium of the seminiferous tubule.11 This may reflect the severity of the reduction in the RARα expression and perhaps the compensatory increase in the expression of RARα and RARγ. More severe degeneration in the glandular tissues in the skin and genitourethral system, as well as emaciation and a slower rate of growth, has been reported in animals fed a vitamin A–deficient diet.
The changes seen in our transgenic mice indicate that RARα is important in the overall maintenance of general homeostatic processes, including the regulation of RARβ and RARγ. This is consistent with the ubiquitous expression reported for the RARα.4 These transgenic mice represent an excellent model system in which to investigate the role of the RARα on the activation and function of other hormone receptors that are known to interact with RARα. These include the RXRs, steroid hormone receptors, peroxisome proliferator-activated receptor, vitamin D3 receptor, and thyroid hormone receptor.
As shown in Table 1, 44% of the homozygous transgenic mice developed lymphoma at an early stage of life. On the basis of morphology, most of these were low-grade lymphomas (Fig 6). However, aggressive subtypes of lymphoma (Burkitt's-type or lymphoblastic) were also noted in some mice. Most of these lymphomas were B-cell lymphomas. The clonality of these tumors was confirmed by Ig gene rearrangement study (Fig 7). Retinoids are known to have significant effects on the immune system in general and on the proliferation and differentiation27-30 of lymphoid cells, but a direct relationship between RARα and lymphomagenesis has not been previously reported. Overexpression of RARγ in T lymphocytes in transgenic mice leads to a significant increase in the CD-8+ T cells in these mice.29 Treatment of some Burkitt's lymphoma cell lines with all-trans–retinoic acid results in a dose-dependent decrease in the growth rate.31 In addition, retinoids are considered potent chemopreventive agents against tumors involving many organs, including the breast, skin, lung, prostate, pancreas, liver, digestive tract, and oral cavity.4 Animals deficient in vitamin A are more susceptible to cancer.4 Administration of retinoids can also reverse malignant epithelial changes.4 This chemopreventive function of retinoids is believed to work through their regulation of cell proliferation and differentiation.4 From our data, it is difficult to determine the direct cause of lymphomagenesis in these mice because RARs are involved in regulating the expression of many genes.
Lymphoma development in RARα null mice was not reported.11 Whether this apparent discrepancy between our mice and null mice is caused by the difference in the genetic background of the mice used in both experiments or caused by the physiologic difference (partial v complete knockout) is currently unknown. The compensatory increase in RARβ and RARγ in our mice may be a factor in this discrepancy. Furthermore, additional differences in other nuclear proteins such as RXRs that were triggered by the low level expression of RARα may also explain some of these discrepancies. The possibility that the RARα antisense independently of the RARα is interfering with other processes cannot be ruled out, but appears unlikely because transgenic mice carrying the sense construct do not show phenotypic abnormality. The transcription of RARβ and RARγ are perhaps the most homologous transcripts to RARα and do not appear to decrease.
Our data from transgenic mice clearly demonstrate that changes in RARα expression can be associated with several phenotypic abnormalities and lymphomagenesis in mice.
ACKNOWLEDGMENT
We thank Dr R. M. Evans of The Salk Institute for providing the human RARα cDNA, Dr T. McDonnell of The University of Texas M.D. Anderson Cancer Center for providing the mouse immunoglobulin JH3-4 heavy chain genomic probes, and Dr P. Chambon of Strasbourg, France, for providing RARγ antibodies.
M.A. is a recipient of the Physician Scientist Award from the National Institutes of Health, Bethesda, MD, Grant No. HL-02229. Animal work is supported in part by M.D. Anderson Cancer Center CORE grant from the National Cancer Institute, Grant No. CA-16672.
Address reprint requests to Maher Albitar, MD, Hematopathology Program, Division of Laboratory Medicine, Box 72, The University of Texas M.D. Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030-4095.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal