Abstract
CD30 ligand (CD30L) is a type-II membrane glycoprotein capable of transducing signals leading to either cell death or proliferation through its specific counterstructure CD30. Although several lines of evidence indicate that CD30L plays a key role as a paracrine- or autocrine-acting surface molecule in the deregulated cytokine cascade of Hodgkin's disease, little is known regarding its distribution and biologic significance in other human hematopoietic malignancies. By analyzing tumor cells from 181 patients with RNA studies and immunostaining by the anti-CD30L monoclonal antibody M80, we were able to show that human hematopoietic malignancies of different lineage and maturation stage display a frequent and broad expression of the ligand. CD30L mRNA and surface protein were detected in 60% of acute myeloid leukemias (AMLs), 54% of B-lineage acute lymphoblastic leukemias (ALLs), and in a consistent fraction (68%) of B-cell lymphoproliferative disorders. In this latter group, hairy cell leukemia and high-grade B-cell non-Hodgkin's lymphoma (B-NHL) expressed a higher surface density of CD30L as compared with B-cell chronic lymphocytic leukemia and low-grade B-NHL. Purified plasmacells from a fraction of multiple myeloma patients also displayed CD30L mRNA and protein. A more restricted expression of CD30L was found in T-cell tumors that was mainly confined to neoplasms with an activated peripheral T-cell phenotype, such as T-cell prolymphocytic leukemia, peripheral T-NHL, and adult T-cell leukemia/lymphoma. In contrast, none of the T-lineage ALLs analyzed expressed the ligand. In AML, a high cellular density of CD30L was detected in French-American-British M3, M4, and M5 phenotypes, which are directly associated with the presence on tumor cells of certain surface structures, including the p55 interleukin-2 receptor α-chain, the αM (CD11b) chain of β2 integrins, and the intercellular adhesion molecule-1 (CD54). Analysis of normal hematopoietic cells evidenced that, in addition to circulating and tonsil B cells, a fraction of bone marrow myeloid precursors, erythroblasts, and subsets of megakaryocytes also express CD30L. Finally, we have shown that native CD30L expressed on primary leukemic cells is functionally active by triggering both mitogenic and antiproliferative signals on CD30+ target cells. As opposed to CD30L, only 10 of 181 primary tumors expressed CD30 mRNA or protein, rendering therefore unlikely a CD30-CD30L autocrine loop in human hematopoietic neoplasms. Taken together, our data indicate that CD30L is widely expressed from early to late stages of human hematopoiesis and suggest a regulatory role for this molecule in the interactions of normal and malignant hematopoietic cells with CD30+ immune effectors and/or microenvironmental accessory cells.
PROLIFERATION OF malignant lymphohematopoietic cells is regulated by a complex network of cytokine-mediated and cell contact-dependent interactions with different cell types, including microenvironmental accessory cells and immune effectors.1-4 These autocrine and paracrine processes are mediated by a number of surface structures on tumor cells whose dysregulated expression may contribute to neoplastic transformation.5-8 Such molecules comprise cytokines and growth factor receptors, surface enzymes, cell adhesion molecules, and structures involved in the regulation of programmed cell death.6,7 9-11
The critical role of homotypic and heterotypic interactions among malignant cells and neighbouring cell populations12,13 is further strengthened by the recent characterization of the membrane-bound counterparts for a number of cytokines previously identified as soluble factors.14 For example, specific ligands (L) for class III tyrosine kinase receptors,15,16 including macrophage colony-stimulating factor, stem cell factor, and Flt-3L, are expressed by bone marrow (BM) stromal cells as membrane-anchored molecules, which are able to act simultaneously as growth factors and adhesion receptors.14-16 Similarly, most members of the emerging tumor necrosis factor (TNF ) ligand superfamily exist as multimeric membrane-bound molecules capable of transducing biologic signals and to act as adhesion molecules in different cell systems.17-19 This superfamily consists of different type-II transmembrane glycoproteins, such as TNF, lymphotoxin (LT)-α, LT-β, CD27L/CD70, CD40L, 4-1BBL, OX40L, CD95L/FASL, TNF-related apoptosis-inducing ligand, and CD30L.17-20 Each ligand elicits its biologic functions by binding to members of the parallel existing TNF/nerve growth factor (NGF ) receptor (R) superfamily, which includes TNFRI, TNFRII, TNFRIII, NGFRp75, CD27, CD40, 4-1BB, OX40, CD95 (FAS/APO-1), and CD30.17-19
CD30L is a 26- to 40-kD glycoprotein with pleiotropic cytokine-like activities as a result of signalling through CD30,21 a transmembrane receptor typically expressed on Hodgkin–Reed-Sternberg (H-RS) cells of Hodgkin's disease (HD) and on malignant cells of anaplastic large-cell lymphoma (ALCL).22,23 CD30L has been previously shown to be expressed on activated T cells, stimulated monocyte-macrophages, granulocytes, eosinophils, and some Burkitt-like lymphoma cell lines.21,24-28 Recombinant CD30L stimulates the proliferation of cultured H-RS cells, either directly or indirectly by enhancing the release of a number of cytokines such as interleukin-6 (IL-6), TNF, and LT-α.21,24,25,29,30 Similarly, CD30L induces the expression of surface accessory molecules (CD54, CD80, and CD86) on activated T cells, along with the secretion of both Th1- and Th2-type cytokines, including TNF, IL-2, interferon-γ, IL-4, and IL-5.27 31-34
As opposed to the great amount of data on CD30L-CD30 interactions in T-cell–mediated immune responses27,31-34 and in the pathophysiology of HD and ALCL,19,21,23,24,26,29,30,35 very little information is yet available on the expression of CD30L in human hematopoietic malignancies, with a special focus for acute leukemias and B- or T-cell lymphoproliferative disorders. By the combination of different methods, including reverse transcriptase-polymerase chain reaction (RT-PCR), Northern blotting, and immunostaining with the anti-CD30L monoclonal antibody (MoAb) M80,26 we have investigated the functional expression of CD30L in a broad series of phenotypically characterized primary human leukemia/lymphoma cells of myeloid or lymphoid origin and compared them with normal cells from different hematopoietic tissues.
MATERIALS AND METHODS
Cell and tissue samples.The study included cellular samples from peripheral blood (PB) or BM of 181 patients with acute myeloid leukemias (AML; n = 67); myeloid blast crisis (MBC) of chronic myeloproliferative disorders (n = 5); and B- and T-cell lymphoproliferations (n = 109), including B- and T-lineage acute lymphoblastic leukemias (ALL), chronic lymphocytic leukemia (CLL), prolymphocytic leukemia (PLL), hairy cell leukemia (HCL), high-grade and low-grade non-Hodgkin's lymphomas (NHL), multiple myeloma (MM), and adult T-cell leukemia/lymphoma (ATLL). Acute leukemias were classified according to the revised French-American-British (FAB) criteria by two different observers.36 FAB assignment was integrated by routine cytochemistry and by the presence of an immunophenotypic profile of blast cells consistent with FAB subgrouping (M0/M1/M2: CD13+, CD33+, CD34+/−, CD14−, CD15+/−, HLA-DR+; M3: CD13+, CD33+, CD34−, CD14−, CD15−/+, CD9+, HLA-DR−; M4/M5: CD13+, CD33+, CD34−/+, CD14+/−, CD15+, HLA-DR+; M6: glycophorin+; and M7: CD41+, CD61+), according to consesus international guidelines.37 NHL and other lymphoproliferative disorders were diagnosed by histopathologic examination of lymph nodes, BM, and PB smears and by immunophenotyping and were classified according to the International Working Formulation,38 taking also into account the featured cell lineage and phenotype.39 In addition, biopsies of uninvolved BM from 11 subjects undergoing staging procedures for solid tumors and 3 BM biopsies from patients affected by HCL were included in the study.
Cell isolation and purification.Anticoagulated PB and BM aspirates were collected from leukemia/lymphoma patients before therapy and after informed consent was obtained. Neoplastic cells were isolated by centrifugation on a Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) gradient and, with the exclusion of T cell-derived malignancies, further purified by T-cell depletion with anti-CD2 immunomagnetic beads (Dynabeads; Dynal, Oslo, Norway).40,41 In the case of MM, tumor cells were further purified by positive indirect immunoselection with the plasma cell-specific MoAb BB-4.42 After purification procedures, all of the samples contained more than 95% of neoplastic cells. Purification of normal cells from PB and tonsils was performed essentially as described.40,41 Briefly, Ficoll-Hypaque–separated mononuclear cells (MNCs) were subjected to a two-step positive selection using anti-CD2– or anti-CD19–conjugated immunomagnetic beads to obtain T and B lymphocytes, respectively.40,41 For immunofluorescence studies, T and B cells were purified with immunomagnetic beads by direct or indirect negative selection with anti-CD19, anti-CD14, and anti-CD16 (for T cells) or anti-CD2, anti-CD14, and anti-CD16 (for B cells). Adherent (Adh) monocytes were purified from MNCs by a two-step negative selection using anti-CD2– and anti-CD19–conjugated immunomagnetic beads, followed by 2 hours of incubation at 37°C in tissue culture dishes. Granulocytes were recovered from pellets of Ficoll-Hypaque–separated PB buffy coats and further purified by 1.2% dextran sedimentation, followed by lysis of contaminating red blood cells. The purity of positively and negatively selected cell fractions was always estimated by morphology and flow cytometric detection of surface lineage-associated antigens.9,40 41
T-cell activation.For activation studies, purified (CD2+, CD3+) T lymphocytes were exposed to 12-O-tetradecanoylphorbol-13-acetate (TPA; Sigma Chemical Co, St Louis, MO; 10 ng/mL) and ionomycin A (Sigma; 1.0 μg/mL) as described.40,41 Cells were collected at different time points from 12 to 72 hours and analyzed for CD30L and CD30 expression. T-cell activation was assessed by 3H-thymidine incorporation and two-color flow cytometry with anti-CD3, anti-CD25, anti-CD38, anti-CD11a, anti-CD54, and anti–HLA-DR MoAbs.40 41
Cell lines and culture conditions.The Epstein-Barr virus–negative Burkitt lymphoma cell line DG-75, the B-ALL cell line MN-60, the HD-derived cell lines HDLM-2 and L-428, and the CD30+ ALCL cell line Karpas 299 were obtained through the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany).25,40,43 The monoblastic cell line THP-1 was obtained through the American Type Culture Collection (Rockville, MD), whereas the BV-173 cell line (early B lymphoblasts obtained from a patient with lymphoid blast crisis of chronic myelogenous leukemia)41 was kindly provided by Dr K.H. Th'ng (Hammersmith Hospital, London, UK). All cell lines were cultured in Iscove's modified Dulbecco medium (GIBCO, Paisley, UK) supplemented with 20% fetal calf serum (GIBCO).
MoAbs, flow cytometry, and immunohistochemistry.Surface expression of CD30L and CD30 was analyzed by single-color or two-color immunofluorescence methods, essentially as described.25,26,40,41,43 Development and characterization of the anti-CD30L MoAb M80 (mouse IgG2b isotype) and the anti-CD30 MoAb M44 (mouse IgG1) were reported elsewhere.24,26 Irrelevant isotype-matched control mouse MoAbs and Igs were purchased from Dako Corp (Glostrup, Denmark) and Jackson's Immunoresearch Laboratories (West Grove, PA). As second-step reagents, fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated F(ab′)2 fragments of goat antimouse Ig (Jackson's Immunoresearch Laboratories) were used. FITC-conjugated anti-CD2 and anti-CD19, along with isotype-matched irrelevant control Igs, were from Becton Dickinson Immunocytometry Systems (San Jose, CA). Viable, antibody-labeled cells were identified according to their forward and side scatter, electronically gated, and assayed for surface fluorescence on a FACScan flow cytometer (Becton Dickinson). The sources and specificities of MoAbs used for immunophenotyping and lineage assessment of primary leukemia/lymphoma cells (CD34, CDw90, CD13, CD33, CD14, CD11b, CD11c, CD15, HLA-DR, CD9, CD41, CD61, CD25, CD7, CD3, CD4, CD2, CD5, CD19, CD20, CD22, CD23, CD21, CD10, BB-4, CD103, FMC-7, CD54, CD56, CD58, CD38, and anti-κ and anti-λ Ig light chains) have been reported in detail previously.40,41,43 BM biopsy cores were immediately fixed in Bouin's solution and then decalcified for an average time of 20 minutes, depending on their size. After thorough rinsing in tap water, biopsies were dehydrated and embedded in paraffin. Immunohistochemistry of deparaffinized tissue with the anti-CD30L MoAb M80 (10 μg/mL) was performed by the alkaline phosphatase antialkaline phosphatase (APAAP) method, as described.40,43 Negative control stains were performed by replacing the primary antibody with an irrelevant isotype-matched (IgG2b) mouse MoAb (10 μg/mL) and with chromogen only, as described.26 28
RNA isolation, RT-PCR, and Northern blot.Total RNA (1 μg), extracted using the guanidium thiocyanate method,40-42,44 was reverse-transcribed by avian myeloblastosis virus (AMV) reverse transcriptase (Promega Co, Madison, WI) in a 20-μL reaction mix containing hexadeoxyribonucleotide random primers (0.4 μg) for 1.0 hours at 42°C. Two microliters of the same cDNA bulks was amplified in a 50-μL volume of final reaction mix in a Perkin Elmer 9600 thermal cycler (Perkin Elmer Cetus, Emeryville, CA) with 25 pmol of primers specific for CD30L21 (sense, 5′-CCC TCC TGG AGA CAC AGC-3′, region 153-170; antisense, 5′-CCT GAA GGC CAA GAG AAA CTG-3′, region 841-821), CD3022 (sense, 5′-CTG TGT CCC CTA CCC AAT CT-3′, region 1121-1140; antisense, 5′-CTT CTT TCC CTT CCT CTT CCA-3′, region 1980-1960), and β-actin (Clontech Laboratories Inc, Palo Alto, CA; sense, region 578-609; antisense, region 1415-1384). PCR conditions for CD30 and CD30L were 4 minutes at 94°C, followed by 35 cycles of 45 seconds at 94°C and 1.5 minutes at 68°C (45 seconds at 62°C and 1 minute at 72°C for CD30), and a final extension of 5 minutes at 72°C. In the case of β-actin, amplification was performed for 30 cycles according to the manufacturer's guidelines. In some experiments, 10-μL aliquots of amplified products specific for CD30, CD30L, and β-actin were removed after 25, 30, or 35 cycles and analyzed separately. In all instances, 10 μL of amplified cDNAs was run on 1.5% agarose gels, blotted onto nylon membranes (Boehringer Mannheim, Mannheim, Germany) and hybridized with 2 × 106 cpm/mL of 5′ end-labeled oligoprobes that were specifically designated to recognize PCR products. The CD30L and CD30 probes spanned nucleotide positions 684-707 and 1216-1241, respectively.21 22
For Northern blot analysis, 10 μg/lane of each RNA sample was size-fractionated on 1% agarose gels containing 6.7% formaldehyde and subsequently blotted onto nylon membranes (Boehringer Mannheim).41 Filters were hybridized in 1.0 mol/L NaCl and 1% sodium dodecyl sulfate at 68°C with 1.0 × 106 cpm/mL of random primed-labeled probes, as described.41 After washing to a final stringency of 0.2× standard sodium cytrate and 0.1% sodium dodecyl sulfate at 65°C, filters were exposed to XAR-5 film (Eastman Kodak, Rochester, NY) at −80°C. The probes used were as follows: 0.7-kb Not I cDNA fragment containing the entire coding region of human CD30L21 (kindly provided by Dr R.G. Goodwin, Immunex Research and Development Corp, Seattle, WA) and 1.3-kb Pst I glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA fragment (courtesy of Dr G. Manfioletti, University of Trieste, Italy).
Proliferation assay.The CD30+ HDLM-2 and Karpas 299 cell lines were used as target cells to investigate the capability of native CD30L expressed on leukemia/lymphoma cells to transduce growth signals.21,24-26 To this end, HDLM-2 or Karpas 299 cells (1 × 105/mL) were cultured for 72 hours in the presence of a serial dilution (2 × 105/mL to 0.25 × 105/mL) of 0.5% paraformaldehyde-fixed primary leukemia/lymphoma cells in 96-well U-bottomed microplates, as previously described.21,24-26 Cultures were pulsed with 0.5 μCi/well 3H-thymidine (specific activity, 25 Ci/mmol; Amersham International, Amersham Place, UK) for the final 12 hours of culture, harvested onto glass fiber membranes, and counted in a liquid scintillator β-counter (Tri-Carb 1600TR; Canberra-Packard, Meriden, CT). To specifically block CD30-CD30L interactions,21,24,26 control experiments were performed in the presence of a fixed concentration (10 μg/mL) of soluble CD30-Fc, which was obtained by fusing the extracellular domains of CD30 and the Fc region of human IgG1.21,24 Results are expressed as mean counts per minute (cpm) ± SEM of quadruplicate cultures.21
Statistical analysis.The association between the expression of CD30L and other antigens in acute leukemias was statistically assessed by means of the χ2 test.45 Medical records of patients with acute leukemias (AML and B-lineage ALL) were reviewed. Clinical data at diagnosis included age, sex, percentage of circulating and BM blasts, white blood cell (WBC) count, absolute number of PB blasts, hemoglobin concentration, platelet count, absolute number of PB neutrophils, serum lactate dehydrogenase (LDH) levels, and the presence of infections. Differences in CD30L expression were assessed by means of the Student's t-test (age), the χ2 test (sex and presence of infections), or the Mann-Whitney test (other continous variables).45 In all cases, a P value less than .05 was considered statistically significant.45
RESULTS
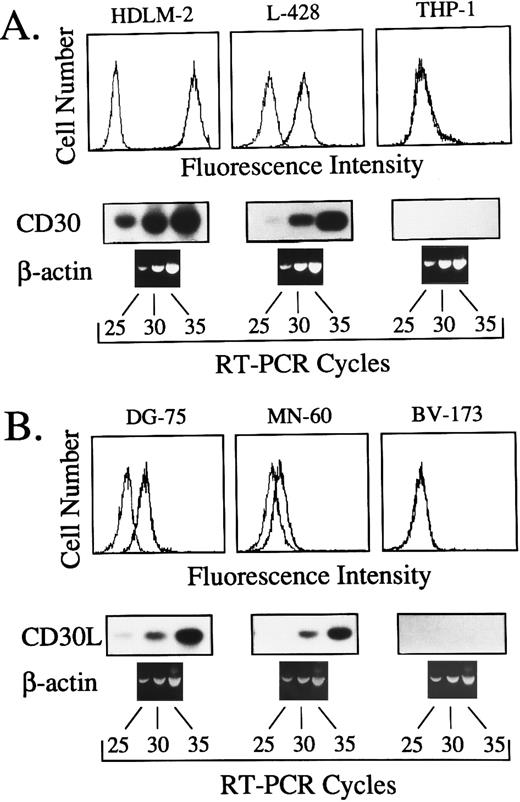
Expression of CD30L and CD30 in normal and malignant human lymphohematopoietic cells was analyzed by RT-PCR and flow cytometry. RT-PCR conditions were optimized by amplification kinetics of cDNAs from human cell lines expressing different amounts of surface CD30 and CD30L, as detected by flow cytometry.24 25 As shown in Fig 1, specific amplified products were clearly detectable after 35 cycles of RT-PCR in cell lines displaying both high and low amounts of surface CD30 (HDLM-2 and L-428) or CD30L (DG-75 and MN-60). Conversely, amplified bands were never detected in CD30 (THP-1) and CD30L (BV-173) negative control cell lines, up to 35 amplification cycles (Fig 1). Owing to the high specificity and sensitivity of our RT-PCR assay, 35 amplification cycles were judged to be optimal for expression studies on primary cells.
(A) Comparison between surface expression of CD30, as detected by the MoAb M44 (upper panels), and the kinetics of cDNA amplification after 25, 30, and 35 cycles with primers specific for CD30 (lower panels) in the human cell lines HDLM-2, L-428 and THP-1. (B) Comparison between surface expression of CD30L, as detected by the MoAb M80 (upper panels), and the kinetics of cDNA amplification performed for 25, 30, and 35 cycles with primers specific for CD30L (lower panels) in the human cell lines DG-75, MN-60, and BV-173. In both cases, the same cDNA bulks were also amplified with primers specific for the housekeeping gene β-actin.
(A) Comparison between surface expression of CD30, as detected by the MoAb M44 (upper panels), and the kinetics of cDNA amplification after 25, 30, and 35 cycles with primers specific for CD30 (lower panels) in the human cell lines HDLM-2, L-428 and THP-1. (B) Comparison between surface expression of CD30L, as detected by the MoAb M80 (upper panels), and the kinetics of cDNA amplification performed for 25, 30, and 35 cycles with primers specific for CD30L (lower panels) in the human cell lines DG-75, MN-60, and BV-173. In both cases, the same cDNA bulks were also amplified with primers specific for the housekeeping gene β-actin.
Expression of CD30L and CD30 by normal mature hematolymphoid cells.Previous studies using CD30-Fc staining and Northern blotting have shown that CD30L is expressed by activated, but not resting, peripheral T cells and monocytes.21,24,25,27 We have therefore used these cell types as controls for our RT-PCR experiments and immunostaining with the MoAb M80. As shown in Fig 2A, a 689-bp amplified product specific for CD30L was detected in circulating granulocytes, CD2−/CD19−/CD14+ adherent macrophages, and CD19+ B cells purified from PB and tonsils of two different donors (a and b). At variance with previous studies,21,25 27 low levels of CD30L mRNA were found in resting peripheral CD2+ T cells by RT-PCR in the absence, however, of membrane-expressed CD30L protein (Fig 2B). CD30L transcripts were strikingly upregulated after T-cell activation for 24 hours by TPA and ionomycin A (Fig 2A), which also resulted in the induction of membrane CD30L on about 40% of activated T cells (Fig 2B). In contrast, T lymphocytes from tonsil tissues displayed a constitutive expression of CD30L (Fig 2A), which was consistent with the presence on these cells of an activated immunoprofile (ie, CD25+, CD38+, CD54+, and HLA-DR+). In agreement with RT-PCR data, membrane CD30L was immunodetected by MoAb M80 on CD19+ B cells from PB (53% of positive cells) and tonsils (60% of positive cells; Fig 2B and data not shown). Finally, the detection of a 860-bp amplified band specific for CD30 was restricted to in vitro-activated circulating T lymphocytes and to tissue T and B cells from both tonsil samples (Fig 2A).
(A) Expression of CD30L and CD30 in normal lymphohematopoietic cells. Adherent (Adh+), CD2−, CD19−, CD14+ monocyte-macrophages were purified from PB by a two-step immunomagnetic selection. Normal T and B lymphocytes were purified from PB and tonsil tissues of two different donors (a and b) by positive immunomagnetic selection with anti-CD2 and anti-CD19 MoAbs. Granulocytes were recovered by dextran sedimentation followed by erythrocyte lysis. Activation of CD2+ T cells was performed by 24 hours of exposure to TPA (10 ng/mL) and ionomycin A (1.0 μg/mL). In all cases, amplification with CD30L, CD30 (upper panels), and β-actin (lower panel) specific primers was performed using the same cDNA bulks. (B) Two-color immunofluorescence showing the expression of CD30L on PB CD19+ B cells, resting CD2+ T cells, and CD2+ T cells after 24 hours of activation by 10 ng/mL TPA and 1.0 μg/mL ionomycin A. Cells were stained by the anti-CD30L MoAb M80 (Y-axis, PE-red fluorescence) and by anti-CD2 or anti-CD19 MoAbs (X-axis, FITC-green fluorescence).
(A) Expression of CD30L and CD30 in normal lymphohematopoietic cells. Adherent (Adh+), CD2−, CD19−, CD14+ monocyte-macrophages were purified from PB by a two-step immunomagnetic selection. Normal T and B lymphocytes were purified from PB and tonsil tissues of two different donors (a and b) by positive immunomagnetic selection with anti-CD2 and anti-CD19 MoAbs. Granulocytes were recovered by dextran sedimentation followed by erythrocyte lysis. Activation of CD2+ T cells was performed by 24 hours of exposure to TPA (10 ng/mL) and ionomycin A (1.0 μg/mL). In all cases, amplification with CD30L, CD30 (upper panels), and β-actin (lower panel) specific primers was performed using the same cDNA bulks. (B) Two-color immunofluorescence showing the expression of CD30L on PB CD19+ B cells, resting CD2+ T cells, and CD2+ T cells after 24 hours of activation by 10 ng/mL TPA and 1.0 μg/mL ionomycin A. Cells were stained by the anti-CD30L MoAb M80 (Y-axis, PE-red fluorescence) and by anti-CD2 or anti-CD19 MoAbs (X-axis, FITC-green fluorescence).
Expression of CD30L by normal BM hematopoietic cells.As indicated by anti-CD30L MoAb M80 staining of BM biopsies, a strong cytoplasmic expression of CD30L was detected in most erythroid precursors (Fig 3A), subsets of megakaryocytes (Fig 3B, arrowhead), and myeloid cells at different maturation stages and eosinophils (arrows in Fig 3A through C). In cells of the erythroid and myeloid lineages, a typical dot-like staining pattern was usually observed, whereas immunoreactivity appeared as large block-like deposits in megakaryocytes (Fig 3). Moreover, in the great majority of the eosinophils, expression of CD30L was characterized by a disperse granular staining of cytoplasms (Fig 3C, arrow). Thus, normal BM hematopoietic cells of different cell lineages constitutively express CD30L with a high cytoplasmic density.
Paraffin-embedded tissue section from normal BM stained with the anti-CD30L MoAb M80 (A, B, and C) or isotype-matched control mouse MoAb (D). A typical paranuclear dot-like staining was detected in cells of the erythroid (A) and myeloid (A and B; arrows) lineages and as large block-like deposits in subset of megakaryocytes (B; arrowhead). In most of eosinophils a disperse granular staining of cytoplasms (C; arrow) was observed. APAAP immunostaining; hematoxylin counterstain. (Original magnification [A through C] × 630; [D] × 200.)
Paraffin-embedded tissue section from normal BM stained with the anti-CD30L MoAb M80 (A, B, and C) or isotype-matched control mouse MoAb (D). A typical paranuclear dot-like staining was detected in cells of the erythroid (A) and myeloid (A and B; arrows) lineages and as large block-like deposits in subset of megakaryocytes (B; arrowhead). In most of eosinophils a disperse granular staining of cytoplasms (C; arrow) was observed. APAAP immunostaining; hematoxylin counterstain. (Original magnification [A through C] × 630; [D] × 200.)
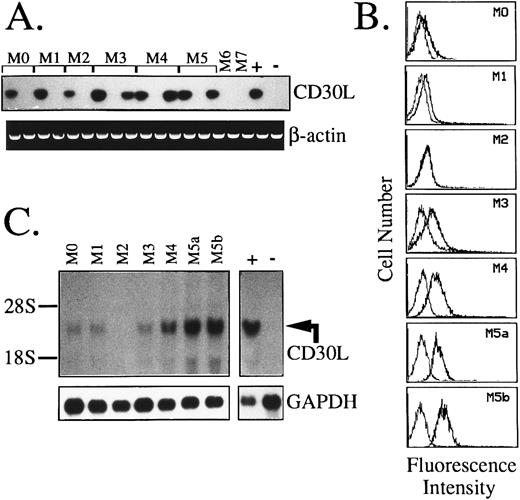
Expression of CD30L and CD30 in AML.Results of RT-PCR and flow cytometric analysis of 67 AML cases and 5 cases of MBC evolved from previous chronic myeloproliferative disorders are summarized in Table 1. As opposed to CD30, which is detectable in less than 6% of cases (4/72), a broad expression of CD30L was found in these malignancies. As shown in Table 1 and Fig 4A, 40 of 67 (60%) AML cases displayed CD30L both at RNA and surface protein level. A very high frequency of CD30L expression (82%) was detected in monocytic and myelomonocytic subtypes of AML (FAB M4 and M5), whereas, among immature-intermediate AML phenotypes (FAB M0, M1, and M2), CD30L+ samples accounted for 36% of the cases (P = .001). In addition, more than 50% of acute promyelocytic leukemias (FAB M3) displayed CD30L RNA and protein, which were also detected in almost all (4/5) blast cell samples from MBC (Table 1). Coexpression of CD30L and CD30 was only detected in 2 AML cases of immature (FAB M1) phenotype (Table 1). The presence of CD30 at a low membrane density in these AML cases was confirmed by dual fluorescence staining with anti-CD33 and anti-CD13 MoAbs (not shown). As shown by representative fluorescence histograms presented in Fig 4B, the relative cellular density of surface CD30L appeared heterogeneous among the different FAB subtypes of AML. In detail, leukemic samples from promyelocytic (FAB M3) and monocytic-oriented leukemias (FAB M4 and M5) displayed a higher percentage of CD30L+ blasts (ranging from 35% to 92% of stained cells), as compared with other AMLs (23% to 38% of positive cells), including M0, M1, and M2 phenotypes (Table 1 and Fig 4B). Northern blot analysis of representative leukemia cases (Fig 4C) showed the presence of a single specific transcript of about 3.0 kb and confirmed the highest expression of CD30L message in monocytic-oriented AMLs.
Expression of CD30 and CD30L in Human Myeloid Hematopoietic Malignancies
| Diagnosis . | CD30 . | CD30L . | ||
|---|---|---|---|---|
| . | No. Positive/No. Tested . | % Stained Cells (range)* . | No. Positive/No. Tested . | % Stained Cells (range) . |
| AML | 4/67 | 22-26† | 40/67 | 23-92 |
| M0 | 0/3 | — | 1/3 | 23 |
| M1 | 2/16 | 22-26 | 7/16‡ | 24-38 |
| M2 | 0/6 | — | 1/6 | 24 |
| M3 | 0/7 | — | 4/7 | 35-43 |
| M4 | 2/17 | 23-25 | 13/17 | 35-72 |
| M5 | 0/16 | — | 14/16 | 48-92 |
| M6 | 0/1 | — | 0/1 | — |
| M7 | 0/1 | — | 0/1 | — |
| MBC | 0/5 | — | 4/5 | 25-35 |
| CML | 0/3 | — | 2/3 | 25-35 |
| PV | 0/1 | — | 1/1 | 28 |
| IM | 0/1 | — | 1/1 | 31 |
| Diagnosis . | CD30 . | CD30L . | ||
|---|---|---|---|---|
| . | No. Positive/No. Tested . | % Stained Cells (range)* . | No. Positive/No. Tested . | % Stained Cells (range) . |
| AML | 4/67 | 22-26† | 40/67 | 23-92 |
| M0 | 0/3 | — | 1/3 | 23 |
| M1 | 2/16 | 22-26 | 7/16‡ | 24-38 |
| M2 | 0/6 | — | 1/6 | 24 |
| M3 | 0/7 | — | 4/7 | 35-43 |
| M4 | 2/17 | 23-25 | 13/17 | 35-72 |
| M5 | 0/16 | — | 14/16 | 48-92 |
| M6 | 0/1 | — | 0/1 | — |
| M7 | 0/1 | — | 0/1 | — |
| MBC | 0/5 | — | 4/5 | 25-35 |
| CML | 0/3 | — | 2/3 | 25-35 |
| PV | 0/1 | — | 1/1 | 28 |
| IM | 0/1 | — | 1/1 | 31 |
CD30 and CD30L expression was evaluated by RT-PCR and immunofluorescence staining using the anti-CD30 MoAb M44 or the anti-CD30L MoAb M80.
Abbreviations: AML, acute myeloid leukemia; MBC, myeloid blast crisis of myeloproliferative disorders; CML, chronic myeloid leukemia; PV, polycythemia vera; IM, idiopathic myelofibrosis.
As evaluated by flow cytometry.
Figures refer to positive cases, ie, displaying more than 20% of stained cells in flow cytometry.
Two cases coexpressed CD30 as evaluated by both RT-PCR and flow cytometry.
Expression of CD30L in leukemic cells of myeloid origin as assessed by RT-PCR (A), flow cytometry (B), and Northern blot analysis (C). (A) cDNA bulks from AML samples (FAB-M0, n = 2; M1, n = 2; M2, n = 2; M3, n = 3; M4, n = 3; M5, n = 3; M6, n = 1; and M7, n = 1) were prepared and amplified with specific primers for CD30L (upper panel) or β-actin (lower panel). The MN-60 and the BV-173 cell lines were used as positive (+) and negative (−) controls, respectively. (B) Flow cytometry analysis of representative AML cases. Cells were incubated with the anti-CD30L MoAb M80 (bold line) and an irrelevant isotype-matched mouse Ig (thin line), followed by PE-labeled goat antimouse Ig. The X- and Y-axes indicate the logarithm of the relative intensity of red fluorescence and relative cell numbers, respectively. (C) Northern blot analysis of the same AML cases shown in (B). Twenty micrograms of total RNA per lane was run on denaturated agarose gels, blotted onto nylon membranes, and hybridized with a CD30L-specific cDNA probe (upper panel) and with a GAPDH cDNA fragment (lower panel); −, negative control (BV-173 cell line); +, positive control (MN-60 cell line).
Expression of CD30L in leukemic cells of myeloid origin as assessed by RT-PCR (A), flow cytometry (B), and Northern blot analysis (C). (A) cDNA bulks from AML samples (FAB-M0, n = 2; M1, n = 2; M2, n = 2; M3, n = 3; M4, n = 3; M5, n = 3; M6, n = 1; and M7, n = 1) were prepared and amplified with specific primers for CD30L (upper panel) or β-actin (lower panel). The MN-60 and the BV-173 cell lines were used as positive (+) and negative (−) controls, respectively. (B) Flow cytometry analysis of representative AML cases. Cells were incubated with the anti-CD30L MoAb M80 (bold line) and an irrelevant isotype-matched mouse Ig (thin line), followed by PE-labeled goat antimouse Ig. The X- and Y-axes indicate the logarithm of the relative intensity of red fluorescence and relative cell numbers, respectively. (C) Northern blot analysis of the same AML cases shown in (B). Twenty micrograms of total RNA per lane was run on denaturated agarose gels, blotted onto nylon membranes, and hybridized with a CD30L-specific cDNA probe (upper panel) and with a GAPDH cDNA fragment (lower panel); −, negative control (BV-173 cell line); +, positive control (MN-60 cell line).
In AML cases, a high cellular density of CD54 (≥50% of positive cells) along with the presence of the p55 IL-2 receptor α-chain (CD25) and the αM (CD11b) chain of β2 integrins were significantly associated with the expression of CD30L on leukemic cells (P < .01). In addition, an inverse association, albeit of borderline statistical significance, emerged with the stem cell antigen CD34. However, it is noteworthy that 5 of 6 acute leukemias (1 CML-MBC and 5 AMLs) with the early stem cell-like phenotype CD34+/CDw90+,46 47 displayed CD30L mRNA and surface protein.
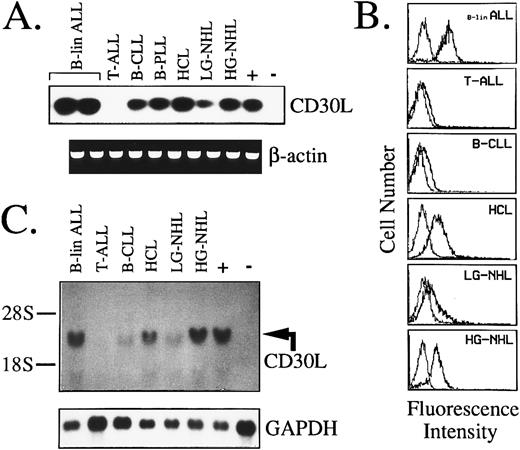
Expression of CD30L and CD30 by malignant cells of the lymphoid lineage.As seen for myeloid cells, in a series of 109 lymphoid tumors of B- and T-cell origin, expression of CD30 mRNA and protein was confined to high-grade B-NHL (3/9) and malignant plasma cells of MM (3/7; Table 2). In contrast, more than 65% of B-cell malignancies expressed CD30L (Table 2 and Fig 5A). In detail, more than 50% of B-lineage ALLs and high-grade B-NHLs, along with 65% to 85% of low-grade lymphoproliferative disorders, including B-CLL, HCL, and B-NHL, expressed CD30L mRNA and surface protein (Table 2). As shown by representative fluorescence histograms presented in Fig 5B, B-lineage ALLs and high-grade B-NHL usually displayed a higher cellular density of CD30L, as opposed to lymphoproliferative disorders such as B-CLL and low-grade B-NHL. In contrast, tumor cells of HCL expressed CD30L at a high surface density, as evidenced by a strong reactivity with the MoAb M80 both in flow cytometry (Fig 5B) and after immunostaining of HCL-involved BM biopsies (data not shown). CD103+/CD30L+ hairy cells were shown by dual fluorescence staining of BM aspirates and PB samples from all HCL patients examined. Immunopurified BB-4+ malignant plasma cells from the BM of MM patients also displayed CD30L expression in about 50% of cases, and in 2 of 7 samples CD30/CD30L coexpression by tumor cells was evidenced (Table 2). Northern blotting analysis overall confirmed immunofluorescence data, with the highest amount of CD30L transcripts being detected in B-lineage ALLs, HCL, and high-grade NHL (Fig 5C). Immature T-cell malignancies appeared overall to lack CD30L expression, because 10 cases of T-ALL and 1 high-grade T-NHL were all negative at both RNA and protein level (Table 2 and Fig 5A through C). However, CD30L message and surface expression were detected in 2 ATLL cases, 1 of 2 T-PLLs, and 1 low-grade T-NHL (Table 2).
Expression of CD30 and CD30L in Human Lymphoid Hematopoietic Malignancies
| Diagnosis . | CD30 . | CD30L . | ||
|---|---|---|---|---|
| . | No. Positive/No. Tested . | % Stained Cells (range)* . | No. Positive/No. Tested . | % Stained Cells (range) . |
| B-cell tumors | 6/93 | 22-26† | 61/93 | 22-94 |
| B-lineage ALL | 0/11 | — | 6/11 | 38-94 |
| B-CLL | 0/47 | — | 33/47 | 22-35 |
| B-PLL | 0/3 | — | 1/3 | 27 |
| HCL | 0/7 | — | 6/7 | 45-79 |
| B-NHL HG | 3/9 | 22-25 | 5/9 | 39-85 |
| LG | 0/9 | — | 6/9 | 25-37 |
| MM | 3/7 | 23-26 | 4/7‡ | 30-45 |
| T-cell tumors | 0/16 | — | 4/16 | 35-56 |
| T-ALL | 0/10 | — | 0/10 | — |
| T-PLL | 0/2 | — | 1/2 | 46 |
| T-NHL HG | 0/1 | — | 0/1 | — |
| LG | 0/1 | — | 1/1 | 35 |
| ATLL | 0/2 | — | 2/2 | 45-56 |
| Diagnosis . | CD30 . | CD30L . | ||
|---|---|---|---|---|
| . | No. Positive/No. Tested . | % Stained Cells (range)* . | No. Positive/No. Tested . | % Stained Cells (range) . |
| B-cell tumors | 6/93 | 22-26† | 61/93 | 22-94 |
| B-lineage ALL | 0/11 | — | 6/11 | 38-94 |
| B-CLL | 0/47 | — | 33/47 | 22-35 |
| B-PLL | 0/3 | — | 1/3 | 27 |
| HCL | 0/7 | — | 6/7 | 45-79 |
| B-NHL HG | 3/9 | 22-25 | 5/9 | 39-85 |
| LG | 0/9 | — | 6/9 | 25-37 |
| MM | 3/7 | 23-26 | 4/7‡ | 30-45 |
| T-cell tumors | 0/16 | — | 4/16 | 35-56 |
| T-ALL | 0/10 | — | 0/10 | — |
| T-PLL | 0/2 | — | 1/2 | 46 |
| T-NHL HG | 0/1 | — | 0/1 | — |
| LG | 0/1 | — | 1/1 | 35 |
| ATLL | 0/2 | — | 2/2 | 45-56 |
CD30 and CD30L expression was evaluated by RT-PCR and immunofluorescence staining using the anti-CD30 MoAb M44 or the anti-CD30L MoAb M80.
Abbreviations: ALL, acute lymphoblastic leukemia; CLL, chronic lymphoblastic leukemia; PLL, prolymphocytic leukemia; HCL, hairy cell leukemia; NHL, non-Hodgkin's lymphoma; HG, high grade; LG, low grade; MM, multiple myeloma; ATLL, adult T-cell leukemia lymphoma.
As evaluated by flow cytometry.
Figures refer to positive cases, ie, displaying more than 20% of stained cells in flow cytometry.
Two cases coexpressed CD30 as evaluated by both RT-PCR and flow cytometry.
Expression of CD30L in malignant cells from lymphoid tumors as assessed by RT-PCR (A), flow cytometry (B), and Northern blot analysis (C). (A) cDNA bulks were amplified with primer pairs specific for CD30L (upper panel) or β-actin (lower panel). The MN-60 and the BV-173 cell lines were used as positive (+) and negative (−) controls, respectively. (B) Flow cytometry analysis of representative cases of lymphoid malignancies. Cells were either incubated with the anti-CD30L MoAb M80 (bold line) or irrelevant isotype-matched mouse Ig (thin line), followed by PE-labeled goat antimouse Ig. The X- and Y-axes indicate the logarithm of the relative intensity of red fluorescence and relative cell numbers, respectively. (C) Northern blot analysis of the same cases shown in (B). Twenty micrograms of total RNA per lane was run on denaturated agarose gel, blotted onto nylon membranes, and hybridized with a CD30L-specific cDNA probe (upper panel) and with a GAPDH cDNA fragment (lower panel); +, positive control (MN-60 cell line); −, negative control (BV-173 cell line).
Expression of CD30L in malignant cells from lymphoid tumors as assessed by RT-PCR (A), flow cytometry (B), and Northern blot analysis (C). (A) cDNA bulks were amplified with primer pairs specific for CD30L (upper panel) or β-actin (lower panel). The MN-60 and the BV-173 cell lines were used as positive (+) and negative (−) controls, respectively. (B) Flow cytometry analysis of representative cases of lymphoid malignancies. Cells were either incubated with the anti-CD30L MoAb M80 (bold line) or irrelevant isotype-matched mouse Ig (thin line), followed by PE-labeled goat antimouse Ig. The X- and Y-axes indicate the logarithm of the relative intensity of red fluorescence and relative cell numbers, respectively. (C) Northern blot analysis of the same cases shown in (B). Twenty micrograms of total RNA per lane was run on denaturated agarose gel, blotted onto nylon membranes, and hybridized with a CD30L-specific cDNA probe (upper panel) and with a GAPDH cDNA fragment (lower panel); +, positive control (MN-60 cell line); −, negative control (BV-173 cell line).
No clear correlations emerged between CD30L expression in B-lineage ALLs and the relative maturation stage of leukemic cells, as indicated by the presence of CD34, CD19, CD22, CD10, and CD20 antigens. However, a significant surface expression of the αM (CD11b) chain of β2 integrins (35% to 50% of blasts) was detected in all CD30L+ ALLs (6/6) but in none of the CD30L− ALL cases.
Correlations of CD30L expression with clinical features in acute leukemias.The clinical features at diagnosis of patients with acute leukemias (AML and B-lineage ALL) were analyzed according to CD30L expression. Interestingly, as compared with CD30L− cases, CD30L+ acute leukemias were characterized by significantly (P < .05) higher values of circulating blasts absolute number (median, 19.7 × 109/L [range, 1.1 to 225.0 × 109/L] v 8.8 × 109/L [range, 0.9 to 203 × 109/L]) and total WBC counts (median, 31.7 × 109/L [range, 1.7 to 250.0 × 109/L] v 13.8 × 109/L [range, 1.2 to 245.0 × 109/L]), along with a lower value of platelet absolute number (median, 38.5 × 103/μL [range, 10.0 to 548.0 × 103/μL] v 69.0 × 103/μL [range, 6.0 to 371.0 × 103/μL]). In addition, although not reaching statistical significance, a more than twofold higher median value of serum LDH was observed in CD30L+ acute leukemias (1,280.5 UI/L [range, 140.0 to 4,900.0 UI/L] v 610.0 UI/L [range, 225.0 to 12,000 UI/L]), with respect to CD30L− cases. When separately analyzed, B-lineage ALL patients with CD30L+ lymphoblasts were also significantly (P = .02) older (mean age, 49.0 years) than those with CD30L− ALLs (mean age, 22.8 years). No stastistically significant differences were conversely found between CD30L expression and the other clinical features analyzed, including the percentage of BM or PB blasts, hemoglobin values, the absolute number of PB neutrophils, and the presence of infections.
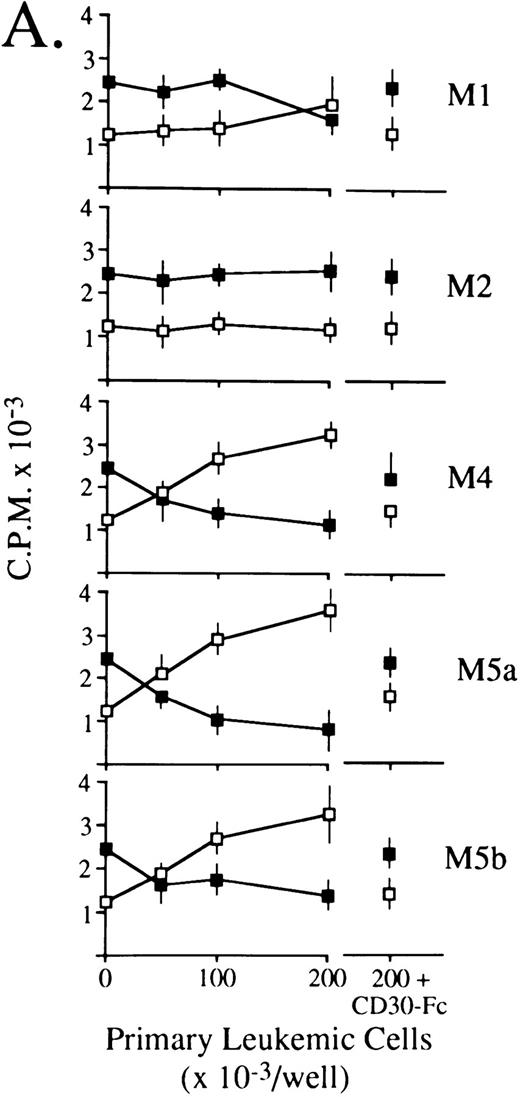
CD30L as expressed by leukemic cells of myeloid and lymphoid origin is functionally active.Previous studies have shown that some CD30+ cell lines respond to membrane-expressed and recombinant CD30L by being either growth-stimulated (HDLM-2) or growth-inhibited (Karpas 299).21,24-26 Accordingly, titrations of 0.5% paraformaldehyde-fixed primary leukemic cells expressing (5 AMLs of different FAB subtype, 1 B-lineage ALL, 1 B-CLL, 1 HCL, and 1 high-grade B-NHL) or lacking (1 AML FAB-M2 and 1 T-ALL) surface CD30L were cocultured with constant aliquots of viable HDLM-2 or Karpas 299 cells in a standard 3H-thymidine incorporation assay. As shown in Fig 6, CD30L-expressing leukemia/lymphoma cells induced a dose-dependent proliferation of HDLM-2 cells (up to 3-fold over baseline values) and inhibited the growth of the Karpas 299 cell line by more than 70%. The extent of optimal stimulatory or inhibitory effects reflected overall the relative cellular density of CD30L on leukemic cells, which is higher in monocytic (FAB M4/M5) AMLs (Fig 6A), B-lineage ALL, HCL, and high-grade B-NHL (Fig 6B) as compared with the other malignant cell types (ie, FAB M1 AMLs, B-CLL, and low-grade B-NHL). Conversely, CD30L− leukemic cells from an FAB M2 AML (Fig 6A) and a T-ALL (Fig 6B) did not elicit any proliferative effect on CD30+ target cell lines. The CD30L-mediated functional effects of leukemia cells appeared to be specific, because both growth-stimulatory (HDLM-2) and -inhibitory (Karpas 299) effects could be blocked by an excess (10 μg/mL) of soluble CD30-Fc fusion protein (Fig 6). Thus, native CD30L on human leukemia cells is a functionally active surface structure able to transduce CD30-mediated growth signals in a fashion that completely overlaps the previously reported biologic activities of human recombinant and native CD30L.21 24-26
Biologic activity of native CD30L expressed on the surface of malignant cells from myeloid (A) and lymphoid (B) tumors as assessed by proliferation assays for HDLM-2 (□) or Karpas 299 (▪) in the presence of a titration of paraformaldehyde-fixed leukemia/lymphoma cells. Primary leukemia/lymphoma cells (0.5 × 105/mL to 2.0 × 105/mL) were fixed in 0.5% paraformaldehyde and cocultured in 96-well U-bottomed microplates with 1 × 105 cells/mL HDLM-2 or Karpas 299 cells for 72 hours. Cultures were pulsed with 0.5 μCi/well 3H-thymidine for the final 12 hours of culture, harvested onto glass fiber membranes, and counted in a liquid scintillator β-counter. As further controls, 2.0 × 105/mL paraformaldehyde-fixed leukemia/lymphoma cells were cocultured as described above with HDLM-2 (□ at the far right) or Karpas 299 (▪ at the far right) cells in the presence of an excess (10 mg/mL) of soluble CD30-Fc fusion protein. Results are expressed as cpm ± SEM of quadruplicate cultures and are representative of three independent experiments.
Biologic activity of native CD30L expressed on the surface of malignant cells from myeloid (A) and lymphoid (B) tumors as assessed by proliferation assays for HDLM-2 (□) or Karpas 299 (▪) in the presence of a titration of paraformaldehyde-fixed leukemia/lymphoma cells. Primary leukemia/lymphoma cells (0.5 × 105/mL to 2.0 × 105/mL) were fixed in 0.5% paraformaldehyde and cocultured in 96-well U-bottomed microplates with 1 × 105 cells/mL HDLM-2 or Karpas 299 cells for 72 hours. Cultures were pulsed with 0.5 μCi/well 3H-thymidine for the final 12 hours of culture, harvested onto glass fiber membranes, and counted in a liquid scintillator β-counter. As further controls, 2.0 × 105/mL paraformaldehyde-fixed leukemia/lymphoma cells were cocultured as described above with HDLM-2 (□ at the far right) or Karpas 299 (▪ at the far right) cells in the presence of an excess (10 mg/mL) of soluble CD30-Fc fusion protein. Results are expressed as cpm ± SEM of quadruplicate cultures and are representative of three independent experiments.
DISCUSSION
Recently, several lines of evidence have indicated that the ligand for the CD30 antigen (CD30L) plays a key role as a paracrine- or autocrine-acting surface molecule involved in the deregulated cytokine cascade characteristic of HD.19,26 35 In contrast, little is known on the distribution and biologic significance of CD30L in other human hematopoietic malignancies, including acute leukemias and lymphoproliferative disorders. Our analysis of malignant cells from 181 leukemia/lymphoma patients showed that hematopoietic tumors of different lineage and maturation stage display a frequent and broad expression of CD30L, which was detected in 60% of AMLs, 54% of B-lineage ALLs, a consistent fraction (68%) of B-cell lymphoproliferative disorders, and in a subset of mature T-cell leukemias.
In acute leukemias, the highest frequency and cellular density of CD30L expression was detected in acute promyelocytic leukemias (FAB M3), monocytic-oriented AMLs (FAB M4 and M5), and B-lineage ALLs. CD30L expression in AMLs was also statistically associated with the presence on leukemic blasts of the p55 IL-2 receptor (CD25), the intercellular adhesion molecule-1 (CD54), and the αM chain of β2 integrins (CD11b).9,10 Interestingly, a consistent surface expression of the CD11b integrin was also found in all CD30L+ B-lineage ALLs. Moreover, even though an inverse association of borderline statistical significance between the CD34 antigen and CD30L emerged from our study, 5 of 6 AML cases coexpressing CD34 and CDw90/Thy-1 displayed surface CD30L. The presence of CD30L in acute leukemias appeared, therefore, associated with phenotypic traits of tumor cells previously shown to identify leukemia subsets with peculiar biologic and clinical features, including the expression of certain adhesion receptors, enhanced proliferative response to cytokines, capability of autonomous growth in vitro, and unfavorable prognostic factors.5 46-52 In this regard, we have found a statistically significant direct association in acute leukemias between the presence of CD30L on tumor cells and the absolute number of circulating blasts and total WBC counts, along with an inverse association with the absolute number of platelets.
A more restricted occurrence of CD30L was found in human T-cell malignancies. In these tumors, expression of the ligand appeared overall confined to neoplasms reflecting activated peripheral T-cells, ie, T-PLL, peripheral T-NHL, and ATLL,39,52 because none of the 10 T-lineage ALL cases analyzed in our study displayed CD30L message or surface protein. In contrast, CD30L was widely detected in B-cell lymphoproliferative disorders, with HCL and high-grade NHL expressing a higher surface density of the ligand as compared with B-CLL and low-grade NHL. In addition, purified BB-4+ plasma cells42 from MM patients displayed CD30L in 4 of 7 cases.
Previous studies from some of us have shown that tissue and circulating granulocytes, eosinophils, activated monocyte/macrophages, and circulating normal B cells, but not resting peripheral T cells express membrane CD30L.21,24-27,28,53 We have shown here that CD30L mRNA and protein are also strongly expressed in normal tonsils by B cells and activated (CD25+/CD38+/CD54+/HLA-DR+) T lymphocytes. Our present detection in resting T cells of a low amount of CD30L mRNA in the absence of surface protein is in agreement with preliminary observations from other groups.54 These data are consistent overall with the expression pattern of the ligand in T- and B-cell lymphoproliferative disorders. In addition, by analyzing normal hematopoietic cells in BM biopsies, we were able to show that a fraction of early myeloid precursors, intermediate myeloid cells, eosinophils, most erythroblasts, and subsets of megakaryocytes also express CD30L. Taken together, our data on normal and leukemic cells suggest that CD30L is widely expressed from early to late stages of human hematopoiesis and may have a role in the growth regulation of some hematopoietic tumors.
The very broad expression of CD30L in human normal and malignant lymphohematopoietic cells, as emerging from our study, appears in striking contrast with the highly restricted expression of its specific counterreceptor, ie, CD30. After its original identification on tumor cells of HD and ALCL,55 several studies and our present data showed that CD30 expression in nonmalignant cells is confined to rarely occurring large mononuclear lymphoid cells in tonsils, lymph nodes, thymic medulla, and in vitro activated T cells.23,27,55 More recently, CD30 expression has been detected on subsets of tissue and circulating CD4+ or CD8+ T cells producing Th2 or Th1 cytokines in healthy and immunoreactive conditions.27,31-33,55,56 Accordingly, by analyzing normal hematopoietic cells with a highly sensitive RT-PCR approach, we were able to detect the constitutive expression of CD30 only on phenotypically activated T or B cells from tonsils. In addition, most human hematopoietic neoplasms, including acute leukemias and B- or T-cell lymphoproliferations, overall lacked CD30 mRNA and protein. However, our detection of CD30 on rare AMLs (4/72), cases of B-cell high-grade lymphoma (3/9), and purified BB-4+ malignant plasmacells (3/7) appears consistent with previous data showing that some AML cases, myeloid and myeloma cell lines, and a fraction of non-ALCL may display low levels of CD30 mRNA and protein.23,25,39,57,58 Moreover, the coexpression of CD30 and CD30L was only found in 4 of the 181 samples analyzed (2 MMs and 2 AMLs), rendering therefore unlikely the idea that CD30-CD30L autocrine loops may have a role in most hematopoietic tumors. Because CD30L expression is highly regulated by cytokines and cellular contacts during physiologic immunoregulatory processes,27,31-33,59 its frequent detection on human leukemia/lymphoma cells may rather suggest a role for CD30L in the regulation of tumor cell interactions with CD30+ immune effectors and/or microenvironmental accessory cells.1-5
Previous data have shown that the interaction of CD30L with its specific receptor is able to induce either cell death or proliferation, depending on the cell type and/or on different intracellular signalling pathways.18,19,21,24,54 CD30-transduced signals may trigger NF-κB–mediated activation and proliferation of leukemic T-cell lines and cultured H-RS cells.19,21,24,60,61 On the other hand, recombinant CD30L also exerts a potent antiproliferative effect on ALCL cell lines, and it has been shown that CD30 is involved in the deletion of autoreactive thymocytes in mice and in the T-cell receptor-dependent cell death of T-cell hybridomas.19,21,24,62,63 Accordingly, it is conceivable that the expression of CD30L may confer to malignant cells a selective growth advantage by either facilitating their interaction with cytokine-producing CD30+ T cells27,31-33,54,59,64 or promoting the cellular death of CD30+ T-cell subsets involved in tumor cell recognition and killing.54,62,63 In this regard, it has been shown that CD30 triggering is part of the human immunodeficiency virus (HIV) replication pathway by causing the apoptotic death of CD4+/CD30+ T lymphocytes and eventually leading to the release of soluble CD30 in HIV patients.54,65 Intriguingly, HCL, a neoplasm in which tumor cells display a very high surface density of CD30L, is associated with a selective decrease of CD45R0+ T cells,66 a major subset of the CD30-expressing immune cells,30 and we have detected elevated serum levels of soluble CD30 in some patients with CD30L+ AML, B-cell lymphoproliferative disorders, and HCL (V. Gattei et al, unpublished observation, January 1996). On the other hand, we have shown that CD30L+ AML cells display a preferential expression of p55 IL-2R and other receptors for CD30+ T-cell–derived cytokines,64 ie, IL-4R, IL-5R, and IL-9R (V. Gattei et al, unpublished observation, May 1996). Whether CD30L on malignant hematopoietic cells is mainly used to trigger effects leading to death of CD30+ immune effectors18,54,62,63 or to gain paracrine or cell contact-dependent stimulation from cytokine-producing CD30+ cells,1-5 remains to be established. Because we have shown here that native CD30L on primary leukemic cells is able to trigger either cellular activation or antiproliferative signals on different CD30+ target cells, both of the mechanisms described above may have a role in the cellular regulation of CD30L+ acute leukemias and lymphoproliferative disorders.
ACKNOWLEDGMENT
We thank our collegues from the Blood Transfusion Center C.R.O. Aviano for kindly providing us with PB apheresis products. We also gratefully acknowledge the excellent assistance of F. Coletto for artwork and graphic support.
Supported in part by the Associazione Italiana per la Ricerca sul Cancro, Milan, Italy; the Ministero della Sanità, Ricerca Finalizzata IRCCS, Rome, Italy; the Consiglio Nazionale delle Ricerche, PF-ACRO (Grant No. 92.02347.PF39), Italy; and the Deutsche Krebshilfe, Bonn, Germany.
Address reprint requests to Valter Gattei, MD, The Leukemia Unit, Department of Medical Oncology, Centro Regionale di Riferimento Oncologico, IRCCS, via Pedemontana Occidentale, Aviano I-33081, Italy.


![Fig. 3. Paraffin-embedded tissue section from normal BM stained with the anti-CD30L MoAb M80 (A, B, and C) or isotype-matched control mouse MoAb (D). A typical paranuclear dot-like staining was detected in cells of the erythroid (A) and myeloid (A and B; arrows) lineages and as large block-like deposits in subset of megakaryocytes (B; arrowhead). In most of eosinophils a disperse granular staining of cytoplasms (C; arrow) was observed. APAAP immunostaining; hematoxylin counterstain. (Original magnification [A through C] × 630; [D] × 200.)](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/6/10.1182_blood.v89.6.2048/3/m_bl_0018f3.jpeg?Expires=1765946229&Signature=X~TbEiDbmxaY7fj-5y5Dtlfca1O7wbP8CJVUwkYeDvnxmRu0zyhIQY4tIij-CmYLPrDb5uQKRL3T68kReZeRawzB403ZNuxOSSUXDn6TFEj0Q0-N3NkmTUUO0EA70eq-UuHdezsAA-Hi8tIFqVCdJHYA9BjRi74PA7HE-BLkhj87aGc2W3U00fqolrNg~ppSbou7blOj78dXgRXzzM79ETq41~wwb5gMZb~62G0tW3tz1CVgsW9yM32oepvIYG7gf6xd~YdgyBxPccdhyQZp7t~RPsr~uO5dlplqe-8UNmAuU0HskZZ5pSZJjX1lJrR0p9o0o9OAsPGFUV7mEC28Sw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal