Abstract
Administration of the immunosuppressive drug cyclosporine after autologous bone marrow transplantation induces a systemic autoimmune syndrome resembling graft-versus-host disease (GVHD). This syndrome termed autologous GVHD has significant antitumor activity. Associated with autologous GVHD is the development of T lymphocytes that recognize major histocompatibility complex (MHC) class II determinants, including self. The present studies attempted to characterize and define the molecular specificity of the effector T lymphocytes in autologous GVHD induced in patients with metastatic breast cancer. The results suggest that the effector cells associated with human autologous GVHD are CD8+ T lymphocytes expressing the α/β T-cell receptor. Additional studies show that the effector T cells recognize MHC class II antigens in association with a peptide from the invariant chain (CLIP). Pretreatment of autologous lymphoblast target cells with anti-CLIP antibody completely blocked lysis mediated by autologous GVHD effector T cells. On the other hand, force loading this peptide markedly enhanced the susceptibility of the target cells to recognition by the autoreactive T cells. The recognition of the MHC class II CLIP complex may account for the novel specificity of the effector T cells associated with human autologous GVHD. Moreover, identification of the target peptide may allow for the development of novel immunotherapeutic strategies to enhance the antitumor efficacy of autologous GVHD.
AUTOLOGOUS bone marrow transplantation (ABMT) is a viable therapeutic option for the treatment of a variety of hematologic malignancies.1,2 This procedure, which uses high-dose chemotherapy and total body irradiation followed by infusion of autologous bone marrow purged of tumor cells, can result in long-term disease-free survival. Recent studies also suggest that ABMT may be effective therapy for patients with drug-responsive solid tumors.3,4 Although long-term disease-free survival can be achieved in a significant number of patients, tumor recurrence remains the major cause for treatment failure of ABMT. Novel approaches are necessary to reduce the incidence of tumor relapse after ABMT. Immunotherapy is a particularly attractive approach for reducing relapse after ABMT because it should be non–cross-resistant with high-dose chemotherapy.5
Recent studies in humans and in rats show that the administration of cyclosporine (CsA) after autologous or syngeneic BMT induces an autoimmune syndrome resembling graft-versus-host disease (GVHD).6-10 This syndrome is termed syngeneic or autologous GVHD.6-10 Results from animal studies suggest that syngeneic GVHD has significant antitumor activity.11,12 Moreover, the antitumor effect of this autoaggression syndrome can be enhanced by the administration of γ-interferon by apparently upregulating the expression of the major histocompatibility complex (MHC) class II target antigen on the tumor cell.11,12 Clinical trials are currently underway to evaluate the antitumor immunotherapeutic potential of autologous GVHD in humans.13-17
Analysis of the effector mechanisms involved in both autologous and syngeneic GVHD unexpectedly showed that there is promiscuous recognition of MHC class II determinants including self by CD8+ T cells.8,9,11,18 The novel specificity and the lack of typical MHC class II restriction element (CD4) on the autoreactive T cells suggest that either a highly conserved structural element or an endogenous superantigen governs recognition of the target antigen.19 Surprisingly, an element commonly associated with MHC class II molecules exists that may satisfy both possibilities.20-24 CLIP, a peptide from the invariant chain, is highly conserved and has a supermotif for binding to MHC class II molecules.20-23
The present studies were undertaken to characterize the effector mechanisms and examine the molecular specificity of the autoreactive T cells in autologous GVHD induced in patients with metastatic breast cancer. The results suggest that CLIP plays a critical role in the recognition of MHC class II molecules in autologous GVHD and may account for the promiscuous activity of the autoreactive T cells. Moreover, defining the specific target antigen, the MHC class II-CLIP complex may allow for the development of strategies to enhance tumor cell recognition and destruction.
MATERIALS AND METHODS
Patients and ABMT induction of autologous GVHD.Women between 18 and 60 years of age with metastatic breast cancer in complete or partial response to chemotherapy were included in this study, as previously described.14 16 The conduct of this trial was approved by the Joint Committee on Clinical Investigation of The Johns Hopkins Hospital. All patients provided informed written consent.
The patients were prepared for ABMT by treatment with cyclosphosphamide (1.5 g/m2) and thiotepa (200 mg/m2; 4× daily) before autologous bone marrow rescue, as previously described.14,16 Autologous GVHD was induced by the intravenous administration of CsA (2.5 mg/kg/d for 28 days; Sandoz LTD, Hanover, NJ) beginning on the day of transplantation. Human recombinant γ-interferon (0.025 mg/m2; CTEP; National Cancer Institute, Bethesda, MD) was administered subcutaneously every other day from days 7 through 28 after transplantation to induce upregulation of MHC class II determinants and enhance tumor cell recognition.11,16 Previous dose escalation studies showed that this dose of CsA was optimal for induction of autologous GVHD in patients with metastatic breast cancer.14 Control patients underwent ABMT using the same preparative regimens but without administration of CsA and γ-interferon.17 Patients were evaluated daily for evidence of autologous GVHD (erythematous rash) and confirmed by skin biopsy, as previously described.14 16 Because autologous GVHD usually occurred during the initial phases of engraftment, the number of peripheral blood mononuclear cells (PBMCs) were limited. Therefore, all assays (ie, identification of effector T cells, CLIP loading, and anti-CLIP blocking) could not be performed in all patients.
Cell-mediated lympholysis (CML) assay.Lytic activity was assessed sequentially after ABMT using PBMCs isolated by Ficoll-Hypaque density centrifugation. The lymphocytes were also separated into distinct T-cell subsets by immunomagnetic bead separation chromatography using monoclonal antibodies (MoAbs) to the α/β T-cell receptor (TCR) and to the CD4 and CD8 cell surface determinants (Becton Dickinson, Mountain View, CA), as previously described.18,25 Target cells used in these studies included phytohemagglutinin (PHA)-stimulated autologous lymphocytes cryopreserved before transplantation, the MHC class II-positive breast cancer cell line T47D,26,27 and the natural killer (NK) target cell line K562. The pretransplantation lymphocytes were thawed and stimulated with PHA for 72 hours (RPMI 1640; 20% normal human serum) before use as targets. The K562 cell line was grown in suspension culture in RPMI 1640 tissue culture medium supplemented with 10% fetal calf serum. The cells were washed three times before the CML assay. T47D is an adherent breast cancer line grown in Dulbecco's modified Eagle's medium (D-MEM) tissue culture medium supplemented with 5% fetal calf serum, glutamine, and sodium pyruvate.26,27 Before assay, the cells were mildly trypsinzed and grown in Nalgene Teflon flasks (Thomas Scientific, Swedesboro, NJ) for 24 hours to allow for the recovery of cell surface antigens. The target cells were labeled with 250 μCi of 51Cr for 1 hour at 37°C. The effector lymphocytes were cocultured with 2.5 × 103 labeled target cells in triplicate in round-bottom microtiter wells for 4 hours at 37°C, as previously described.18 25
Spontaneous and maximum 51Cr release was assessed by adding complete medium (RPMI 1640, heat-inactivated 10% pooled normal human serum) and 1.0 N HCl, respectively, to triplicate wells of labeled target cells. The percentage of specific 51Cr release was calculated according to the following formula: % Specific 51Cr Release = 100 × (cpm Experimental − cpm Spontaneous)/(cpm Maximum − cpm Spontaneous). To assess the specificity of the effector T lymphocytes, the target cells were pretreated (1 hour at 4°C) with either anti-MHC class II (anti-DR; Becton Dickinson), antihuman Ia (American Type Culture Collection, Rockville, MD), and anti-MHC class I (Dako Corp, Carpinteria, CA) MoAbs or with antihuman CLIP (affinity purified rabbit antihuman CLIP and murine monoclonal antihuman CLIP; see below). The anti-DR and anti-MHC class I antibodies were used at a final concentration of 1:200, whereas the antihuman Ia (ascites fluid) was used at 1:1,000 dilution. Fab fragments of antihuman CLIP were prepared by papain digestion and sephadex G-200 size chromatography.28 Target cells were pretreated with 1.5 μg of the antihuman CLIP Fab preparation. As controls, the target cells were also pretreated with either normal mouse serum or rabbit prebleed IgG. After antibody pretreatment, the cells were washed three times before assay.
CLIP peptides and antibody production.The peptide from the human MHC class II invariant chain (human sequence aa 82-110: PKPPKPVSKMRMATPLLMQALP) and the truncated variant containing the MHC class II binding domain (aa 91-101: MRMATPLLMR) were synthesized by Quality Controlled Biochemicals Co (Hopkinton, MA) and purified by high-performance liquid chromatography.20-23 The peptides (>92% purity) were dissolved in RPMI 1640 before use. Force loading of CLIP was accomplished by incubating the cells in graded concentrations of the peptide for 2 hours at 4°C. Dose-response studies showed that maximal enhancement of lytic activity was achieved by pretreating the target cells with ≥1 μmol/L of peptide. Binding was confirmed flow cytometrically using the rabbit antihuman CLIP antibody. Antibody was prepared to the CLIP peptide by Quality Controlled Biochemicals Co. The peptides were conjugated to keyhole limpet hemocyanin (KLH). Rabbits were immunized with the peptide-KLH conjugate. The antibody was affinity purified using peptide-conjugated sepharose matrix columns. Fab fragments from the affinity-purified antibody were prepared by limited papain digestion and sephadex G-200 column chromatography.28 The specificity of the affinity purified polyclonal antibody was confirmed by enzyme-linked immunosorbent assay (ELISA), comparing its ability to recognize the parent CLIP and three truncated variants (V1, aa 82-103; V2, aa 90-110; and V3, aa 91-101) containing either flanking region or just the MHC class II binding domain. The polyclonal antibody recognized both flanking regions and the MHC class II binding domain but did not recognize an irrelevant peptide. Moreover, the addition of free excess CLIP or the variants but not the irrelevant peptide inhibited the ELISA. Murine MoAb to human CLIP was also obtained from Dr Peter Cresswell (Yale University, New Haven, CT). This MoAb recognized the N-terminal flanking region (aa 81-87) on CLIP.29 30
Flow cytometry.Expression of MHC class II determinants and CLIP were assessed flow cytometrically. The cells were stained with either murine antihuman MHC class II antibodies or the rabbit anti-CLIP antibody for 1 hour at 4°C. The cells were washed twice in phosphate-buffered saline and counter-stained with fluorescein isothiocyanate (FITC)-conjugated sheep antimouse IgG (absorbed with rat IgG; Sigma Chemical Co, St Louis, MO) or FITC-conjugated goat antirabbit IgG (Sigma Chemical Co) for 1 hour at 4°C, as previously described.18 25 The cells were incubated as controls with normal mouse serum or rabbit prebleed IgG, followed by counter-staining with the FITC-conjugated secondary reagents. After washing twice in phosphate-buffered saline, the cells were analyzed on an Coulter Epics 752 flow cytometer (Coulter, Hialeah, FL).
RESULTS
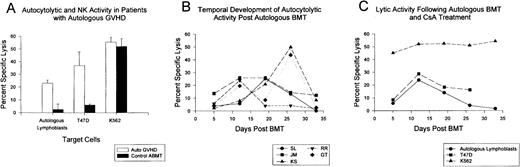
Autocytotoxic activity and autologous GVHD.PBMCs from the ABMT patients on the autologous GVHD induction protocol were serially monitored for autocytolytic activity tested against PHA-stimulated, pretransplant lymphocytes and for their ability to lyse the breast cancer cell line, T47D. Because of the limited number of cells that could be isolated early after ABMT, all assays were standardized using a constant effector to target ratio (100:1). Figure 1A summarizes the data from patients with biopsy-confirmed autologous GVHD and control ABMT patients (non–CsA-treated) evaluating maximal lytic activity during the course of treatment. The results show that there was significant lysis of pretransplantation lymphoblasts in the patients with autologous GVHD. Autocytotoxicity ranged from 11% to 47%. Comparable lytic activity against the T47D cell line was observed (range, 12% to 43%). In contrast, the control ABMT patients did not show significant levels of autocytolytic and anti-T47D activity after transplantation. NK activity was comparable in both groups of patients. The temporal development of autocytolytic activity in 5 ABMT patients developing autologous GVHD after transplantation is shown in Fig 1B. Autocytolytic activity could be readily detected in these patients and developed concurrently with the initial onset of the clinical manifestations of autologous GVHD (erythematous rash and positive skin biopsy). The median time to onset of autologous GVHD in this particular group of patients was 12 days, with a duration of 2 to 4 weeks. Autocytolytic activity subsided upon resolution of autologous GVHD. Similarly, the sequential evaluation of autocytotoxic and NK activity and the ability to lyse the T47D breast cancer cell line is shown in Fig 1C, which details the results from a representative patient who developed autologous GVHD. The ability of PBMCs from this patient to lyse pretransplantation lymphoblasts and the T47D cell line exhibited an identical temporal relationship with maximal lytic activity against both targets observed on day 12. In contrast, NK activity recovered rapidly in most patients and was relatively constant during the intervals tested.
Development of autocytolytic activity in patients with autologous GVHD. (A) Maximum autocytolytic (n = 18), anti-T47D (n = 15) and NK cell (n = 18) activity observed during the interval (days 5 through 33) for patients who developed autologous GVHD confirmed by skin biopsy. The results from 6 control ABMT patients not on the autologous GVHD induction protocol are presented for comparison. (B) Peripheral blood lymphocytes from ABMT patients on the autologous GVHD induction protocol were sequentially monitored for their ability to lyse pretransplantation, PHA-stimulated lymphoblasts. The results from 5 representative patients are expressed as the percentage of specific lysis at a 100:1 effector to target cell ratio. Initial time to onset of clinical autologous GVHD confirmed by skin biopsy for patients S.L., J. M., K.S., R. R., and G.T. was on days 17, 10, 10, 12, and 12, respectively. (C) Peripheral blood lymphocytes from patient C.N. who developed autologous GVHD beginning on day 11 were serially monitored for the development of lytic activity against pretransplant lymphocytes, the T47D breast cancer cell line, and the NK target cell line, K562. All lytic assays were performed at a 100:1 effector to target cell ratio with the results expressed as the percentage of specific lysis.
Development of autocytolytic activity in patients with autologous GVHD. (A) Maximum autocytolytic (n = 18), anti-T47D (n = 15) and NK cell (n = 18) activity observed during the interval (days 5 through 33) for patients who developed autologous GVHD confirmed by skin biopsy. The results from 6 control ABMT patients not on the autologous GVHD induction protocol are presented for comparison. (B) Peripheral blood lymphocytes from ABMT patients on the autologous GVHD induction protocol were sequentially monitored for their ability to lyse pretransplantation, PHA-stimulated lymphoblasts. The results from 5 representative patients are expressed as the percentage of specific lysis at a 100:1 effector to target cell ratio. Initial time to onset of clinical autologous GVHD confirmed by skin biopsy for patients S.L., J. M., K.S., R. R., and G.T. was on days 17, 10, 10, 12, and 12, respectively. (C) Peripheral blood lymphocytes from patient C.N. who developed autologous GVHD beginning on day 11 were serially monitored for the development of lytic activity against pretransplant lymphocytes, the T47D breast cancer cell line, and the NK target cell line, K562. All lytic assays were performed at a 100:1 effector to target cell ratio with the results expressed as the percentage of specific lysis.
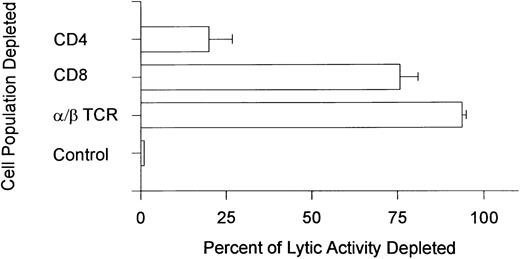
Identification of effector cells and target antigen.Studies were undertaken to characterize the effector cell population that mediated the autocytotoxicity. The results shown in Fig 2 show that depletion of cells expressing the α/β TCR and the CD8 cell surface determinant eliminated the ability of PBMCs from patients with autologous GVHD to lyse pretransplant lymphoblasts. Depletion of the CD4 subset of cells had only a minimal effect. However, in 1 patient, autocytoxic function was significantly eliminated (>70%) when the PBMCs were depleted of either CD4 or CD8 cells. These results suggest that either both CD4+ and CD8+ were required for killing or that the effector cell in this single patient expressed both cell surface accessory molecules.
Phenotypic analysis of the autocytolytic lymphocytes. Peripheral blood lymphocytes from 6 patients with autologous GVHD were depleted of cells expressing the α/β TCR (n = 3) and the CD4 or CD8 cell surface determinants (n = 3) by immunomagnetic bead separation chromatography. Control cells were treated with normal mouse serum before separation. The depleted cell populations were tested for their ability to lyse pretransplantation, PHA-stimulated lymphoblasts. The results are expressed as the percentage of lytic activity depleted and were standardized against the control treated cells (100% activity; 0% lytic activity depleted) that had lytic activity ranging from 17.5% to 43.7% (mean ± SE percentage of specific lysis, 25.2% ± 4.9%).
Phenotypic analysis of the autocytolytic lymphocytes. Peripheral blood lymphocytes from 6 patients with autologous GVHD were depleted of cells expressing the α/β TCR (n = 3) and the CD4 or CD8 cell surface determinants (n = 3) by immunomagnetic bead separation chromatography. Control cells were treated with normal mouse serum before separation. The depleted cell populations were tested for their ability to lyse pretransplantation, PHA-stimulated lymphoblasts. The results are expressed as the percentage of lytic activity depleted and were standardized against the control treated cells (100% activity; 0% lytic activity depleted) that had lytic activity ranging from 17.5% to 43.7% (mean ± SE percentage of specific lysis, 25.2% ± 4.9%).
A series of antibody blocking studies was also performed to identify the antigen or restricting element recognized on the target cell by the autoreactive lymphocytes. The pretransplant lymphoblasts and the T47D breast cancer cell line were pretreated with MoAbs to either MHC class I or MHC class II determinants. The results detailed in Table 1 show that pretreatment of both target cells with antibody to MHC class II determinants significantly blocked lysis mediated by the PMBCs from patients with autologous GVHD. On the other hand, MHC class I antibodies were ineffective.
Recognition of MHC Class II Determinants by Autocytotoxic T Cells Associated With Autologous GVHD
| Target Cells . | Percentage of Specific Lysis of Treated Target Cells Mediated by Autologous GVHD Effector Lymphocytes . | |||||
|---|---|---|---|---|---|---|
| . | Experiment No. . | Pretreatment of Target Cells . | . | . | ||
| . | . | Normal Mouse Serum . | Anti-MHC Class I . | Anti-MHC Class II . | . | . |
| Autologous lymphoblasts | 1 | 43.7 | 40.6 | 10.5 | ||
| 2 | 23.7 | 21.3 | 5.4 | |||
| T47D | 1 | 28.7 | 29.6 | 4.9 | ||
| 2 | 48.2 | NT | 8.3 | |||
| Target Cells . | Percentage of Specific Lysis of Treated Target Cells Mediated by Autologous GVHD Effector Lymphocytes . | |||||
|---|---|---|---|---|---|---|
| . | Experiment No. . | Pretreatment of Target Cells . | . | . | ||
| . | . | Normal Mouse Serum . | Anti-MHC Class I . | Anti-MHC Class II . | . | . |
| Autologous lymphoblasts | 1 | 43.7 | 40.6 | 10.5 | ||
| 2 | 23.7 | 21.3 | 5.4 | |||
| T47D | 1 | 28.7 | 29.6 | 4.9 | ||
| 2 | 48.2 | NT | 8.3 | |||
Autologous pretransplant lymphoblasts and T47D cells were labeled with 51Cr and pretreated with either normal mouse serum or murine MoAbs to the MHC class I and MHC class II determinants for 1 hour at 4°C. The cells were washed in RPMI 1640 before use as targets in the 51Cr release assay. Effector T cells were derived from patients with biopsy confirmed autologous GVHD. The effector to target ratio was 100:1.
Abbreviation: NT, not tested.
CLIP and target cell recognition.The apparent promiscuous recognition of MHC class II determinants by the autoreactive CD8+ T cells suggests the possibility that a highly conserved element governs recognition.8 9 CLIP, a peptide from the MHC class II invariant chain, is a potential candidate because of its unusual affinity and promiscuous specificity for MHC class II molecules. 20-23 A series of studies was undertaken to determine whether CLIP was involved in the recognition of MHC class II determinants by the autologous GVHD effector T cells.
Initially, the affinity-purified antibody to CLIP was assessed for its ability to block lysis mediated by the autoreactive T cells derived from patients with autologous GVHD. Pretreatment of either autologous lymphoblasts or the T47D tumor cell line with the anti-CLIP antibody completely blocked lysis (Table 2). In every patient tested, lysis of pretransplant lymphoblasts and the T47D cell line was completely blocked by pretreatment of the target cells with the affinity-purified anti-CLIP antibody. Blocking of lysis (>95% reduction) was confirmed in 3 patients using a murine MoAb to human CLIP that recognizes the N-termal flanking region (aa 81-87).29 30 Moreover, addition of free excess CLIP (10 μmol/L) but not an irrelevant peptide to the assay specifically inhibited the antibody from blocking recognition (data not shown). On the other hand, anti-CLIP antibody pretreatment of target cells did not block lysis mediated by allospecific cytolytic T cells sensitized in a mixed lymphocyte response (n = 3; mean ± SD 27.3% ± 6.2% specific lysis of control mouse Ig treated target cells v 25.9% ± 5.4% specific lysis of anti-CLIP–treated target cells at a 75:1 effector:target ratio).
Recognition by Autologous GVHD Effector T Lymphoctes Is Blocked by Pretreating the Target Cells With Anti-CLIP Antibody
| Target Cells . | Percentage of Specific Lysis of Treated Target Cells Mediated by the Autologous GVHD Effector Lymphocytes . | ||
|---|---|---|---|
| . | Pretreatment of Target Cells . | . | |
| . | Control . | Anti-CLIP . | . |
| Autologous lymphoblasts (n = 11) | 18.0 ± 3.9 | 1.5 ± 0.6 | |
| T47D (n = 4) | 26.6 ± 6.5 | 0.5 ± 2.9 | |
| Target Cells . | Percentage of Specific Lysis of Treated Target Cells Mediated by the Autologous GVHD Effector Lymphocytes . | ||
|---|---|---|---|
| . | Pretreatment of Target Cells . | . | |
| . | Control . | Anti-CLIP . | . |
| Autologous lymphoblasts (n = 11) | 18.0 ± 3.9 | 1.5 ± 0.6 | |
| T47D (n = 4) | 26.6 ± 6.5 | 0.5 ± 2.9 | |
Pretransplant lymphobasts and T47D cells were labeled with 51Cr and pretreated with (1.5 μg) rabbit antibody to CLIP (Fab) or prebleed IgG for 1 hour at 4°C before use as targets in a 51Cr release assay. Effector T cells were derived from patients with biopsy-confirmed autologous GVHD. The effector to target ration was 100:1.
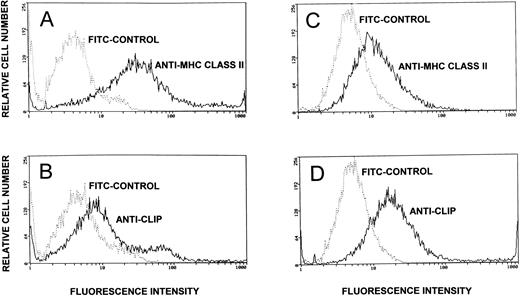
Expression of CLIP and MHC class II determinants on the lymphoblasts and on the T47D tumor cell line was assessed by flow cytometry. As shown in Fig 3, approximately 25% of the MHC class II-positive lymphoblasts expressed significant levels of CLIP as detected by staining with the anti-CLIP antibody. The remainder of the lymphoblasts showed minimal staining with the anti-CLIP antibody, indicating low expression of CLIP. Forced loading of CLIP (1 μmol/L) not only resulted in an increase in the relative intensity of staining of the population but also led to a twofold to threefold increase in the number of cells that intensely stain with the anti-CLIP antibody (data not presented). Comparatively, the T47D cells that also expressed MHC class II determinants showed significant staining with the anti-CLIP antibody. CLIP expression on the tumor cell line, as detected flow cytometrically, was more homogeneous compared with the lymphoblasts.
Cell surface expression of CLIP and MHC class II determinants on lymphoblasts and the T47D breast cancer cell line. PHA lymphoblasts (identified by electronic gating for cell size and scatter) and the T47D cell line were assessed flow cytometrically for expression of the MHC class II determinants and for cell surface CLIP. MHC class II expression was assessed by staining the cells with murine anti–HLA-DR followed by counterstaining with FITC-conjugated sheep antimouse IgG. Control cells were pretreated with normal mouse serum before counterstaining (A, lymphoblasts; C, T47D). CLIP expression was assessed by staining with affinity-purified rabbit anti-CLIP antibody and counterstaining with FITC-conjugated goat antirabbit IgG. As controls, the lymphoblasts and the T47D cell line were pretreated with rabbit prebleed IgG before counterstaining with FITC goat antirabbit IgG (B, lymphoblasts; D, T47D breast cancer cell line).
Cell surface expression of CLIP and MHC class II determinants on lymphoblasts and the T47D breast cancer cell line. PHA lymphoblasts (identified by electronic gating for cell size and scatter) and the T47D cell line were assessed flow cytometrically for expression of the MHC class II determinants and for cell surface CLIP. MHC class II expression was assessed by staining the cells with murine anti–HLA-DR followed by counterstaining with FITC-conjugated sheep antimouse IgG. Control cells were pretreated with normal mouse serum before counterstaining (A, lymphoblasts; C, T47D). CLIP expression was assessed by staining with affinity-purified rabbit anti-CLIP antibody and counterstaining with FITC-conjugated goat antirabbit IgG. As controls, the lymphoblasts and the T47D cell line were pretreated with rabbit prebleed IgG before counterstaining with FITC goat antirabbit IgG (B, lymphoblasts; D, T47D breast cancer cell line).
Because only a fraction of the autologous PHA blast cells expressed significant levels of CLIP, studies were undertaken to assess whether force loading of this peptide onto the target cells enhances their ability to be recognized and lysed by the autoreactive T lymphocytes. Pretransplant lymphocytes stimulated with PHA were incubated for 2 hours at 4°C with 1 μmol/L of CLIP before assay. The results in Table 3 show that force loading of CLIP onto the PHA lymphoblast target cells significantly enhanced their susceptibility to lysis mediated by the autologous GVHD-associated effector T cells. Lysis was enhanced twofold after force loading of CLIP. Similarly, as shown in Table 3, lysis of the T47D cell line by the autologous GVHD effector T cells was also enhanced by force loading of CLIP. In contrast, loading an irrelevant MHC class II binding peptide did not enhance lysis.
Susceptibility to Recognition and Lysis Mediated by the Autologous GVHD Effector Cells Is Enhanced by Force Loading CLIP
| Pretreatment . | Percentage of Specific Lysis . | |
|---|---|---|
| . | of Treated Target Cells . | |
| . | PHA Blast Cells . | T47D Cells . |
| CLIP loaded (n = 3) | 30.2 ± 3.9 | 34.9 ± 2.7 |
| Control (n = 3) | 15.3 ± 2.1 | 16.4 ± 1.3 |
| Pretreatment . | Percentage of Specific Lysis . | |
|---|---|---|
| . | of Treated Target Cells . | |
| . | PHA Blast Cells . | T47D Cells . |
| CLIP loaded (n = 3) | 30.2 ± 3.9 | 34.9 ± 2.7 |
| Control (n = 3) | 15.3 ± 2.1 | 16.4 ± 1.3 |
Pretransplant PHA-stimulated lymphoblasts or T47D cells labeled with 51Cr were loaded with CLIP (1.0 μmol/L) or an irrelevant peptide for 2 hours at 4°C. The cells were washed three times in RPMI 1640 before use as target cells in a 51Cr release assay. Effector lymphocytes were from patients with biopsy-confirmed autologous GVHD. The effector to target ratio was 100:1.
DISCUSSION
The present studies characterized the effector mechanisms associated with autologous GVHD induced in patients with metastatic breast cancer as immune therapy after ABMT. The results show that the onset of autologous GVHD is associated with the development of α/β TCR-positive CD8+ effector T cells that recognize MHC class II determinants on autologous pretransplantation lymphoblasts. The induction of these CD8+ autoreactive T cells was dependent on the administration of CsA and not related to the administration of γ-interferon.12 14
The autoimmune recognition of MHC class II determinants by the autologous GVHD effector cells in the present studies is in accord with the initial observations of Jones et al.8 Moreover, in their studies, the autologous GVHD effector T cells recognized and lysed PHA blast cells from individuals disparate for MHC class II antigens, findings that were confirmed by Carella et al.31 These data suggest that there is promiscuous recognition of MHC class II determinants by the autologous GVHD-associated effector lymphocytes. This premise is also supported by the finding in the present studies that the autologous GVHD effector T cells targeted the MHC class II determinants on the T47D tumor cell line and did not require MHC class II identity. Moreover, recent studies suggest that repertoire of autoreactive T cells is highly conserved. Analysis of the Vβ TCR repertoire of T cells infiltrating the skin in patients with autologous GVHD showed an apparent predominance of lymphocytes expressing the Vβ 15 determinant.18 Comparatively, recent studies in the rat model of autologous GVHD show that the autoreactive TCR repertoire is highly conserved with the effector lymphocytes expressing the Vβ 8.5 TCR determinant. It is important to note that the autoreactive T cells in humans and in rats express a Vβ determinant that allows responsiveness to the soluble superantigen, staphylococcal enterotoxin B (SEB).32 33
The mechanisms accounting for the novel specificity of the CsA-induced autoreactive T cells that do not express the appropriate surface accessory molecule for classical MHC class II restriction are unclear. The results from the present studies suggest that CLIP, a peptide from the invariant chain, is critically involved in the recognition of MHC class II determinants by the CsA-induced autoreactive T cells. This protein controls assembly and cell surface expression of MHC class II gene products and has a supermotif for binding to MHC class II determinants.20-23,29,30 During assembly and transport of the MHC class II molecule to the cell surface, the invariant chain is enzymatically degraded, leaving CLIP in the peptide binding groove. CLIP, by virtue of its high affinity for MHC class II molecules, can inhibit binding of other peptides.20-23 Pretreatment of the target cells with antibody to this peptide completely blocks lysis mediated by the autoreactive T cells associated with autologous GVHD. Additionally, susceptibility to recognition and lysis by the autoreactive T lymphocytes is specifically enhanced by the forced loading of CLIP onto target cell MHC class II molecules. These data suggest that the MHC class II-CLIP complex is the target antigen in the CsA-induced autoaggression syndrome. The recognition of this highly conserved element may override restriction of the T-cell response to a specific MHC haplotype (ie, allowing promiscuous recognition of MHC class II molecules).
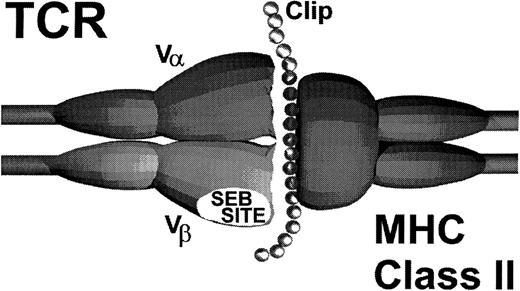
Alternatively, it is possible that CLIP provides an additional interaction with the autoreactive T cells that may explain the promiscuous recognition of MHC class II determinants. In support of this hypothesis are preliminary studies using truncated variants of CLIP. Forced loading a variant containing only the MHC class II binding domain did not enhance target cell recognition. Rather, this truncated peptide modestly inhibited (50%) target cell recognition, presumably due to the competitive displacement of native CLIP.The flanking regions on CLIP that extend beyond the limits of the peptide binding groove on MHC class II molecules appear to play a critical role in the recognition of MHC class II antigens in autologous GVHD.22,24,25,29 Significant enhancement of target cell recognition only occurred when the parent molecule containing the flanking regions was force loaded, suggesting that these regions may interact with the restricted autoreactive TCR as schematically shown in Fig 4. These data are consistent with the observations in the rat model of autologous GVHD that show that the flanking region identified by amino acids 86-91 is critical for recognition.24 Moreover, this region appears to directly interact with the Vβ TCR determinant. In this regard, recent studies show that CLIP modifies the T-cell response to SEB by disrupting the ligation of the TCR with MHC class II determinants.23 Based on the findings that the autoreactive T cells primarily express a Vβ TCR element that confers responsiveness to SEB, in both humans and rats,18,32,33 it is tempting to speculate that a portion of CLIP interacts with the TCR on the autoreactive T cells at or near the SEB binding site. Studies in the rat model showing that SEB pretreatment of the MHC class II autoreactive cytolytic lymphocytes inhibits their activity are consistent with this hypothesis.24 This additional interaction between the peptide target antigen and the TCR may allow recognition of MHC class II molecules despite the fact that these cells have a surface accessory molecule (CD8) that is classically associated with responses to MHC class I gene products. Thus, in the setting of CsA-induced autoaggression, CLIP may be acting like a superantigen.19
Schematic model of autologous GVHD effector T-cell receptor and MHC class II-CLIP target antigen interaction.
Schematic model of autologous GVHD effector T-cell receptor and MHC class II-CLIP target antigen interaction.
Of additional importance in the present studies is the finding that tumor cell recognition by the CsA-induced autoreactive T cells, at least in an in vitro model, is also dependent on the MHC class II-CLIP complex. These findings may account for the relative antitumor activity of autologous GVHD and the potentiating effects of γ-interferon.11,12 Effective tumor cell targeting would be dependent on expression of MHC class II determinants and CLIP. Our initial animal studies indicate that the induction of autologous GVHD is associated with a significant antitumor effect.11,12 However, administration of γ-interferon enhances both the intensity of clinical disease16 and the antitumor effect of autologous GVHD.12 In this setting and based on our findings, tumor cell recognition would not only be enhanced by the increased expression of MHC class II determinants but also by the fact that newly upregulated MHC class II antigens are more likely to be associated with CLIP. Current studies are underway to evaluate MHC class II-CLIP expression in patient tumor samples to correlate expression of this target antigen complex with response. Moreover, identification of the target antigen complex may allow for the development of immunotherapeutic strategies to augment the antitumor effect of CsA-induced autologous GVHD, including the administration of CLIP to enhance tumor cell targeting.
Supported by National Institutes of Health Grants No. AI24319, CA67800, CA15396, and CA/ES 66204.
Address reprint requests to Allan D. Hess, PhD, Oncology Center 3-127, The Johns Hopkins University, 600 N Wolfe St, Baltimore, MD 21287-8985.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal