Abstract
B-chronic lymphocytic leukemia (BCLL) is a lymphoproliferative disease that is characterized by clonal expansion of CD5+ B cells. BCLL is associated with secondary immunodeficiency and hypogammaglobulinemia. It has been suggested that T-cell dysregulation may play a role in the hypogammaglobulinemia and in the increased incidence of autoimmunity in BCLL patients. We attempted to transfer human peripheral blood mononuclear cells (PBMC) from BCLL patients in different stages of the disease into immunodeficient mice. PBMC from BCLL patients in stage 0, stages I to II, and stages III to IV were transplanted into the peritoneal cavity of lethally irradiated Balb/c or beige/nude/Xid (BNX) mice radioprotected with bone marrow (BM) from severe combined immunodeficiency (SCID) mice. Different engraftment profiles were found in the chimeric mice 2 weeks after transplantation of PBMC according to the disease stage of the BCLL donors. Infusion of PBMC from donors in stage 0 led to marked engraftment of human T cells, whereas the human tumor cells could hardly be detected. In contrast, chimeric mice receiving PBMC from patients in stage III to IV disease exhibited engraftment with a dominance of tumor cells, compared with a miniscule level of T cells. The ability of the engrafted cells to produce human Ig was also found to be correlated with the disease stage of the donor, although all donors had the same magnitude of hypogammaglobulinemia. Total human Ig production in the chimeric mice was normal in mice receiving PBMC from donors in stage 0, whereas in chimeric mice engrafted with PBMC from donors in stages III to IV almost no human Igs could be detected. This differential reconstitution of antibody production in the mouse model according to the stage of the patient's disease will allow further studies on possible cellular interactions between malignant and immune cells in BCLL.
B-CHRONIC LYMPHOCYTIC leukemia (BCLL) is a lymphoproliferative disease that is characterized by clonal expansion of small mature B lymphocytes that express low surface Igs, as well as the CD5 antigen.1-3 Clonality is suggested by the expression of a single Ig light chain, either λ or κ, on the membrane of BCLL cells.2 BCLL is associated with secondary immunodeficiency, and in particular with hypogammaglobulinemia. In addition, some patients may demonstrate features of autoimmunity such as autoimmune hemolytic anemia during the course of the disease.3,4 The mechanisms responsible for the development of autoimmune phenomena or hypogammaglobulinemia in BCLL patients are poorly understood.3-5 However, it has been suggested that T-cell dysregulation may play a role in the hypogammaglobulinemia and in the increased incidence of autoimmunity in BCLL patients.2,3 In the past few years, severe combined immunodeficiency (SCID) mice engrafted with human peripheral blood mononuclear cells (PBMC) were used as in vivo models for studying normal and malignant human hematopoietic cells (reviewed in Mosier6 ). BCLL cell lines7,8 and BCLL cells from patients were transferred into SCID mice by intraperitoneal injection, but these animals were reported to develop a lethal human B-cell tumor within a few weeks.9 Recently, Lubin et al10 described a new approach that enables the adoptive transfer of human PBMC into lethally irradiated normal strains of mice radioprotected with SCID bone marrow (BM). Dissemination of engrafted lymphocytes is rapid, and both T and B lymphocytes are found in significant numbers in the lymphoid tissues as early as 24 hours following transplantation. Polyclonal human antibodies were found in large quantities as early as 10 days posttransplant,10 and in contrast to the human-SCID mice, human/Balb chimera did not develop Epstein-Barr virus (EBV) lymphoma.11
In the present study, we investigated in such radiation chimera the relative engraftment potential of malignant and normal PBMC from BCLL patients in different stages of the disease.
MATERIALS AND METHODS
Mice.The animals used were 6 to 10 weeks old. Balb/c mice were obtained from Olac (Shaw's Farm, Blackthorn, Bicester, Oxon., UK), outbred immune-deficient beige/nude/Xid (BNX) mice from Harlan Sprague-Dawley (Indianapolis, IN), and NOD/SCID mice from The Weizmann Institute Animal Breeding Center. All mice were kept in small cages (five animals per cage) and fed sterile food and acid water containing ciprofloxacin (20 μg/mL).
Conditioning regimen for mice.Balb/c mice were exposed to split-dose (4 Gy followed 3 days later by 10 Gy) total-body irradiation from a gamma-beam 150 A 60Co source (produced by Atomic Energy of Canada, Kanata, Ontario) with focal skin distance of 75 cm and a dose rate of 0.7 Gy/min. BNX mice were exposed to total-body irradiation of 12 Gy (single dose) 1 day before transplantation.
Preparation and transplantation of BM cells.BM cells were obtained from NOD/SCID mice (4 to 8 weeks old) according to the method used by Levite et al.12 Recipient mice were injected with 2 to 3 × 106 SCID BM cells (intravenously in 0.2 mL phosphate-buffered, saline [PBS]) 1 day after irradiation.
CLL patients.The patient group included 23 patients treated at the Kaplan Hospital Hematology Institute (Rehovot, Israel). Diagnosis of CLL was based on sustained lymphocytosis, peripheral blood smear, BM biopsy, and expression of CD5. Some patients had morphologic features consistent with activated CLL or prolymphocytoid transformation at diagnosis. Two patients had prolymphocytic leukemia (PLL) and did not express CD5. Each patient donated 20 to 75 mL blood after providing informed consent. Blood was drawn using heparin-washed sterile syringes, and PBMC were separated (as described later) within 24 hours. Patient charts were reviewed for age, disease duration, CLL stage according to the Rai classification,13 and current treatment. Complete blood cell counts and serum Ig levels were obtained on the day of blood sampling (Table 1). Fifteen of the patients were not treated, seven were treated with prednisone and/or chlorambucil, and one was treated with 2-chlorodeoxyadenosine.
Patient Characteristics
| Patient No. . | Age (yr) . | Sex (M/F) . | Disease Duration . | Stage . | WBC Count (× 109/L) . | Morphologic Features . | Serum Ig (mg/dL)* . | ||
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | IgG . | IgM . | IgA . |
| 1 | 82 | M | 12 yr | II | 77 | Typical CLL | 945 | 41 | 56 |
| 2 | 73 | M | 10 yr | 0 | 34 | Typical CLL | 900 | 105 | 117 |
| 3†‡ | 70 | F | 2 yr | III | 250 | Typical CLL | 1,040 | 21 | 34 |
| 4 | 51 | M | 2 yr | II | 38 | Typical CLL | 1,000 | 57 | 111 |
| 5 | 65 | F | 3 mo | 0 | 44 | Typical CLL | 662 | 33 | 22 |
| 6ρ | 67 | M | 3 yr | I | 40 | Typical CLL | 4,340 | 20 | 85 |
| 7 | 62 | M | 2 yr | I | 35 | Typical CLL | 1,490 | 34 | 202 |
| 8 | 70 | M | 2 mo | IV | 20 | Activated CLL/PL | 1,490 | 185 | 112 |
| 9 | 58 | M | 12 yr | 0 | 70 | Typical CLL | 513 | 72 | 110 |
| 10† | 76 | F | 6 yr | II | 30 | Typical CLL | 929 | 88 | 153 |
| 11 | 75 | F | 9 yr | IV | 70 | Typical CLL | 1,120 | 30 | 113 |
| 12 | 69 | M | 7 yr | IV | 110 | Typical CLL | 425 | 27 | 12 |
| 13 | 78 | M | 20 yr | 0 | 88 | Typical CLL | 936 | 22 | 67 |
| 14† | 75 | M | 11 yr | II | 12 | Typical CLL | 644 | 25 | 68 |
| 15 | 67 | M | 3 mo | I | 31 | Activated CLL/PL | 733 | 25 | 135 |
| 161-154 | 47 | M | 2 yr | 0 | 73 | Typical CLL | 1,130 | 82 | 266 |
| 17†‡ | 78 | F | III | 300 | Typical CLL | ||||
| 18#∥ | 50 | M | 7 yr | III | 27 | Typical CLL | 461 | 48 | 32 |
| 19† | 82 | M | 14 yr | III | 90 | PLL | 971 | 102 | 350 |
| 20† | 80 | F | II | 40 | Activated CLL/PL | 808 | 27 | 103 | |
| 21† | 89 | F | 9 yr | II | 45 | Activated CLL/PL | 378 | 17 | 29 |
| 22 | 80 | M | 9 yr | II | 57 | PLL | 897 | 38 | 61 |
| 231-167 | 70 | M | 4 yr | II | 35 | Activated CLL/PL | 852 | 28 | 125 |
| Patient No. . | Age (yr) . | Sex (M/F) . | Disease Duration . | Stage . | WBC Count (× 109/L) . | Morphologic Features . | Serum Ig (mg/dL)* . | ||
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | IgG . | IgM . | IgA . |
| 1 | 82 | M | 12 yr | II | 77 | Typical CLL | 945 | 41 | 56 |
| 2 | 73 | M | 10 yr | 0 | 34 | Typical CLL | 900 | 105 | 117 |
| 3†‡ | 70 | F | 2 yr | III | 250 | Typical CLL | 1,040 | 21 | 34 |
| 4 | 51 | M | 2 yr | II | 38 | Typical CLL | 1,000 | 57 | 111 |
| 5 | 65 | F | 3 mo | 0 | 44 | Typical CLL | 662 | 33 | 22 |
| 6ρ | 67 | M | 3 yr | I | 40 | Typical CLL | 4,340 | 20 | 85 |
| 7 | 62 | M | 2 yr | I | 35 | Typical CLL | 1,490 | 34 | 202 |
| 8 | 70 | M | 2 mo | IV | 20 | Activated CLL/PL | 1,490 | 185 | 112 |
| 9 | 58 | M | 12 yr | 0 | 70 | Typical CLL | 513 | 72 | 110 |
| 10† | 76 | F | 6 yr | II | 30 | Typical CLL | 929 | 88 | 153 |
| 11 | 75 | F | 9 yr | IV | 70 | Typical CLL | 1,120 | 30 | 113 |
| 12 | 69 | M | 7 yr | IV | 110 | Typical CLL | 425 | 27 | 12 |
| 13 | 78 | M | 20 yr | 0 | 88 | Typical CLL | 936 | 22 | 67 |
| 14† | 75 | M | 11 yr | II | 12 | Typical CLL | 644 | 25 | 68 |
| 15 | 67 | M | 3 mo | I | 31 | Activated CLL/PL | 733 | 25 | 135 |
| 161-154 | 47 | M | 2 yr | 0 | 73 | Typical CLL | 1,130 | 82 | 266 |
| 17†‡ | 78 | F | III | 300 | Typical CLL | ||||
| 18#∥ | 50 | M | 7 yr | III | 27 | Typical CLL | 461 | 48 | 32 |
| 19† | 82 | M | 14 yr | III | 90 | PLL | 971 | 102 | 350 |
| 20† | 80 | F | II | 40 | Activated CLL/PL | 808 | 27 | 103 | |
| 21† | 89 | F | 9 yr | II | 45 | Activated CLL/PL | 378 | 17 | 29 |
| 22 | 80 | M | 9 yr | II | 57 | PLL | 897 | 38 | 61 |
| 231-167 | 70 | M | 4 yr | II | 35 | Activated CLL/PL | 852 | 28 | 125 |
Abbreviations: WBC, white blood cell; AIHA, autoimmune hemolytic anemia; 2CDA, 2-chlorodeoxyadenosine.
Normal values: IgG 1,020 to 1,460; IgM 85 to 350; IgA 210 to 350.
Received treatment (prednisone, chlorambucil, or both).
Coombs+, AIHA.
ρ Patient also suffers from IgG κ myeloma.
Coombs+, no hemolysis.
IgA nephropathy.
Received treatment (2CDA).
Preparation and transplantation of human PBMC.Buffy coats from normal volunteers or whole blood from CLL patients were layered onto Lymphoprep solution (Nycomed, Oslo, Norway) and centrifuged at 2,000 rpm for 20 minutes. The interlayer was collected, washed twice, counted, and resuspended in PBS, pH 7.4, to the desired cell concentration. Human PBMC (100 to 1,000 × 106 cells in 0.5 to 1.0 mL PBS) were injected intraperitoneally into recipient mice conditioned as described earlier. Control mice did not receive human PBMC.
Cell, plasma, and organ collection from human/mouse chimera.Animals were bled from the retro-orbital vein using heparin-coated glass capillary tubes. Plasma was kept for human Ig determination. Peritoneal cells were obtained by lavage with 10 mL PBS. Organs were removed after the animals were killed by cervical dislocation, and the peritoneum was washed again. The organs were cut into pieces and pressed through stainless steel sieves to make a cell suspension in PBS, and cells were then isolated using Lymphoprep.
FACS analysis of donor PBMC and human cell engraftment in chimeric mice.Single cell suspensions were incubated for 30 minutes on ice with a mixture of appropriate fluorescently labeled monoclonal antibodies. After washing, two- or three-color flow cytometric analysis of human antigens was performed on a FACScan analyzer (Becton Dickinson, Mountain View, CA). The labeled antibodies used recognize specific surface molecules: CD45-fluorescein isothiocyanate (FITC) and CD45-peridinin chlorophyll protein (PerCP); CD3-PerCP and CD3-phycoerythrin (PE); CD14-PE; CD19-FITC; CD20-FITC and CD20-PE; CD5-FITC and CD5-PE; CD25-FITC; CD4-PE; CD8-FITC; HLA-DR-PE; CD45RA-FITC; CD45RO-PE. All of these antibodies were purchased from Becton Dickinson. CD45 (CY-chrome) antibody was purchased from Pharmingen (San Diego, CA). Antihuman κ light chain FITC and antihuman λ chain FITC were purchased from Kallestad (Chaska, MN).
Human Ig determination.Sera were tested for total human Ig production. Total human Igs were quantified by sandwich enzyme-linked immunosorbent assay (ELISA) using goat F(ab)2-purified antihuman IgG+IgM+IgA (Zymed Laboratories, San Francisco, CA) as the capture agent and peroxidase-conjugated purified goat antihuman Ig (G+M+A) (Zymed Laboratories) as the detection reagent. Human serum of known Ig concentration was used as the standard. Microplates (Nunc, Roskilde, Denmark) precoated with the capture reagent (5 μg/mL, 50 μL/well) and blocked with 1% bovine serum albumin in PBS were incubated overnight at 4°C with dilutions of plasma ranging from 1/500 to 1/16,000 or the standard from 0.5 to 0.05 μg/mL, and then washed three times with 0.05% Tween solution in PBS. The detection reagent was added, and the plates were incubated for 2 hours at 37°C and then washed again three times. Fresh substrate solution, 2,2′-Azino-bis(3-ethylbenz-thiazoline-6-sulfonic acid) (Sigma, St Louis MO), was added, and after peroxidase-catalyzed color development, absorbency at 405 nm was quantified on an ELISA reader (Dynatech, Port Guernsey, Channel Islands, UK).
Serum electrophoresis and immunofixation.Electrophoresis and immunofixation of serum samples were performed using commercial kits (Silenus immunofixation kit; Silenus Laboratories, Hawthorn, Australia).
Statistical analysis.Statistical analyses were performed using the Stat View II program (Abacus Concepts Inc, Berkeley, CA) on a Macintosh IICi (Apple Computer Inc, Cupertino, CA). The student t test, analysis of variance, and regression analysis were used to calculate probability (P ) values. Results are presented as the mean ± SE.
RESULTS
CLL donors.The study included 23 patients (Table 1), 16 men and seven women with a mean age of 70 ± 11 years (range, 47 to 89). The mean disease duration was 7 ± 5 years (range, 2 months to 20 years). The mean white blood cell count was 70 ± 68 × 109/L (12 to 300 × 109). Five patients were classified as stage 0, three as stage I, eight as stage II, four as stage III, and three as stage IV. All patients had sustained lymphocytosis, which in 16 patients consisted of small mature lymphocytes. Five patients designated as activated CLL (CLL/PL)2 exhibited, in addition to BCLL cells, a subgroup of larger prolymphocytes consisting of less than 55% of the cells; two patients had PLL.
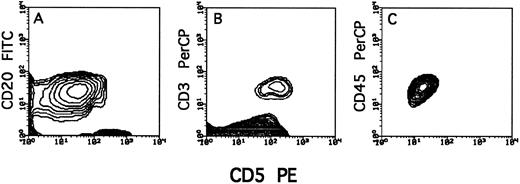
All 21 CLL patients were CD5+ as found by FACS analysis (Fig 1). The two PLL patients were CD5−. Tumor cells were differentiated from CD5+ T cells by CD20 positivity or CD3 negativity (Fig 1). Normal B cells (CD5−CD20+) were not detected by this analysis (probably due to their low incidence), although their presence was indicated by the ability to produce human antibodies.
Typical staining of T cells (CD3+CD5+) and tumor cells (CD20+CD5+) in PBMC of lymphocytes from a BCLL donor before transplantation.
Typical staining of T cells (CD3+CD5+) and tumor cells (CD20+CD5+) in PBMC of lymphocytes from a BCLL donor before transplantation.
Engraftment of human CLL cells in human/mouse chimera.PBMC from the CLL patients were transplanted into the peritoneal cavity of lethally irradiated Balb/c or BNX mice radioprotected with SCID BM (Table 2). To study the engrafted human cells in the peritoneum of the chimeric mice, the human cells were distinguished from the murine cells by triple staining in which the human origin of the cells is indicated by staining with antihuman CD45 and the specific subpopulations are defined by antibodies that are also specific for human cells and are not cross-reactive with mouse cells. Therefore, the numbers in Table 2 represent the fraction of human cells of the total cells recovered from the peritoneum. The other cells are murine cells, mainly of monocyte/macrophage origin (I. Lubin, unpublished results, 1994). Similar to the results obtained by Lubin et al10 with normal human PBMC, large numbers of human cells (about 10% of the initial number infused, data not shown) could be recovered from chimeric Balb/c mice up to 4 weeks after transplantation, and thereafter the human cells gradually disappeared.
Engraftment Characteristics According to Donor Stage of Disease
| Group . | Human Donor . | Human/Mouse Chimera . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | Patient No. . | Stage . | Total Ig . | Transplanted Cells (×106) . | Transplanted T Cells (×106) . | No. of Mice . | Tumor Engraftmentρ . | T-cell Engraftmentρ . | T Cell/Tumor . | Total Human Ig . |
| . | . | . | (μg/mL) . | . | . | . | . | . | . | (μg/mL) . |
| A | 3 | III | 10,950 | 600 | 20 | 13 | 10 (60) | 0.3 (1.5) | 0.03 | 7 ± 9 |
| 8* | IV | 17,870 | 350 | 9 | 3 | 50 (70) | 6 (8) | 0.12 | 530 ± 203 | |
| 12 | IV | 4,640 | 500 | 15 | 3 | 12 (40) | 0.1 (0.3) | 0.01 | 0 | |
| 17 | III | 1,000 | 7 | 2 | 88 (93) | 0.0 (0) | 0.00 | 0 | ||
| 18 | III | 5,410 | 150 | 9 | 4 | 40 (65) | 5 (8) | 0.13 | 7 ± 12 | |
| 19† | III | 14,230 | 350 | 4 | 5 | 75 (95) | 1 (1.4) | 0.01 | 40 ± 32 | |
| Mean ± SE | 11,220 ± 2,235 | 492 ± 119.3 | 10.7 ± 2.3 | 30 | 45.5 ± 12.8 | 2.07 ± 1.1 | 0.05 ± 0.02 | 97.3 ± 86.7 | ||
| B | 1 | II | 10,420 | 500 | 20 | 2 | 8 (16) | 13 (25) | 1.60 | 270 ± 45 |
| 4 | II | 11,680 | 400 | 40 | 7 | 10 (13) | 50 (70) | 5 | 2,410 ± 683 | |
| 6 | I | 10,050‡ | 150 | 20 | 1 | 4 (20) | 7 (36) | 1.80 | 750 | |
| 7 | I | 17,260 | 400 | 32 | 6 | 1.5 (6) | 10 (42) | 7 | 950 ± 460 | |
| 10 | II | 11,700 | 150 | 12 | 3 | 0.2 (1.8) | 4 (40) | 20 | 1,188 ± 98 | |
| 15* | I | 8,930 | 200 | 6 | 2 | 14 (33) | 13 (32) | 0.90 | 1,170 ± 760 | |
| 20* | II | 9,380 | 500 | 28 | 7 | 13 (34) | 8 (22) | 0.60 | 170 ± 112 | |
| 21* | II | 4,240 | 500 | 18 | 4 | 55 (88) | 4.5 (7) | 0.08 | 65 ± 21 | |
| 22† | II | 9,960 | 500 | 18 | 3 | 3.5 (7.5) | 20 (42) | 6 | 2,590 ± 592 | |
| 23* | II | 10,050 | 200 | 6 | 3 | 23 (46) | 13 (26) | 0.60 | 67 ± 70 | |
| Mean ± SE | 9,314 ± 1,348 | 350 ± 49.4 | 20 ± 3.4 | 38 | 13.22 ± 5.13 | 14.25 ± 4.25 | 4.35 ± 1.91 | 963 ± 290.5 | ||
| C | 2 | 0 | 11,220 | 350 | 16 | 1 | 5 (7) | 13 (19) | 2.6 | 1,130 |
| 5 | 0 | 7,170 | 250 | 10 | 3 | 0 (0) | 21 (67) | >20 | 4,100 ± 160 | |
| 9 | 0 | 6,950 | 450 | 3 | 2 | 0.7 (3) | 15 (64) | 21 | 5,496 ± 1,048 | |
| 16 | 0 | 14,780 | 200 | 12 | 2 | 7 (12) | 28 (52) | 4 | 748 ± 12 | |
| Mean ± SE | 10,030 ± 1,863 | 312 ± 55.4 | 10.25 ± 2.72 | 8 | 3.17 ± 1.69 | 19.25 ± 3.37 | 12.4 ± 5.26 | 2,868.5 ± 1,152.5 | ||
| Group . | Human Donor . | Human/Mouse Chimera . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | Patient No. . | Stage . | Total Ig . | Transplanted Cells (×106) . | Transplanted T Cells (×106) . | No. of Mice . | Tumor Engraftmentρ . | T-cell Engraftmentρ . | T Cell/Tumor . | Total Human Ig . |
| . | . | . | (μg/mL) . | . | . | . | . | . | . | (μg/mL) . |
| A | 3 | III | 10,950 | 600 | 20 | 13 | 10 (60) | 0.3 (1.5) | 0.03 | 7 ± 9 |
| 8* | IV | 17,870 | 350 | 9 | 3 | 50 (70) | 6 (8) | 0.12 | 530 ± 203 | |
| 12 | IV | 4,640 | 500 | 15 | 3 | 12 (40) | 0.1 (0.3) | 0.01 | 0 | |
| 17 | III | 1,000 | 7 | 2 | 88 (93) | 0.0 (0) | 0.00 | 0 | ||
| 18 | III | 5,410 | 150 | 9 | 4 | 40 (65) | 5 (8) | 0.13 | 7 ± 12 | |
| 19† | III | 14,230 | 350 | 4 | 5 | 75 (95) | 1 (1.4) | 0.01 | 40 ± 32 | |
| Mean ± SE | 11,220 ± 2,235 | 492 ± 119.3 | 10.7 ± 2.3 | 30 | 45.5 ± 12.8 | 2.07 ± 1.1 | 0.05 ± 0.02 | 97.3 ± 86.7 | ||
| B | 1 | II | 10,420 | 500 | 20 | 2 | 8 (16) | 13 (25) | 1.60 | 270 ± 45 |
| 4 | II | 11,680 | 400 | 40 | 7 | 10 (13) | 50 (70) | 5 | 2,410 ± 683 | |
| 6 | I | 10,050‡ | 150 | 20 | 1 | 4 (20) | 7 (36) | 1.80 | 750 | |
| 7 | I | 17,260 | 400 | 32 | 6 | 1.5 (6) | 10 (42) | 7 | 950 ± 460 | |
| 10 | II | 11,700 | 150 | 12 | 3 | 0.2 (1.8) | 4 (40) | 20 | 1,188 ± 98 | |
| 15* | I | 8,930 | 200 | 6 | 2 | 14 (33) | 13 (32) | 0.90 | 1,170 ± 760 | |
| 20* | II | 9,380 | 500 | 28 | 7 | 13 (34) | 8 (22) | 0.60 | 170 ± 112 | |
| 21* | II | 4,240 | 500 | 18 | 4 | 55 (88) | 4.5 (7) | 0.08 | 65 ± 21 | |
| 22† | II | 9,960 | 500 | 18 | 3 | 3.5 (7.5) | 20 (42) | 6 | 2,590 ± 592 | |
| 23* | II | 10,050 | 200 | 6 | 3 | 23 (46) | 13 (26) | 0.60 | 67 ± 70 | |
| Mean ± SE | 9,314 ± 1,348 | 350 ± 49.4 | 20 ± 3.4 | 38 | 13.22 ± 5.13 | 14.25 ± 4.25 | 4.35 ± 1.91 | 963 ± 290.5 | ||
| C | 2 | 0 | 11,220 | 350 | 16 | 1 | 5 (7) | 13 (19) | 2.6 | 1,130 |
| 5 | 0 | 7,170 | 250 | 10 | 3 | 0 (0) | 21 (67) | >20 | 4,100 ± 160 | |
| 9 | 0 | 6,950 | 450 | 3 | 2 | 0.7 (3) | 15 (64) | 21 | 5,496 ± 1,048 | |
| 16 | 0 | 14,780 | 200 | 12 | 2 | 7 (12) | 28 (52) | 4 | 748 ± 12 | |
| Mean ± SE | 10,030 ± 1,863 | 312 ± 55.4 | 10.25 ± 2.72 | 8 | 3.17 ± 1.69 | 19.25 ± 3.37 | 12.4 ± 5.26 | 2,868.5 ± 1,152.5 | ||
CLL/PLL.
PLL.
Not including paraprotein (patient also had myeloma).
ρ Percentage of total cells in peritoneal wash (% of lymphocytes in peritoneal wash).
Transplantation of BNX mice resulted in a similar engraftment pattern, yet when tumor cell engraftment was achieved (Table 2, patients no. 3 and 12), it lasted for about 70 days (data not shown), presumably as a result of a reduced capacity for host T-cell recovery in this immunodeficient strain.
The identification of engrafted CD5+CD20+ cells in the chimeric mice, as the original tumor cells used for transplantation, has been established by expression of the light chain in 29 chimeric mice that were typical for the tumor cells of their six respective donors (Fig 2).
Phenotypic characterization of light chain expressed on CD5+CD20+ lymphocytes recovered from human-<Balb/c chimera. Chimera were prepared by conditioning Balb/c mice with split irradiation followed by radioprotection with SCID BM; thereafter, the mice were transplanted with 100 to 1,000 × 106 human PBMC from BCLL patients. Cells were recovered from the peritoneum wash 14 days after transplantation.
Phenotypic characterization of light chain expressed on CD5+CD20+ lymphocytes recovered from human-<Balb/c chimera. Chimera were prepared by conditioning Balb/c mice with split irradiation followed by radioprotection with SCID BM; thereafter, the mice were transplanted with 100 to 1,000 × 106 human PBMC from BCLL patients. Cells were recovered from the peritoneum wash 14 days after transplantation.
Altogether, four different patterns of engraftment were found in the recipient mice: (1) engraftment mainly of human CLL cells, seven patients; (2) engraftment of human T cells, five patients; (3) combined engraftment of both human CLL cells and human T cells, eight patients; and (4) no human cell engraftment, three patients.
Rai's revised criteria2 13 include three prognostically important groups: stage 0, stage I to II, and stage III to IV. The tumor stage of the donor was found to be correlated with the pattern of engraftment, although similar numbers of T cells or tumor cells were infused (Table 2).
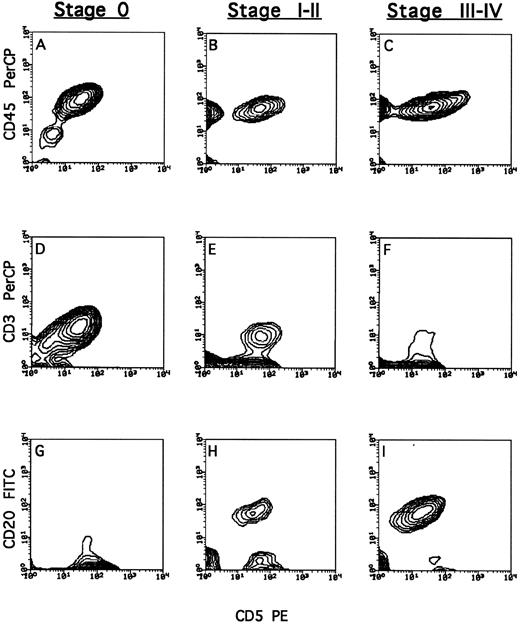
Transplantation of PBMC from patients in stage 0 led to marked engraftment of human T cells (19.3% ± 3.4% of total cells recovered from the peritoneal cavity), whereas human tumor cells could hardly be detected (3.2% ± 1.7%) (Fig 3A, D, and G). In contrast, chimeric mice receiving PBMC from patients in stage III to IV disease exhibited a dominance of tumor cells (45.5% ± 12.8%), compared with a miniscule level of T cells (2.1% ± 1.1%) (Fig 3C, F, and I). Infusion of PBMC from patients in stage I to II resulted in engraftment of both T cells (13.2% ± 5.1%) and tumor cells (14.2% ± 4.2%) (Fig 3B, E, and H).
Phenotypic characterization of lymphocytes recovered from human-<Balb/c chimera. Chimera were prepared by conditioning Balb/c mice with split irradiation followed by radioprotection with SCID BM; thereafter, the mice were transplanted with 100 to 1,000 × 106 human PBMC from BCLL patients. Cells were recovered from the peritoneum wash 14 days after transplantation. The total population of human lymphocytes (A, B, and C) was typed by staining with anti-CD45. T cells (D, E, and F ) were typed by triple staining of CD45+ cells with anti-CD3 and anti-CD5. BCLL cells (G, H, and I) were typed by triple staining of CD45+ cells with anti-CD20 and anti-CD5. Typical FACS analysis of cells from chimeric mice generated from patients in stage 0, I to II, and III to IV disease; patients no. 5 (A, D, and G), 20 (B, E, and H), and 18 (C, F, and I), respectively.
Phenotypic characterization of lymphocytes recovered from human-<Balb/c chimera. Chimera were prepared by conditioning Balb/c mice with split irradiation followed by radioprotection with SCID BM; thereafter, the mice were transplanted with 100 to 1,000 × 106 human PBMC from BCLL patients. Cells were recovered from the peritoneum wash 14 days after transplantation. The total population of human lymphocytes (A, B, and C) was typed by staining with anti-CD45. T cells (D, E, and F ) were typed by triple staining of CD45+ cells with anti-CD3 and anti-CD5. BCLL cells (G, H, and I) were typed by triple staining of CD45+ cells with anti-CD20 and anti-CD5. Typical FACS analysis of cells from chimeric mice generated from patients in stage 0, I to II, and III to IV disease; patients no. 5 (A, D, and G), 20 (B, E, and H), and 18 (C, F, and I), respectively.
The lack of engraftment following transplantation of PBMC from three of the total of 23 donors was probably related to technical difficulties rather than to disease characteristics.
As previously reported for Balb/c recipients of human PBMC,11 none of the transplanted mice in the present study developed detectable lymphoma.
Organ distribution of engrafted human cells.Lubin et al10 showed that human lymphocytes could be found as early as 1 day after transplantation not only in the peritoneum but also in the liver, lungs, and spleen of recipient mice. Studies of the organ distribution of engrafted cells, in the present investigation showed a major difference between chimeric mice transplanted with normal PBMC versus BCLL PBMC.
In contrast to the marked dissemination of normal T and B cells in recipients of normal PBMC,10 engraftment of the tumor B cells, in 15 independent experiments in which the dissemination of infused cells was analyzed, was restricted to the peritoneum, and the pathologic cells could not be detected in the internal organs tested. Extraperitoneal CLL cell dissemination was found only in mice receiving cells from four unique patients, three of whom had prolymphocytic features (patients no. 19, 20, and 22 in Table 1).
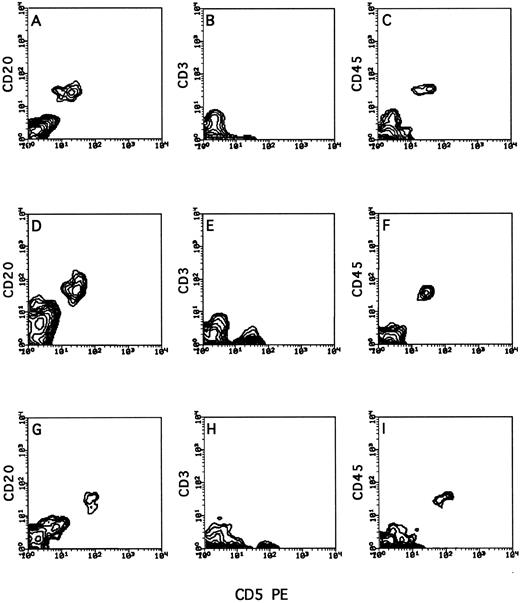
As can be seen in Fig 4, in which a typical engraftment pattern of tumor cells from an activated CLL/PL patient is shown, tumor cells were found not only in the peritoneum but also in the spleen (A, B, and C), liver (D, E, and F ), and lungs (G, H, and I). In the mice receiving PBMC from the five patients who were in the low stage of the disease (stage 0 to II), the engrafted human cells were uniformly T cells (Fig 5) and were found in the spleen, liver, lungs, and kidney, but only rarely in the BM and peripheral blood. In contrast, extremely low T-cell engraftment in the peritoneum or in any other internal organ was found in the recipients of 100% (six of six) of the high-stage CLL patients (Fig 3) or of the PLL patients (Fig 4), although the inoculum infused into the mice consisted of a total number of T cells similar to that infused into the recipients of PBMC from low-stage donors.
Typical organ distribution of human lymphocytes in human/mouse chimera following transplantation of PBMC from a stage II BCLL patient with prolymphocytic features. Human/mouse chimera were prepared as described in Fig 2. Two weeks after transplantation of human BCLL cells, mice were killed, and the recovered cells from different organs were isolated on Ficoll and tested by cytofluorometry for triple staining with anti-CD45, anti-CD3, and anti-CD20. Shown are cells recovered from the spleen (A, B, and C), liver (D, E, and F ), and lung (G, H, and I) of a chimera generated from patient no. 20.
Typical organ distribution of human lymphocytes in human/mouse chimera following transplantation of PBMC from a stage II BCLL patient with prolymphocytic features. Human/mouse chimera were prepared as described in Fig 2. Two weeks after transplantation of human BCLL cells, mice were killed, and the recovered cells from different organs were isolated on Ficoll and tested by cytofluorometry for triple staining with anti-CD45, anti-CD3, and anti-CD20. Shown are cells recovered from the spleen (A, B, and C), liver (D, E, and F ), and lung (G, H, and I) of a chimera generated from patient no. 20.
Typical organ distribution of human lymphocytes in human/mouse chimera following transplantation of PBMC from a stage 0 BCLL patient. Human/mouse chimera were prepared as described in Fig 2. Two weeks after transplantation of human BCLL cells, mice were killed, and the recovered cells from different organs were isolated on Ficoll and tested by cytofluorometry for triple staining with anti-CD45, anti-CD3, and anti-CD20. Shown are cells recovered from the spleen (A, B, and C), liver (D, E, and F ), and lung (G, H, and I) of a chimera generated from patient no. 2.
Typical organ distribution of human lymphocytes in human/mouse chimera following transplantation of PBMC from a stage 0 BCLL patient. Human/mouse chimera were prepared as described in Fig 2. Two weeks after transplantation of human BCLL cells, mice were killed, and the recovered cells from different organs were isolated on Ficoll and tested by cytofluorometry for triple staining with anti-CD45, anti-CD3, and anti-CD20. Shown are cells recovered from the spleen (A, B, and C), liver (D, E, and F ), and lung (G, H, and I) of a chimera generated from patient no. 2.
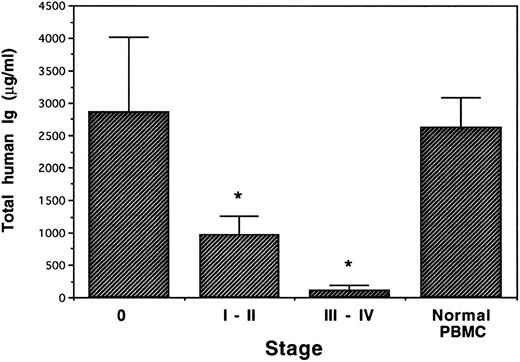
PBMC of BCLL patients exhibit different Ig production capacity in human/mouse chimera according to the stage of the disease.Human Ig production was determined 14 days after transplant. The production of human Ig by the engrafted cells was also found to be correlated with the donor disease stage (Fig 6), although all donors had a similar magnitude of hypogammaglobulinemia and there was no statistical difference in total leukocyte or T-cell number transplanted. Thus, although the mean IgG serum level of stage 0 donors before transplantation was significantly depressed (8,010 ± 1,350 μg/mL; Table 1), total human Ig production in the chimeric mice receiving PBMC from donors in stage 0 was similar (2,868 ± 1,152 μg/mL) to that found in control mice receiving PBMC from normal donors (2,627 ± 451 μg/mL). In contrast, following engraftment of PBMC from donors in stage III to IV (whose mean IgG serum level was 8,680 ± 1,710 μg/mL; Table 1), almost no human Ig could be detected (97 ± 86.7 μg/mL). Chimeric mice receiving PBMC from patients in stage I to II showed a moderately decreased human Ig production (963 ± 290 μg/mL).
Total human Ig in human/Balb chimera. PBMC from BCLL patients in different stages of disease and from normal donors were transplanted into conditioned Balb/c mice (4 patients in stage 0, 10 in stage I to II, six in stage III to IV, and 3 normal donors). Human antibody production was measured 2 weeks after transplantation by ELISA. *Significant in F test.
Total human Ig in human/Balb chimera. PBMC from BCLL patients in different stages of disease and from normal donors were transplanted into conditioned Balb/c mice (4 patients in stage 0, 10 in stage I to II, six in stage III to IV, and 3 normal donors). Human antibody production was measured 2 weeks after transplantation by ELISA. *Significant in F test.
DISCUSSION
Our results demonstrate that transplantation of PBMC from BCLL patients in different stages of disease into immunodeficient mice affords a sensitive in vivo model for investigation of human BCLL, and in particular for the study of the immune dysregulation that is characteristic of this disease.
SCID mice have been used previously as models for studying normal and malignant human hematopoietic cells (reviewed by Mosier6 ). Certain malignant lymphoid tumors have been successfully transplanted into SCID mice,14 including acute lymphoblastic leukemia (ALL),15-17 T-ALL,15 Burkitt's lymphoma line (Daudy),18 EBV lymphoma,19 multiple myeloma,20 and acute myeloid leukemia.21 Studies on BCLL have been hampered by the difficulty in establishing BCLL cell lines, since they are highly refractory to transformation by EBV.22 Zhu et al8 and Kawata et al7 have established BCLL cell lines by combined treatment with EBV, cyclosporin A, and serial passages in culture. Transplantation into SCID mice resulted in a lethal disease of two forms: malignant ascites when administered intraperitoneally, and multiple organ dissemination after intravenous inoculation, both resembling aggressive lymphoma or EBV-positive lymphoma, and not the stable phase of BCLL. Only one group described transplantation of fresh BCLL cells in SCID mice: when injected intraperitoneally, BCLL cells survived in the peritoneal cavity for several weeks but did not migrate to other organs, and the animals developed lethal human B-cell tumors that originated from EBV transformation of bystander (non-CLL) B cells.9 23
In the present study, we used the model recently described by Lubin et al10 that enables the adoptive transfer of human PBMC into lethally irradiated normal strains of mice radioprotected with SCID BM. It has been demonstrated that in these chimeric mice dissemination of engrafted lymphocytes is rapid, and toward the second week posttransplant engrafted B and T cells form follicles in the spleen and lymph nodes (T. Burakova, unpublished results, 1996). Polyclonal human antibodies were found in large quantities as early as 10 days posttransplant,10 and most importantly, the mice could mount a primary antibody response.24 In contrast to the human-SCID mice, the human/Balb chimera did not develop EBV lymphoma.11
We have now investigated the engraftment potential of T cells and tumor cells from BCLL patients and found a close correlation between the engraftment pattern of human cells in the peritoneal cavity and the stage of the disease according to Rai's criteria.2,13 Stage 0 BCLL cells were poorly engrafted, stage I to II cells exhibited only partial engraftment, and transplantation of cells from stage III to IV patients led to a marked engraftment of leukemic cells. The identification of engrafted CD5+CD20+ cells in the chimeric mice as cells expanded from the original tumor cells used for transplantation has been established by our finding that in 29 chimeric mice infused with PBMC from six different donors the engrafted CD5+CD20+ cells expressed the light chain typical for the tumor cells of their respective donors. Moreover, our previous results based on hundreds of human/mouse chimera transplanted with normal human PBMC repeatedly showed that (1) although CD20+ cells can be detected in the peritoneum or in the spleen in very low numbers,10 it is impossible to find double-stained CD5+CD20+ cells at these sites; and (2) both λ and κ light chains are always found in the serum of human/mouse chimera.24 A reversed pattern was found when analyzing T-cell engraftment following infusion of CLL PBMC. Good engraftment of T cells was associated with stage 0 donors, partial engraftment with stage I to II donors, and very poor engraftment with stage III to IV donors, although similar numbers of T cells were infused in all three groups.
Human Ig production determined 14 days after transplantation was also found to be correlated with the stage of the disease. It was correlated positively with T-cell engraftment and negatively with BCLL engraftment. Thus, although all three patient groups had originally displayed the same degree of hypogammaglobulinemia, chimeric mice transplanted with PBMC from stage 0 BCLL patients showed human Ig production similar to that found in control mice receiving the equivalent T-cell dose from PBMC of healthy donors. In contrast, transplantation with stages III to IV BCLL resulted in a very low human Ig titer and stages I to II a moderate level.
Considering that T-cell engraftment was not significantly different between recipients of cells from stage 0 donors or stage I to II donors, the observed difference in human Ig production was most likely related to the difference in BCLL cell engraftment.
Hypogammaglobulinemia is a distinct feature of BCLL and is rare in other B-cell malignancies.2,3 Regulatory abnormalities of T cells have been reported in BCLL, including decreased T helper function, increased suppressor activity, and abnormal response to cytokines such as interleukin-2.2 3 Although these abnormalities could induce decreased Ig production, their role in the hypogammaglobulinemia is unclear.
Previous studies showed a normal function of helper T cells from BCLL patients to allogeneic B cells, and suggested that there is an intrinsic B-cell defect in CLL.25,26 Natural killer cells from BCLL patients were shown to downregulate Ig production.27,28 Sera and supernatants from BCLL cells inhibit a variety of T-cell functions,29 but others could not demonstrate such suppressive activity.30 However, the preferred theory postulates that the accumulation of neoplastic B cells leads to hypogammaglobulinemia by diluting out normal B cells.30,31 It is also possible that inert pathologic B cells consume growth factors or perturb normal communication within the immune system, or that B cells may serve as unprofessional antigen-presenting cells that turn off naive T cells.32 Our present observation that normal Ig levels are attained in mice infused with cells from low-stage BCLL patients, compared with very low Ig production in chimera generated from high-stage BCLL (although the donors had the same magnitude of hypogammaglobulinemia or intrinsic T-cell dysfunction), rules out an intrinsic B-cell defect, at least in low-stage BCLL, and suggests that the engraftment of tumor cells or of normal B and T cells in the recipient mice is mutually exclusive or, alternatively, the BCLL cells may exert a suppressive effect on T-cell proliferation and Ig production. The selective engraftment of BCLL cells in the mouse model can be explained by two alternative mechanisms. Our preferred one is that low-stage BCLL cells exhibit a low engraftment capability in mouse peritoneum due to specific cytokine dependency or other unknown causes, whereas high-stage CLL cells are more malignant and therefore show better survival/proliferation. Alternatively, the differences in T-cell function might be the primary explanation. According to this theory, low-stage BCLL T cells have normal proliferating capacity in the human/mouse chimera, allowing them to induce normal Ig production and to reject BCLL cell engraftment, whereas high-stage T cells are defective and allow BCLL cell proliferation. Both theories may prove to be correct.
In addition, if the depressed Ig production in CLL patients is mediated by the tumor cells, the removal of low-stage BCLL by failure to engraft in the recipient mice may result in disappearance of the immune suppression and consequently in normal Ig production. This speculation is reminiscent of the observation by Saxon et al33 and Smith et al34 who found that transplantation of PBMC from patients with common variable immunodeficiency (known to be affected by T-cell– or monocyte-mediated suppression) into SCID mice results in normalized human antibody production comparable to that found in SCID mice transplanted with normal PBMC.
Clearly, further studies using adoptive transfer of isolated tumor cells and nonmalignant lymphocyte subpopulations at different ratios are required to verify the role of active suppression in this model.
Finally, our initial results regarding cell dissemination in the chimeric mice indicate that BCLL cells display unique homing properties. In contrast to normal PBMC, which can migrate outside the peritoneum to different internal organs, we found BCLL cell dissemination in only four patients, three of whom had prolymphocytic features. These results are in agreement with reports by Kobayashi et al,9 who showed that BCLL cells remain in the peritoneum of SCID mice and that organ dissemination could not be found even by using sensitive polymerase chain reaction techniques. Thus, it seems that BCLL cells may lack certain homing or adhesion molecules that are necessary for homing in the human/mouse chimera, whereas prolymphocytic cells retain this ability. Further studies might explain this observation by comparing the different phenotypes of CLL, PLL, and normal B cells vis-à-vis homing receptors and adhesion molecules.
In summary, the present study shows that PBMC of BCLL patients can be effectively engrafted into human/mouse chimera, generating an in vivo model of human BCLL. Using this model, we demonstrated that the different engraftment capacity of BCLL cells is correlated with the disease stage. Furthermore, the usefulness of this model for investigating the pathophysiology of BCLL is illustrated by our finding that in the chimeric mice generated by infusion of stage 0 BCLL PBMC the hypogammaglobulinemia of the donors is reversed.
Supported in part by grants from XTL Biopharmaceuticals Ltd, Rehovot, Israel.
Address reprint requests to Yair Reisner, PhD, Department of Immunology, The Weizmann Institute of Science, Rehovot 76100, Israel.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal