Abstract
Fifteen patients with refractory Hodgkin's disease were treated in a phase I/II trial with the natural killer (NK)-cell–activating bispecific monoclonal antibody HRS-3/A9, which is directed against the Fcγ-receptor III (CD16 antigen) and the Hodgkin's-associated CD30 antigen, respectively. Median counts of NK cells and of all lymphocyte subsets were considerably decreased in the patients before therapy. HRS-3/A9 was administered 4 times every 3 to 4 days, starting with 1 mg/m2. The treatment was well tolerated, and the maximum tolerated dose was not reached at 64 mg/m2, the highest dose administered because of the limited amounts of HRS-3/A9 available. Side effects were rare and consisted of fever, pain in involved lymph nodes, and a maculopapulous rash. A total of 9 patients developed human antimouse Ig antibodies, and 4 patients developed an allergic reaction after attempted retreatment. A total of 1 complete and 1 partial remission (lasting 16 and 3 months, respectively), 3 minor responses (1 to 11+ months), and 1 mixed response were achieved. There was no clear-cut dose-side effect or dose-response correlation. Our results encourage further clinical trials with this novel immunotherapeutic approach and emphasize the necessity to reduce the immunogenicity of the murine bispecific antibodies.
ALTHOUGH HODGKIN'S DISEASE (HD) is highly curable by chemotherapy and/or radiotherapy, conventional treatment will eventually fail in at least one third of all patients with advanced disease.1 Immunotherapy with bispecific monoclonal antibodies (BiMoAbs)2 might be a valuable treatment option for these patients. These antibodies can bridge human effector cells to tumor cells. To induce tumor-specific cytolysis, the antibody arm binding to the effector cell must be directed against triggering molecules shown on the surface of effector cells, such as natural killer (NK) cells, phagocytes, or T lymphocytes.3
Recently, we have developed a set of BiMoAbs for tumor-specific recruitment of NK cells4 and T lymphocytes5,6 for the treatment of HD. The anti-CD16/CD30 BiMoAb HRS-3/A9 binds with one arm to the CD30 antigen, which is expressed by the Hodgkin and Reed-Sternberg cells in the majority of cases with HD and only a small proportion of activated lymphocytes.7 With its second arm, the anti-CD16/CD30 BiMoAb binds to the Fcγ-receptor III (CD16 antigen) extracellular domain on NK cells and mononuclear phagocytes. HRS-3/A9 can induce specific lysis of CD30+ tumor cells in vitro. Moreover, in a severe combined immunodeficiency mouse model we have shown that treatment with the HRS-3/A9 BiMoAb has the capacity to direct and activate human NK cells to CD30+ Hodgkin's tumors in vivo, resulting in a 100% complete remission rate of subcutaneously established tumors after 1 single injection.4 We now report on the clinical results of a phase I/II dose escalation study with this anti-CD16/CD30 BiMoAb in 15 patients with advanced refractory HD.
MATERIALS AND METHODS
Patients.The study had been approved by the local ethics review committee (Ärztekammer des Saarlandes, Saarbrüken, Germany). Patients were eligible if they had histologically proven CD30+ HD at second or higher relapse or were refractory to at least two standard polychemotherapy protocols not curable by radiotherapy alone. Rebiopsy for confirmation of histology and CD30 expression immediately before study entry was recommended but not mandatory. Further inclusion criteria were age of 18 to 60 years; Karnofsky performance status ≥50%; measurable tumor; and no other chemotherapy, radiotherapy, or immunotherapy within the preceding 4 weeks. All patients gave written informed consent.
BiMoAb treatment.HRS-3/A9 had been produced under good manufacturing practice (GMP) conditions by Biotest Pharma GmbH (63276 Dreieich, Germany) and contained greater than 95% intact murine IgG1 BiMoAb. The treatment consisted of a cycle of 4 HRS-3/A9 infusions administered intravenously every 3 to 4 days over 1 hour in 250 mL 5% human albumin solution after intracutaneous testing before the first application of the BiMoAb. The starting dose was 1 mg/m2/infusion. Two patients were treated at each dose level, and the dose was doubled for the next 2 patients if no side effects of the National Cancer Institute Common Toxicity Criteria grade 3 and 4 were observed, and treatment was continued until the maximum dose (because of the limited amount of BiMoAb available) of 64 mg/m2 was reached.
Clinical assessments.Toxicity was evaluated using the National Cancer Institute Common Toxicity Criteria as described.8 The response criteria were defined as follows: Complete remission (CR), disappearance of all known disease for at least 4 weeks; partial remission (PR), ≥50% reduction of total tumor mass for at least 4 weeks and no appearance of new lesions or progression of any lesion; minor response (MR), reduction of total tumor mass by ≥25% but less than 50% for at least 4 weeks and no appearance of new lesions or progression of any lesion; disease stabilization, decrease or increase of less than 25% of all lesions and no appearance of new lesions for at least 8 weeks; progressive disease, increase of greater than 25% in any measurable tumor lesion or appearance of new lesions; mixed response, reduction of one lesion by ≥50% in combination with an increase of another lesion of greater than 25% or the appearance of a new lesion.
Blood counts, lymphocyte subsets, and human antimouse antibody (HAMA).Blood counts with differentiation (Coulter STKS; Coulter, Hialeah, FL) and analysis of circulating lymphocyte subsets (FACScan; Becton Dickinson, Heidelberg, Germany) were performed before and after the end of the BiMoAb infusion, as well as 1, 3, 6, 24, 48, and 72 hours thereafter. Antibodies used were fluorescein isothiocyanate-conjugated anti-CD3, anti-CD4, anti-CD57, and goat antimouse Ig (all from Becton Dickinson); fluorescein isothiocyanate-conjugated anti-CD16 (Dako, Hamburg, Germany); phycoerythrin-conjugated anti-CD8 and anti-CD19 (Becton Dickinson); and phycoerythrin-conjugated anti-CD56 (Coulter-Immunotech, Hamburg, Germany). HAMA response to HRS-3/A9 was assayed as described previously,9 with minor modifications. Briefly, enzyme-linked immunosorbent assay (ELISA) plates were coated with BiMoAb HRS-3/A9 (2 μg/mL; 50 μL/well) overnight at 4°C; nonspecific binding was blocked by 1.5% gelatin (wt/vol) in phosphate-buffered saline at room temperature (RT). Dilutions of patient sera were incubated for 1 hour at RT. After washing, 50 μL/well of a 1:1,000 diluted biotinylated goat antihuman-F(ab′)2 was added as the secondary antibody for 1 hour at RT. After extensive washing, a 1:50,000 dilution of alkaline phosphatase-conjugated streptavidin (Boehringer Mannheim, Mannheim, Germany) was incubated for 15 minutes at RT (100 μL/well), and, after additional washing, the reaction product was developed using nitrophenyl-phosphate (Sigma, St Louis, MO) as a substrate. The reaction was stopped with HCl and read at 405 nm on an ELISA reader (Dynatech MR 4000; Dynatech Laboratories, Inc, Chantilly, VA).
Characteristics of Patients Treated With HRS-3/A9
| Patient No. . | Gender . | Age (yr) . | Histology* . | Involved Sites . | Relapse No. . | Previous Therapy . |
|---|---|---|---|---|---|---|
| 1 | Male | 37 | Nod-scl | OSS | 2 | 3× COPP/ABVD, radiotherapy, 2× Vbl |
| 2 | Male | 37 | Nod-scl | OSS, LN | 2 | 6× COPP, radiotherapy, 6× ABVD |
| 3 | Male | 44 | Nod-scl | PUL, HEP, SPL, LN | 6 | 6× COPP, 5× COPP/ABVD, 1× Mtx/VP-16/Ifo, radiotherapy, 4× Vbl/Adr/Dex, 3× DexaBEAM, 1× ADR/CDDP/Ara-C |
| 4 | Female | 44 | Mixed | BM, LN | 4 | 5× COPP, radiotherapy, 2× COPP/ABVD/IEV, radiotherapy, 5× IEV, 3× PVB |
| 5 | Male | 42 | Mixed | PUL, OSS, LN | 2 | 4× COPP/ABVD, radiotherapy, 1× DexaBEAM |
| 6 | Female | 57 | Mixed | BM, LN | 2 | 4× COPP/ABV/IMEP, radiotherapy, 7× CEP |
| 7 | Female | 36 | Nod-scl | PUL, LN | 3 | 2× COPP/ABVD, radiotherapy, 2× DexaBEAM, PBSCT (BEAM), 4× Ifo/VP-16, 6× VBL/Pred |
| 8 | Male | 45 | Mixed (NA) | PUL | 4 | 6× ABVD, radiotherapy, 4× ABVD, 6× MOPP, 6× CEVD, 2× DexaBEAM, PBSCT (CVB), interleukin-4 |
| 9 | Female | 29 | Nod-scl | OSS, Soft tissue | 4 | Radiotherapy, 3× COPP/ABVD, radiotherapy, 2× DexaBEAM, ABMT (CVB) |
| 10 | Male | 25 | Unclassifiable (NA) | OSS, SPL, LN | 2 | 4× COPP/ABVD, VIP-E, PBSCT (BEAM), 7× Taxol, 6× Vbl/Pred |
| 11 | Female | 54 | Unclassifiable (NA) | PUL | 4 | 4× COPP, radiotherapy, CEVD, 2× ACOP-E, IMEP, ABMT (EBAC), 4× Vbl, 4× CEP, Taxol, VACOP-B, 2× Dex/Procarbacin |
| 12 | Male | 26 | Nod-scl (NA) | PUL, HEP, BM, LN | 4 | 3× COPP/ABVD, 4× DexaBEAM, radiotherapy, 10× Pm, 2× A-SHAP |
| 13 | Female | 26 | Nod-scl (NA) | PUL, LN | 2 | 1× COPP/ABVD, 3× DexaBEAM, ABMT (Cy/VP-16), 2× CCNU/Pred, 2× CDDP, 5× IEV, 4× COPP |
| 14 | Female | 58 | Mixed (NA) | PUL, HEP | 3 | Radiotherapy, 3× MOPP/ABVD, 2× MOPP/ABVD, 2× MINE, ABMT (BEAM), 2× Vbl, MEVAC |
| 15 | Male | 41 | Nod-scl | OSS, LN | 1 | COPP, DexaBEAM, 6× CEP |
| Patient No. . | Gender . | Age (yr) . | Histology* . | Involved Sites . | Relapse No. . | Previous Therapy . |
|---|---|---|---|---|---|---|
| 1 | Male | 37 | Nod-scl | OSS | 2 | 3× COPP/ABVD, radiotherapy, 2× Vbl |
| 2 | Male | 37 | Nod-scl | OSS, LN | 2 | 6× COPP, radiotherapy, 6× ABVD |
| 3 | Male | 44 | Nod-scl | PUL, HEP, SPL, LN | 6 | 6× COPP, 5× COPP/ABVD, 1× Mtx/VP-16/Ifo, radiotherapy, 4× Vbl/Adr/Dex, 3× DexaBEAM, 1× ADR/CDDP/Ara-C |
| 4 | Female | 44 | Mixed | BM, LN | 4 | 5× COPP, radiotherapy, 2× COPP/ABVD/IEV, radiotherapy, 5× IEV, 3× PVB |
| 5 | Male | 42 | Mixed | PUL, OSS, LN | 2 | 4× COPP/ABVD, radiotherapy, 1× DexaBEAM |
| 6 | Female | 57 | Mixed | BM, LN | 2 | 4× COPP/ABV/IMEP, radiotherapy, 7× CEP |
| 7 | Female | 36 | Nod-scl | PUL, LN | 3 | 2× COPP/ABVD, radiotherapy, 2× DexaBEAM, PBSCT (BEAM), 4× Ifo/VP-16, 6× VBL/Pred |
| 8 | Male | 45 | Mixed (NA) | PUL | 4 | 6× ABVD, radiotherapy, 4× ABVD, 6× MOPP, 6× CEVD, 2× DexaBEAM, PBSCT (CVB), interleukin-4 |
| 9 | Female | 29 | Nod-scl | OSS, Soft tissue | 4 | Radiotherapy, 3× COPP/ABVD, radiotherapy, 2× DexaBEAM, ABMT (CVB) |
| 10 | Male | 25 | Unclassifiable (NA) | OSS, SPL, LN | 2 | 4× COPP/ABVD, VIP-E, PBSCT (BEAM), 7× Taxol, 6× Vbl/Pred |
| 11 | Female | 54 | Unclassifiable (NA) | PUL | 4 | 4× COPP, radiotherapy, CEVD, 2× ACOP-E, IMEP, ABMT (EBAC), 4× Vbl, 4× CEP, Taxol, VACOP-B, 2× Dex/Procarbacin |
| 12 | Male | 26 | Nod-scl (NA) | PUL, HEP, BM, LN | 4 | 3× COPP/ABVD, 4× DexaBEAM, radiotherapy, 10× Pm, 2× A-SHAP |
| 13 | Female | 26 | Nod-scl (NA) | PUL, LN | 2 | 1× COPP/ABVD, 3× DexaBEAM, ABMT (Cy/VP-16), 2× CCNU/Pred, 2× CDDP, 5× IEV, 4× COPP |
| 14 | Female | 58 | Mixed (NA) | PUL, HEP | 3 | Radiotherapy, 3× MOPP/ABVD, 2× MOPP/ABVD, 2× MINE, ABMT (BEAM), 2× Vbl, MEVAC |
| 15 | Male | 41 | Nod-scl | OSS, LN | 1 | COPP, DexaBEAM, 6× CEP |
Abbreviations: NA, histology at presentation because no rebiopsy taken immediately before BiMab treatment was available; Nod-scl, nodular sclerosis; mixed, mixed cellularity; OSS, bone; LN, lymph nodes; PUL, lung; HEP, liver; SPL, spleen; BM, bone marrow; ABMT, Autologous bone marrow transplantation; PBSCT, PB stem cell transplantation; ACOP-E, adriamycin, cyclophosphamide, vincristine, prednisone, etoposide; ABVD, adriamycin, bleomycin, vinblastin, dacarbacine; A-SHAP, adriamycin, prednisolone, cytosine-arabinoside, cisplatin; BEAM, BCNU, etoposide, cytosine-arabinoside, melphalan; CEP, CCNU, etoposide, prednimustine; CEVD, CCNU, etoposide, vinblastine, dexamethasone; CVB, cyclophosphamide, etoposide, BCNU; COPP, cyclophosphamide, vincristine, procarbazine, prednisone; DexaBEAM, dexamethasone, BCNU, etoposide, cytosine-arabinoside, melphalan; EBAC; etoposide, BCNU, cytosine-arabinoside, cyclophosphamide; IEV, ifosfamide, etoposide, vinblastin; IMEP, ifosfamide, methotrexate, etoposide, prednisone; MEVAC, mitoguazone, etoposide, vindesine, adriamycin, prednisone; MINE, mitoguazone, ifosfamide, vinorelbine, etoposide; MOPP, nitrogen mustard, vincristine, procarbazine, prednisone; PVB, cisplatin, vinblastin, bleomycin; VACOP-B, etoposide, adriamycin, cyclophosphamide, vincristine, prednisone, bleomycin; VIP-E, etoposide, ifosfamide, carboplatin, epirubicin; ADR, doxorubicin; Ara-C, cytosine-arabinoside; CDDP, cisplatin; Cy, cyclophosphamide; Dex, dexamethasone; Ifo, ifosfamide; MTX, methotrexate; Pm, prednimustine; Pred, prednisone; VBL, vinblastine; VP-16, etoposide.
Only CD30+ histologies were treated.
RESULTS
Patient characteristics are shown in Table 1. Only patients with CD30+ histologies at first presentation were treated. CD30 positivity was confirmed by a rebiopsy obtained immediately before BiMoAb treatment whenever possible. Although it cannot be definitely excluded, it is unlikely that any of the patients from whom rebiopsy was not possible had developed CD30− disease, because, to date, no loss of CD30 positivity has been observed in cases originally CD30+ on immunohistologic re-evaluation during the course of the disease. All patients had stage IV disease, with half of them having involvement of the lung or the liver. The median age was 41 years (range, 25 to 58 years), and the median Karnofsky performance status was 80% (range, 50% to 100%). All patients were in second relapse or higher (median, third) or refractory to ≥2 chemotherapy protocols. All patients had had extensive pretreatment with a median of 4 (range, 2 to 8) different previous chemotherapy regimens. The majority (12 of 15) had a history of extended-field radiotherapy and 7 had undergone high-dose chemotherapy with autologous bone marrow transplantation or peripheral blood (PB) stem cell support. In the remaining 8 patients high-dose chemotherapy had not been feasible because of total refractoriness to chemotherapy or failure to harvest sufficient numbers of stem cells.
Toxicity.HRS-3/A9 treatment was well tolerated, with the maximum tolerated dose (MTD) not being reached at 64 mg/m2 administered 4 times. Further dose escalations were not possible because of the limited amount of BiMoAb available. Mild and moderate side effects (CTC grades I and II only) were observed in 6 patients and consisted of fever in 4 patients, a temporary decrease in blood pressure in 1 patient, pain in involved lymph nodes beginning shortly after BiMoAb infusion and lasting a few hours in 2 patients, and a maculopapulous skin rash after the last HRS-3/A9 application in 1 patient, respectively. All side effects except for the allergic exanthema disappeared within 8 hours after the termination of the BiMoAb treatment. No changes in routinely evaluated blood and serum laboratory values were observed after therapy. There was no correlation between BiMoAb dose and kind or severity of observed side effects (Table 2).
Treatment With HRS-3/A9 — Response and Side Effects
| Patient No. . | Dose HRS-3/A9 . | Response . | Response Duration (mo) . | HRS-3/A9–Related Side Effects* . |
|---|---|---|---|---|
| 1 | 1 mg/m2 ×4 | Progressive | None | |
| 2 | 1 mg/m2 ×4 | MR | 2 | None |
| 3 | 2 mg/m2 ×2 | Progressive | Pain in involved LNs (II°) | |
| 4 | 2 mg/m2 ×2 | Progressive | Pain in involved LNs (I°), fever (II°) | |
| 5 | 2 mg/m2 ×4 | MR | 1 | None |
| 6 | 4 mg/m2 ×4 | PR | 3 | None |
| 7 | 4 mg/m2 ×4 | Mixed response | Allergic exanthema (II°) | |
| 8 | 8 mg/m2 ×4 | MR | 11+ | None |
| 9 | 8 mg/m2 ×4 | Stable disease | 10+ | None |
| 10 | 16 mg/m2 ×4 | Progressive | Fever (I°) | |
| 11 | 16 mg/m2 ×4 | Progressive | Fever (I°) | |
| 12 | 32 mg/m2 ×4 | Stable disease | 2 | None |
| 13 | 32 mg/m2 ×4 | Progressive | Fever (II°), hypotension (I°) | |
| 14 | 64 mg/m2 ×4 | Progressive | None | |
| 15 | 64 mg/m2 ×4 | CR | 6 | None |
| Patient No. . | Dose HRS-3/A9 . | Response . | Response Duration (mo) . | HRS-3/A9–Related Side Effects* . |
|---|---|---|---|---|
| 1 | 1 mg/m2 ×4 | Progressive | None | |
| 2 | 1 mg/m2 ×4 | MR | 2 | None |
| 3 | 2 mg/m2 ×2 | Progressive | Pain in involved LNs (II°) | |
| 4 | 2 mg/m2 ×2 | Progressive | Pain in involved LNs (I°), fever (II°) | |
| 5 | 2 mg/m2 ×4 | MR | 1 | None |
| 6 | 4 mg/m2 ×4 | PR | 3 | None |
| 7 | 4 mg/m2 ×4 | Mixed response | Allergic exanthema (II°) | |
| 8 | 8 mg/m2 ×4 | MR | 11+ | None |
| 9 | 8 mg/m2 ×4 | Stable disease | 10+ | None |
| 10 | 16 mg/m2 ×4 | Progressive | Fever (I°) | |
| 11 | 16 mg/m2 ×4 | Progressive | Fever (I°) | |
| 12 | 32 mg/m2 ×4 | Stable disease | 2 | None |
| 13 | 32 mg/m2 ×4 | Progressive | Fever (II°), hypotension (I°) | |
| 14 | 64 mg/m2 ×4 | Progressive | None | |
| 15 | 64 mg/m2 ×4 | CR | 6 | None |
Abbreviation: LN, lymph node.
All side effects (except for the allergic exanthema) disappeared within less than 8 hours.
Immunological evaluation.The majority of patients had a significant decrease of all lymphocyte subsets before therapy, which was most pronounced for B lymphocytes and helper T cells but also for suppressor/cytotoxic T cells and NK cells (Table 3), whereas values for granulocytes and monocytes were within the normal range. After BiMoAb infusion, no consistent changes of PB counts of any subpopulation including NK cells and monocytes was observed. Despite the pronounced immunosuppression, 9 patients (60%) developed a human antibody response against the murine Ig (HAMAs) as determined by ELISA 4 weeks after treatment. At that time, a second treatment cycle with HRS-3/A9 was intended in 4 patients, of whom 3 were HAMA-positive. After intracutaneous challenge with HRS-3/A9, only the patient who had had the allergic skin rash after the last BiMoAb infusion of the first treatment cycle developed a marked skin reaction with erythema and induration and, therefore, was excluded from further treatment. The remaining 3 patients had negative skin tests and received BiMoAb infusions at the dose level that they had tolerated during the first treatment cycle. In all of them (including 1 who had been negative for HAMAs), moderate systemic reactions such as shivering, hypotension, low back pain, and chest tightness occurred despite pretreatment with antihistaminics and prednisone, which led to the termination of treatment after 1 to 3 additional BiMoAb infusions.
Pretreatment Values of Lymphocyte Subsets
| Patient No. . | NK Cells . | B Lymphocytes . | CD4+ Lymphocytes . | CD8+ Lymphocytes . |
|---|---|---|---|---|
| 1 | 69 | 30 | 115 | 169 |
| 2 | 434 | 462 | 347 | 1,821 |
| 3 | 49 | 49 | 592 | 690 |
| 4 | 34 | 20 | 40 | 475 |
| 5 | 41 | 20 | 61 | 57 |
| 6 | 61 | 54 | 314 | 291 |
| 7 | 132 | 91 | 125 | 192 |
| 8 | 166 | 139 | 41 | 166 |
| 9 | 77 | 2 | 63 | 91 |
| 10 | 69 | 34 | 1,322 | 240 |
| 11 | 118 | 3 | 294 | 395 |
| 12 | 62 | 25 | 241 | 235 |
| 13 | 322 | 25 | 223 | 1,757 |
| 14 | 109 | 41 | 205 | 999 |
| 15 | 148 | 87 | 222 | 816 |
| Median | 77 | 34 | 205 | 291 |
| Normal range (95% CI) | 80-450 | 150-500 | 550-1,200 | 500-1,050 |
| Patient No. . | NK Cells . | B Lymphocytes . | CD4+ Lymphocytes . | CD8+ Lymphocytes . |
|---|---|---|---|---|
| 1 | 69 | 30 | 115 | 169 |
| 2 | 434 | 462 | 347 | 1,821 |
| 3 | 49 | 49 | 592 | 690 |
| 4 | 34 | 20 | 40 | 475 |
| 5 | 41 | 20 | 61 | 57 |
| 6 | 61 | 54 | 314 | 291 |
| 7 | 132 | 91 | 125 | 192 |
| 8 | 166 | 139 | 41 | 166 |
| 9 | 77 | 2 | 63 | 91 |
| 10 | 69 | 34 | 1,322 | 240 |
| 11 | 118 | 3 | 294 | 395 |
| 12 | 62 | 25 | 241 | 235 |
| 13 | 322 | 25 | 223 | 1,757 |
| 14 | 109 | 41 | 205 | 999 |
| 15 | 148 | 87 | 222 | 816 |
| Median | 77 | 34 | 205 | 291 |
| Normal range (95% CI) | 80-450 | 150-500 | 550-1,200 | 500-1,050 |
Values are shown in cells per microliter.
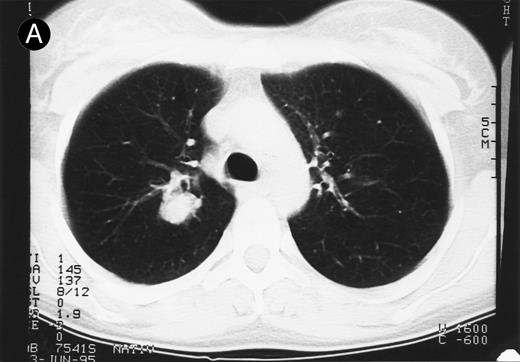
Response to therapy.In 15 of 15 evaluable patients, 1 CR and 1 PR with entire relief of B symptoms were observed after therapy with 64 mg/2 and 4 mg/m2, respectively, that lasted for 6 months and for 3 months, respectively. In addition, 3 MRs and 2 disease stabilizations (after documented preceding progressive disease) were observed, with 2 of them still ongoing after 10 and 11 months, respectively. One mixed response with a considerable reduction of a pulmonary mass (Fig 1) and left cervical lymph nodes but with growth of lymph nodes at distant sites was observed. No clear dose-response correlation could be established, although the CR occurred at the maximum dose administered (Table 2).
Response to HRS-3/A9 treatment of patient no. 7. Computed tomography (CT) scans of a pulmonary infiltration with HD in the right lung just above the tracheal bifurcation level is shown before (A) HRS-3/A9 therapy and (B) 5 weeks after the last BiMoAb infusion (4 mg/m2 × 4). The response was determined a mixed response because the partial response in the lung was accompanied by the simultaneous growth of peripheral lymph nodes.
Response to HRS-3/A9 treatment of patient no. 7. Computed tomography (CT) scans of a pulmonary infiltration with HD in the right lung just above the tracheal bifurcation level is shown before (A) HRS-3/A9 therapy and (B) 5 weeks after the last BiMoAb infusion (4 mg/m2 × 4). The response was determined a mixed response because the partial response in the lung was accompanied by the simultaneous growth of peripheral lymph nodes.
DISCUSSION
This is the first clinical evaluation of an NK-cell–activating BiMoAb in a defined patient population suffering from a distinct hematologic malignancy. Despite abundant in vitro data and results of experimental therapy in several animal tumor models that suggest tumor-specific immune recruitment with BiMoAbs as an effective option for cancer treatment,3-6,10-13 clinical experience with BiMoAbs is scarce and is limited to a few clinical trials of BiMoAbs with reactivity against the CD3/T-cell receptor complex for the recruitment of T cells,14-19 BiMoAbs against the high affinity Fcγ-receptor I (CD64) on monocytes and neutrophils,20 and BiMoAbs against the Fcγ-receptor III (CD16) on NK cells and monocytes.21
HRS-3/A9 induced no dose-limiting but only short-lasting mild to moderate side effects in a minority of patients, and the MTD was not reached at 64 mg/m2, the highest dose administered. When starting the study, we had expected that the available amounts of BiMoAb would be sufficient for the determination of the MTD, because two recently published studies had reported an MTD of 5 and 10 mg/m2, respectively, after systemic application of BiMoAbs with reactivity to CD1621 and CD6420 in patients with a variety of solid tumors. Principal toxicities of the chemically cross-linked F(ab′)2 anti-Her2/anti-CD64 BiMoAb MDX-210 used in that study consisted of transient monocytopenia and fever, malaise, nausea, and hypotension, the latter being dose-limiting.20 The anti-Her2/anti-CD16 BiMoAb 2B1 induced transient fever, rigor, nausea, diarrhea, dyspnea, hypotension, leukopenia, and dose-limiting thrombocytopenia, particularly in extensively pretreated patients, which was not caused by direct binding of the antibody to the platelets.21 These toxicities resemble those observed after cytokine therapy, and, indeed, induction of release of considerable amounts of cytokines, eg, tumor necrosis factor-α, interleukin-6 (IL-6), IL-8, and granulocyte colony-stimulating factor, was shown in these BiMoAb trials. The phenomenon of cytokine induction is somewhat surprising because BiMoAbs should not trigger a significant cytokine release in the PB, as they bind only monovalently to effector cells. In contrast, in our study we observed only moderate fever with or without hypotension in 3 patients after administration of 16 and 32 mg/m2 BiMoAb, respectively. In particular, we observed no hematologic toxicity. The low toxicity might be explained by the high purity of our antibody preparation, but also in part may be caused by the quantitiative and functional impairment of the cellular immune system with consecutive impairment of cytokine release in our patients, which is commonly observed in advanced HD.22
The majority of clinical studies with T-cell–recruiting BiMoAbs used the local application of BiMoAb together with ex vivo expanded and activated lymphocytes with or without IL-2. In these studies, a considerable objective local response rate of various solid tumors, eg, brain,14 peritoneal cavity,15,16,19 and pleura,16 at the treatment site was observed. In contrast, the systemic administration of T-cell–targeting BiMoAbs18 in lymphoma patients and CD6420 or CD1621 in patients with solid tumors resulted in only minor and transient responses. Compared with the results of these studies, the response rate (1 CR, 1 PR, and 3 MRs in 15 patients) in our trial with the NK-cell–activating HRS-3/A9 BiMoAb is surprising, especially in the light of the pronounced NK-cell deficiency, that has been consistently described in patients with heavily pretreated HD.22 That these responses, which lasted from 1 to 11+ months, have been achieved with a single cycle of HRS-3/A9 application confirms the tumoricidal potential of this BiMoAb that had been expected from in vitro and animal studies.4 Although a dose-response correlation cannot be derived from our study, it is intriguing that the complete response was observed in the patient who received the highest dose.
Because neither antitumor effects nor toxicities of HRS-3/A9 showed a clear dose-dependency, several further trials are needed to encompass the optimal dose and schedule of NK-cell–activating BiMoAbs. In contrast to cytotoxic drugs or immunotoxins, in which the MTD can be regarded as the optimal dose, there are several unknowns when designing a trial with effector-cell–activating BiMoAbs. There are no data available as to which total dose, mode of application (push or continuous infusion), and time interval between the applications result in the most efficient targeting and activation of effector cells against tumor cells. With these data missing, we chose a treatment schedule of 4 BiMoAb infusions within 2 weeks at a 3- to 4-day interval based on an expected serum elimination half-life for whole murine IgG antibodies in humans of 6 to greater than 48 hours23,24 and an anticipated occurrence of a potentially counteractive HAMA response after 2 weeks. Valone et al20 recently emphasized the term “optimal biologic dose,” which they defined in their study as the BiMoAb dose that results in maximal Fcγ receptor saturation, optimal cytokine release, and changes in leukocyte subsets.20 In our study, saturation of circulating NK cells with HRS-3/A9 peaked at 6 hours and 3 to 6 hours after the first and last BiMoAb infusions, respectively. Maximal saturation of circulating NK cells showed a considerable interindividual variation ranging from 21% to 100% and 31% to 100% after the first and last doses, respectively. However, the situation in the PB does not necessarily mirror the conditions at the tumor site, because BiMoAbs should be able to bind to CD30 first at the tumor site. In this regard, it is of interest that pain occurred in involved lymph nodes shortly after BiMoAb-infusion in 2 patients who received doses as little as 2 mg/m2.
A major obstacle against a successful BiMoAb therapy, which eventually could preclude prolonged and repeated treatment, might be the development of HAMAs in the majority of patients, which has also been observed in other BiMoAb trials,20,25 and the evolvement of allergic reactions even in HAMA-negative patients who are re-exposed to BiMoAbs. However, this problem should be resolvable by the construction of less immunogenic bispecific single-chain antibodies26 or so-called “diabodies,”27 which should also result in a considerable reduction of costs when compared with those of BiMoAbs produced by the tetradoma technology.
In conclusion, the results of this phase I/II study have shown the safety and encouraging antitumor activity of an immunotherapeutic approach with an NK-cell–activating BiMoAb in refractory HD. The results of this trial justify further studies for the optimization of this approach. Such studies should aim at defining the optimal dose and schedule of HRS-3/A9 and the value of costimulation of effector cells with cytokines such as IL-228 or IL-1229 in this patient population characterized by a pronounced cellular immunodeficiency.
Supported by Grants PF135/3-1 of the Deutsche Forschungs gemeinschaft and W5/93/PF 3 of the Deutsche Krebshilfe/Dr Mildred-Scheel-Stiftung to M.P.
F.H. and C.R. contributed equally to this work.
Address reprint requests to Michael Pfreundschuh, PhD, MD, Medizinische Klinik und Poliklinik, Innere Medizin I, Universitätskliniken des Saarlandes, D-66421 Homburg, Germany.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal