Abstract
A subset of Hodgkin's disease (HD) patients have detectable Epstein-Barr virus (EBV) genomes in the malignant Reed-Sternberg (R-S) cells. R-S cells express only a limited set of latent EBV proteins, but only LMP1 and LMP2 can potentially elicit a CD8+ cytotoxic T-lymphocyte (CTL) response. We have evaluated if either of these proteins could be used as targets for specific adoptive T-cell therapy for EBV-positive (EBV+) HD. The success of this strategy requires that R-S cells are susceptible to lysis by CD8+ CTL, and that CTL specific for LMP1 and LMP2 can be detected and potentially amplified in HD patients. Antigen presentation and CTL sensitivity was evaluated with an in vitro maintained, phenotypically representative R-S cell line, HDLM-2. The R-S cells were able to process and present viral proteins, and to be efficiently lysed by specific CTL in a Class I–restricted manner. Since CTL responses to LMP1 and LMP2 do not represent the dominant responses to EBV, we examined if CTL clones specific for these proteins could be isolated despite the presence of weak or nondetectable responses in polyclonal T-cell lines. LMP-specific clones were generated from individuals either by cloning from the polyclonal EBV-reactive T-cell lines or by direct stimulation of peripheral blood mononuclear cells (PBMC) with cells expressing LMP1 or LMP2 as the only EBV protein. Our ability to isolate CTL specific for LMP proteins from individuals with HD and the sensitivity of R-S cells for CTL-mediated lysis suggest that the pursuit of specific adoptive immunotherapy represents a viable strategy for the subset of HD patients with EBV+ tumors.
HODGKIN'S DISEASE (HD) is a lymphoma characterized by the presence of the large multinucleated Reed-Sternberg (R-S) cell or its mononuclear Hodgkin cell variant within a cellular background of reactive leukocytes in the affected lymph node. Current therapeutic regimens combining radiation and chemotherapy have rendered early-stage disease imminently curable1-3 and achieved a significant improvement in cure rates for advanced-stage disease. However, patients with recurrent or refractory disease treated with intensive regimens continue to have a long-term disease-free survival of less than 30%,4-6 and alternative therapeutic strategies are thus needed, particularly ones that lack overlapping toxicities with chemoradiotherapy.
Our laboratory has evaluated the treatment of human viral diseases by the adoptive transfer of virus-specific T-cell clones and demonstrated the potential efficacy of this strategy.7,8 Application of this approach to the treatment of human malignancies has been hindered by difficulties in identifying immunogenic target antigens on tumor cells, although some success has recently been achieved, particularly with melanoma, by using tumor-reactive T cells isolated from patients to probe for relevant target antigens.9,10 In viral-associated malignancies, the expressed viral proteins represent potential targets for specific tumor therapy. A well-known transforming virus, Epstein-Barr virus (EBV), has been linked epidemiologically to HD for decades,11-13 and more recently, EBV genomes have been detected in the malignant R-S cells of 40% to 60% of EBV-seropositive (EBV+) HD patients.14-17 The pattern of viral protein expression in (EBV+) R-S cells differs from EBV-transformed B cells in that it is restricted to a limited set of latent proteins, EBNA 1, LMP1, and LMP2,16,18-20 two of which, EBNA 1 and LMP1, have been shown to have oncogenic potential.21 22
Effective treatment of HD with specific CD8+ T cells requires satisfying several conditions in addition to expression of novel viral proteins by the tumor. First, R-S cells must not evade immune recognition by losing the expression of Class I molecules or the ability to process and present the viral antigens with Class I molecules on the cell surface for recognition by potentially reactive T cells. Analysis of Class I expression in R-S cells isolated from HD lymph nodes has shown that R-S cells express abundant Class I molecules in at least 50% of the cases.23 Second, the viral antigens must be immunogenic and capable of eliciting CD8+ responses. Studies have suggested that the EBV proteins expressed in R-S cells are, at best, poor immunogens. Several laboratories have evaluated T-cell responses to EBV and demonstrated that most individuals exhibit an immunodominant cytotoxic T-lymphocyte (CTL) response specific for the EBNA 2 and EBNA 3 proteins.24,25 Weaker CTL responses to LMP1 and LMP2, two of the proteins expressed in R-S cells, have been detected in the peripheral blood, and analysis of T cells derived from lymph nodes of six HD patients with EBV+ tumors failed to detect LMP-reactive T cells.26 CD8+ CTL responses to EBNA 1, the third EBV protein expressed in R-S cells, have not been isolated, apparently because this protein contains a region of unique glycine-alanine repeats that may interfere with the processing of the protein via the Class I pathway.27 Additional but less likely mechanisms of evasion used by EBV+ R-S cells may include defects in the processing of EBV proteins or in T-cell signaling that could account for defective immune-mediated killing.
We have initiated preclinical studies with the purpose of developing a specific T-cell therapy for HD. Our results suggest that despite the presence of only weak or nondetectable responses to LMP1 and LMP2 in the CTL polyclonal population reactive to EBV, it is possible to isolate and expand such CTLs from the peripheral blood of both normal individuals and HD patients. Moreover, using an in vitro derived R-S cell line that has been extensively characterized and shown to be morphologically, antigenically, and molecularly representative of R-S cells isolated from biopsies,28 we demonstrate that Class I–positive R-S cells can present viral antigens and be sensitive to EBV-specific CTL. Thus, the adoptive transfer of viral-specific CTL may be a feasible strategy for a subset of (EBV+) HD patients.
MATERIALS AND METHODS
R-S cell lines.The R-S cell line HDLM-2 established in 1982 from the pleural effusion of a 74-year-old man with stage IV nodular sclerosing HD was obtained from Dr Hans Drexler (German Collection of Microorganisms and Cell Cultures, Department of Human and Animal Cell Cultures, Braunschrreig, Germany). The line is maintained in vitro as a single-cell suspension in RPMI 1640 supplemented with 20% fetal calf serum (FCS) (Gemini Bio-Products Inc, Calabasas, CA), 50 U/mL penicillin, 50 μg/mL streptomycin, 4 mmol/L L-glutamine, and 25 mmol/L β-mercaptoethanol.
HLA typing.HDLM-2 and HD patients were typed serologically by the HLA tissue-typing laboratory at the Fred Hutchinson Cancer Research Center (FHCRC). Molecular HLA typing of HDLM was performed in the Immunogenetics Laboratories at the FHCRC. HLA-A, -B, and -C alleles were assigned by amplification and direct sequencing of exons 2 (α1 domain) and 3 (α2 domain) from genomic DNA.29,30 All alleles recognized by the World Health Organization Nomenclature Committee31 are identified by this method, with the exception of A*0201 and A*0209, which differ only in exon 4 (α3 domain). In brief, locus-specific amplification of HLA-A genes was performed with a 5′ intron 1 and 3′ intron 3 consensus primer. Four 5′ primers designed to exon 2 were paired with the 3′ intron primer to amplify specific groups of HLA-A alleles.32 Amplification of HLA-B and HLA-C genes was performed as previously described.29,30 Amplified templates were sequenced and HLA-A, HLA-B, and HLA-C alleles were assigned by previously published methods.29 30
Immunophenotyping.Class I expression by HDLM-2 was determined by staining with monoclonal antibodies (MoAbs) specific for either the Class I molecule (W6/32; Sigma, St Louis, MO) or the A1 or B8 alleles (generously provided, respectively, by Drs Daniel Geraghty and Thomas Spies, FHCRC). Briefly, 5 × 105 cells were incubated with MoAbs diluted 1:50 in a total volume of 100 μL Hanks balanced salt solution (HBSS) supplemented with 2% FCS for 45 minutes at 4°C. Cells were washed two times with HBSS, and a second-step fluorescein-conjugated goat antimouse antibody (Zymed Laboratories Inc, South San Francisco, CA) diluted 1:50 was added to cell pellets in a total volume of 100 μL. Cells were again incubated at 4°C for 45 minutes, washed twice, and fixed with 1% paraformaldehyde. The stained cells were then analyzed by flow cytometry on a Coulter Epics FACS machine (Coulter Electronics, Hialeah, FL). Analysis of surface expression of accessory molecules was similarly performed with MoAbs specific for ICAM-1, LFA1, LFA3, CD80, CD86, and CD30 (Pharmingen, San Diego, CA).
Generation of alloreactive T-cell clones.Alloreactive T-cell clones were generated as previously described.33 Polyclonal lines were established by stimulating 5 × 106 peripheral blood mononuclear cells (PBMC) obtained by Ficoll-Hypaque gradient separation from an HLA-A2 B57,60 individual with 1 × 106 irradiated EBV-transformed B cells (LCL) from an HLA-A1 B8 homozygous individual, in medium consisting of RPMI 1640 (GIBCO, Grand Island, NY) supplemented with 10% pooled and heat-inactivated human serum, 25 mmol/L HEPES, 4 mmol/L L-glutamine, 50 U/mL penicillin, 50 μg/mL streptomycin, and 25 mmol/L β-mercaptoethanol (CTL media). Responding cells were stimulated for three weekly cycles by plating 1 × 106 responding cells with 3 × 105 stimulating cells. Recombinant interleukin-2 (IL-2) (generously provided by Hoffman-LaRoche, Nutley, NJ) was added on days 2 and 4 at a final concentration of 2 to 5 U/mL. After the third in vitro stimulation, cultures were enriched for CD8+ cells by negative selection with αCD4 MoAb-coated flasks (Applied Immune Sciences, Santa Clara, CA). CD8+ enriched cells were cloned by limiting dilution at 0.3 cells per well in CTL media supplemented with IL-2 at 50 U/mL final concentration in the presence of 3 × 104 allogeneic LCL per well in 96-well round-bottom microtiter plates (Corning, Corning, NY). Wells demonstrating growth after 10 to 14 days were assayed for lysis of allogeneic but not autologous target cells by standard 4-hour chromium (51Cr)-release assay (CRA). Clones demonstrating specific lysis were restimulated every 9 days with antigen in 24-well plates in CTL media supplemented with IL-2 at 40 U/mL final concentration on days 1 and 3 and 20 U/mL on day 5 of the 9-day stimulation cycle. The Class I target of the clones was assessed by measuring the lysis of targets matched at only one HLA allele.
Generation of EBV-specific CTL clones.EBV-specific CTL clones were isolated by two different protocols. With the first method, polyclonal lines were generated by stimulating 2 × 106 PBMC with 5 × 105 autologous irradiated (8,000 rad) LCL in 24-well plates (Costar, Cambridge, MA) in 2 mL CTL media. Responding cells were stimulated in weekly cycles by plating 1 × 106 T cells and 3 × 105 LCL per well in 24-well plates in CTL media supplemented with IL-2 at 2 U/mL final concentration on days 2 and 4 after restimulation. After the third stimulation, cells were enriched for CD8+ cells by selection on αCD4 MoAb-coated flasks (Applied Immune Sciences) and clones were isolated by plating the enriched responding cells at a limiting dilution of 0.3 cells per well in the presence of 3 × 104 autologous LCL per well. Isolated clones were characterized for HLA restriction by lysis of allogeneic LCL matched at only one HLA allele and for specificity by lysis of target cells infected with vac recombinants encoding individual EBV proteins by standard CRA. With the second method, PBMC obtained from patients were separated into adherent cell (AC) and nonadherent cell (NAC) fractions by plastic adherence. The ACs were infected at a multiplicity of infection (MOI) of 5:1 for 16 hours with vaccinia recombinants encoding individual EBV protein genes (vac/LMP1 or vac/LMP2) (generously supplied by Mike Kurilla, University of Virginia, Charlottesville, VA) and then UV-irradiated to inactivate residual vaccinia. The vac/LMP-infected ACs were cocultured with the NAC fraction for 7 days in CTL media. After 7 days, responding cells were restimulated weekly with fresh vac/LMP-infected, UV-inactivated ACs and irradiated PBMC feeder cells in CTL media supplemented with 2 to 5 U/mL IL-2 on days 2 and 4 after restimulation. After the third restimulation, cells were enriched for CD8+ cells by depleting CD4+ cells using αCD4 MoAb-coated flasks (Applied Immune Sciences) and cloned by limiting dilution using 3 × 104 autologous LCL per well as stimulators to avoid expansion of vaccinia-reactive cells, in CTL media supplemented with IL-2 at 50 U/mL final concentration. Wells demonstrating growth after 10 to 14 days were screened for lysis of autologous but not allogeneic LCL. Clones demonstrating EBV-specific lytic activity were then expanded for further characterization.
Cytotoxicity assay.Standard 4-hour CRAs were used to assess lytic activity of cells. Target cells were labeled overnight with 10 μCi 51Cr, washed three times with HBSS, resuspended in RPMI supplemented with 10% FCS, and plated at 5 × 103 cells per well in 96-well V-bottom plates (Costar, Cambridge, MA). Target cells for specificity determination were infected with vac recombinants at MOI of 10 and labeled with 51Cr for 16 to 18 hours, and then prepared for use as described. Effector cells were plated in triplicate at various E:T ratios. Samples were assayed in triplicate, and specific lytic activity was calculated using the standard equation.
RESULTS
Phenotypic characterization of R-S cells as potential targets for CD8+ CTL.HDLM-2, an in vitro–derived R-S cell line, was characterized for Class I expression for evaluation of lysis by Class I–restricted CTLs. Traditional serologic HLA typing could not be performed, due to the sensitivity of the cells to nonspecific lysis by complement. Therefore, HLA Class I genotype was determined by sequencing genomic DNA selectively amplified by reverse transcriptase–polymerase chain reaction with primers to exons 2 and 3 of the HLA-A and -B alleles. Using this method, HDLM-2 was determined to be HLA-A1, 2 B8, 44 (data not shown).
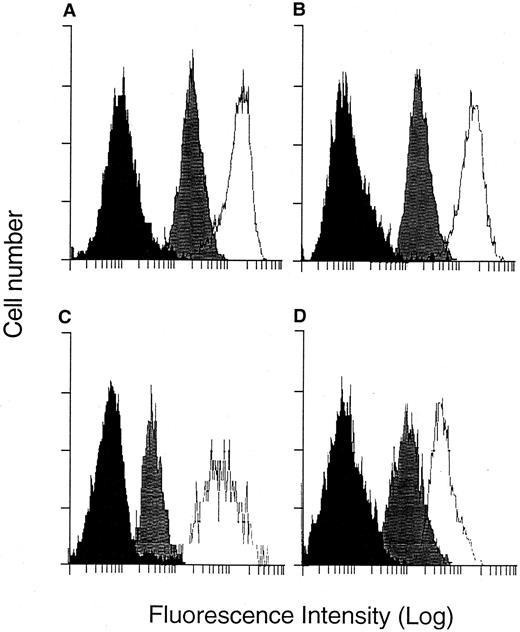
Downregulation of Class I expression on the surface of target cells is one potential mechanism of immune escape used by malignant cells.34-36 Therefore, HDLM-2 was evaluated for surface expression of Class I molecules with a fluorescein-conjugated MoAb specific for the public domain of the Class I molecule (W6/32), and flow cytometry showed a strong expression of Class I molecules that was approximately equivalent to CD30 expression (Fig 1A). Additionally, since we had identified donors who could potentially generate CTL responses restricted to A1 and B8, expression of these alleles was directly assessed with monospecific MoAbs, and expression similar to that observed with the framework antibody was observed (Fig 1B).
FACS analysis of Class I and adhesion molecule surface expression. HDLM-2 cells were suspended in FACS media and stained with MoAbs specific for surface molecules. After addition of a second-step antibody conjugated to fluorescein, cells were analyzed by flow cytometry. (A) Negative control (▪), anti–Class I (w6/32) (), anti-CD30 (□). (B) Negative control (▪), anti-A1 (), anti-B8 (□). (C) Negative control (▪), anti-LFA2 (), anti-ICAM1 (□). (D) Negative control (▪), anti-CD80 (), anti-CD86 (□).
FACS analysis of Class I and adhesion molecule surface expression. HDLM-2 cells were suspended in FACS media and stained with MoAbs specific for surface molecules. After addition of a second-step antibody conjugated to fluorescein, cells were analyzed by flow cytometry. (A) Negative control (▪), anti–Class I (w6/32) (), anti-CD30 (□). (B) Negative control (▪), anti-A1 (), anti-B8 (□). (C) Negative control (▪), anti-LFA2 (), anti-ICAM1 (□). (D) Negative control (▪), anti-CD80 (), anti-CD86 (□).
Expression of other surface molecules demonstrated to participate in effector and target cell interactions was also evaluated, including the adhesion molecules ICAM-1 and LFA-2 and the costimulatory molecules CD80 and CD86, which can also function as adhesion molecules to improve target lysis.37 HDLM-2 demonstrated surface expression of all of these molecules (Fig 1C and D).
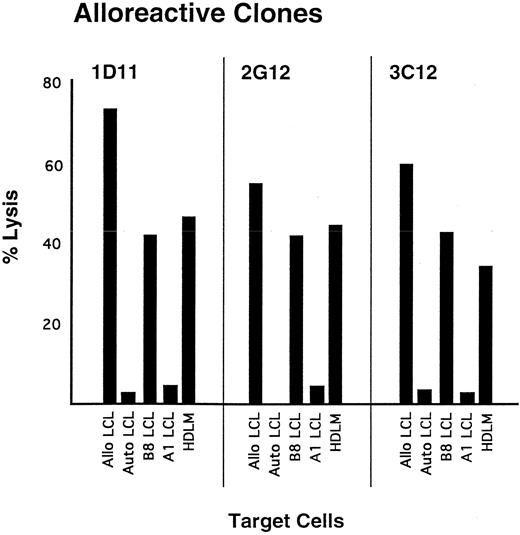
Analysis of HDLM-2 as a target for CTL-mediated lysis.The susceptibility of HDLM-2 to lysis by CD8+ CTL was initially evaluated with alloreactive CTL. Allospecific CTL lines were generated by stimulating responder PBMC obtained from an HLA-A2 B57,60 donor with stimulator cells obtained from an HLA-A1 B8 homozygous individual, and clones were isolated following plating at 0.3 cells per well in the presence of irradiated A1 B8 stimulator cells. Three clones demonstrating greater than 50% specific lysis at an E:T of 10:1 were evaluated for Class I specificity, and all exhibited lysis of target cells expressing B8 but not target cells expressing A1 (Fig 2). These three CTL clones were then tested for recognition of HDLM-2 cells, and all demonstrated greater than 35% specific lysis at an E:T of 10:1 (Fig 2).
Lysis of HLA-B8–positive target cells by B8-allospecific CTL clones. Polyclonal lines were generated by stimulating PBMC with allogeneic B lymphoblastoid cells derived from a homozygous A1, B8 donor. Specific lytic activity was determined in a standard CRA. Target cells include autologous LCL (negative control), LCL generated from the A1,B8 donor, allogeneic LCL matched with the stimulating LCL at only 1 class I allele, and HDLM-2. Effector cells were added at an E:T of 10:1.
Lysis of HLA-B8–positive target cells by B8-allospecific CTL clones. Polyclonal lines were generated by stimulating PBMC with allogeneic B lymphoblastoid cells derived from a homozygous A1, B8 donor. Specific lytic activity was determined in a standard CRA. Target cells include autologous LCL (negative control), LCL generated from the A1,B8 donor, allogeneic LCL matched with the stimulating LCL at only 1 class I allele, and HDLM-2. Effector cells were added at an E:T of 10:1.
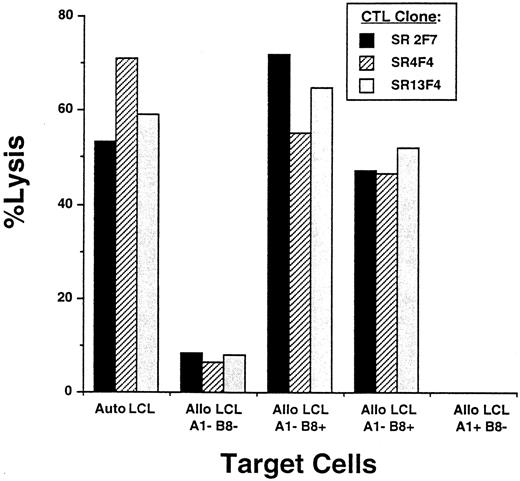
Analysis of antigen-processing function in R-S cells.Lysis of R-S cells expressing EBV genes by virus-specific CD8+ CTLs requires that viral proteins be processed and presented appropriately in the context of Class I for CTL recognition. CTL clones reactive with LMP1, one of the EBV proteins expressed in R-S cells, and restricted to one of the HLA-A or -B alleles expressed on HDLM-2 were generated by obtaining PBMC from a normal EBV+ homozygous A1 B8 individual and stimulating with autologous monocytes infected with vac/LMP1. Clones were isolated by plating responder cells enriched for CD8+ cells at a limiting dilution of 0.3 cells per well in the presence of autologous LCL stimulators. Clones demonstrating lysis of HLA-A1 B8 target cells expressing LMP1 as the only EBV gene were selected for further study. Clones restricted to B8 were identified by testing for lysis of allogeneic LCL matched with the stimulator cell at only this HLA allele. Three representative CTL clones that exhibited greater than 50% specific lysis of autologous LCL at E:T of 20:1 demonstrated similar lysis of allogeneic LCL matched only at the HLA-B8 locus (Fig 3).
Determination of HLA class I restriction of LMP-specific T-cell clones. LMP-specific clones were isolated by stimulating in vitro PBMC obtained from an HLA-A1,B8 homozygous individual with autologous monocytes infected with vac/LMP1 recombinants and cloning by limiting dilution. Clones were characterized for Class I restriction using a standard CRA. Autologous LCL and allogeneic LCL mismatched and matched at either the A1 or B8 alleles were prepared as previously described. Effector cells were added at an E:T of 10:1. Three representative CTL clones restricted to the B8 allele are shown: SR2F7 (▪), SR4F4 (▨), and SR13F4 ().
Determination of HLA class I restriction of LMP-specific T-cell clones. LMP-specific clones were isolated by stimulating in vitro PBMC obtained from an HLA-A1,B8 homozygous individual with autologous monocytes infected with vac/LMP1 recombinants and cloning by limiting dilution. Clones were characterized for Class I restriction using a standard CRA. Autologous LCL and allogeneic LCL mismatched and matched at either the A1 or B8 alleles were prepared as previously described. Effector cells were added at an E:T of 10:1. Three representative CTL clones restricted to the B8 allele are shown: SR2F7 (▪), SR4F4 (▨), and SR13F4 ().
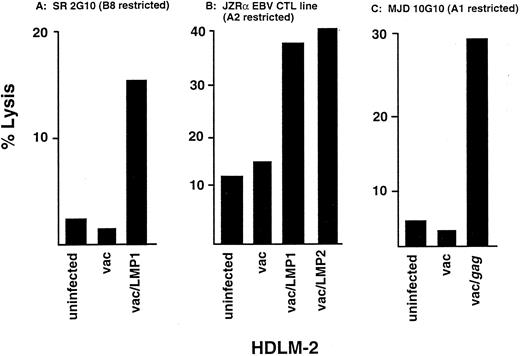
Since HDLM-2 expresses B8 but does not contain an EBV genome or express any endogenous viral proteins, we used a recombinant vaccinia virus to introduce individual viral genes to determine whether HDLM-2 could process and present the viral proteins for recognition by CTL. HDLM-2 cells infected with vac/LMP1 were lysed by a B8-restricted LMP1-specific CTL clone described earlier, whereas the clone did not lyse uninfected or vac-infected HDLM-2 targets (Fig 4A). Since we did not have an EBV-specific CTL clone restricted to HLA-A1 or -A2, the presentation of LMP1 and LMP2 in the context of the HLA-A2 allele was examined using a polyclonal EBV-reactive CTL line generated by stimulating PBMC from an HLA-A2 B13,18 individual with autologous LCL. The CD8+ enriched CTLs lysed HDLM-2 cells infected with vac/LMP1 or vac/LMP2, but not uninfected or vac control–infected HDLM-2 targets (Fig 4B), indicating that this R-S cell can process and present the two LMP proteins in the context of the HLA-A2 allele. The presentation of viral antigens in the context of HLA-A1 was evaluated using an A1-restricted HIVgag-specific CTL clone generated in our laboratory using previously described methods.38 This clone lysed vac/gag-infected HDLM-2 cells but not uninfected or vac-infected control target cells (Fig 4C). These results indicate that this R-S cell can process and present viral antigens, including LMP1 and LMP2, in the context of multiple HLA Class I alleles.
Lysis of HDLM-2 expressing viral proteins by CTL. Virus-specific lines and clones were generated from normal, healthy donors who shared HLA alleles with HDLM-2 by stimulating PBMC with autologous APC infected with vac recombinants. Lysis of HDLM-2 after infection with vac recombinants was determined in a standard CRA. (A) Lytic activity of a HLA-B8–restricted LMP1-specific CTL clone, 2G10 for HDLM-2 target cells infected with vac/LMP1, vac alone, or uninfected at an E:T of 2:1. (B) Lytic activity of an EBV-reactive CTL line generated from a HLA-A2,B13,18 individual for HDLM-2 target cells infected with vac/LMP1, vac/LMP2, vac alone, or uninfected at an E:T of 10:1. (C) Lytic activity of a HLA-A1–restricted HIVgag-specific CTL clone for HDLM-2 target cells infected with vac/gag, vac alone, or uninfected at an E:T of 10:1.
Lysis of HDLM-2 expressing viral proteins by CTL. Virus-specific lines and clones were generated from normal, healthy donors who shared HLA alleles with HDLM-2 by stimulating PBMC with autologous APC infected with vac recombinants. Lysis of HDLM-2 after infection with vac recombinants was determined in a standard CRA. (A) Lytic activity of a HLA-B8–restricted LMP1-specific CTL clone, 2G10 for HDLM-2 target cells infected with vac/LMP1, vac alone, or uninfected at an E:T of 2:1. (B) Lytic activity of an EBV-reactive CTL line generated from a HLA-A2,B13,18 individual for HDLM-2 target cells infected with vac/LMP1, vac/LMP2, vac alone, or uninfected at an E:T of 10:1. (C) Lytic activity of a HLA-A1–restricted HIVgag-specific CTL clone for HDLM-2 target cells infected with vac/gag, vac alone, or uninfected at an E:T of 10:1.
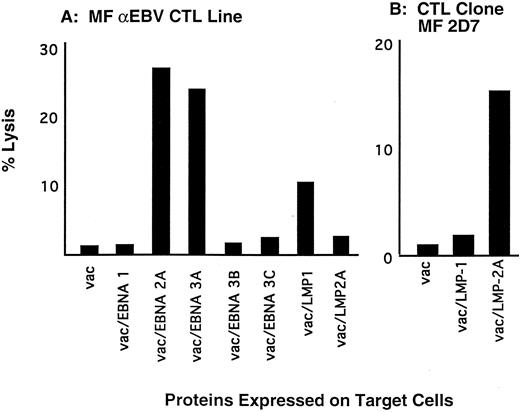
CTL responses to EBV proteins expressed in R-S cells from patients with HD.Eradication of EBV+ R-S cells in HD patients depends on the presence of a cellular immune response to the EBV proteins expressed in R-S cells. Therefore, CTL responses to the latent EBV proteins were evaluated in HD patients by stimulating PBMC with autologous LCL that express all of the latent proteins. After four weekly restimulations with autologous LCL, responding cells from the polyclonal cultures were screened for recognition of individual EBV proteins using a panel of autologous fibroblasts infected with vac-recombinants encoding single EBV genes. The CTL responses detected from a representative patient with HD demonstrated CD8+ responses predominantly to EBNA 2 and EBNA 3A, as reflected by specific lysis of targets expressing these genes of 27% and 25%, respectively, at an E:T of 5:1. A weak response to LMP1 was present, but the response to LMP2 was measured at background levels (Fig 5A).
CTL responses to latent EBV proteins. (A) Polyclonal lines were generated from a patient with HD by stimulating PBMC with autologous LCL. After 3 in vitro stimulations, responding cells were enriched for CD8+ cells, and the repertoire of CTL responses to the latent EBV proteins was determined by lysis of autologous fibroblasts infected with vac recombinants encoding individual latent EBV genes. Specific lysis was determined by a standard CRA. Effector cells were added at an E:T of 10:1. (B) Responding cells from the polyclonal lines described above were cloned by limiting dilution in the presence of autologous LCL stimulators. Specificity of the clones was determined in a CRA by measuring specific lysis of autologous fibroblasts infected with vac recombinants. Clone MF 2D7 lysed vac/LMP2-infected target cells at an E:T of 5:1.
CTL responses to latent EBV proteins. (A) Polyclonal lines were generated from a patient with HD by stimulating PBMC with autologous LCL. After 3 in vitro stimulations, responding cells were enriched for CD8+ cells, and the repertoire of CTL responses to the latent EBV proteins was determined by lysis of autologous fibroblasts infected with vac recombinants encoding individual latent EBV genes. Specific lysis was determined by a standard CRA. Effector cells were added at an E:T of 10:1. (B) Responding cells from the polyclonal lines described above were cloned by limiting dilution in the presence of autologous LCL stimulators. Specificity of the clones was determined in a CRA by measuring specific lysis of autologous fibroblasts infected with vac recombinants. Clone MF 2D7 lysed vac/LMP2-infected target cells at an E:T of 5:1.
We then examined if LMP-reactive CTL clones could be isolated from the CD8+ enriched polyclonal line derived from this HD patient, despite the presence of only weak or nondetectable responses in the polyclonal cultures. A total of 3,000 wells were plated at 0.3 cells per well with autologous LCL as stimulators, and yielded 30 CTL clones that lysed autologous LCL but not mismatched allogeneic LCL. One of these 30 clones, 2D7, demonstrated specific lysis of vac/LMP2-infected target cells (Fig 5B). None of the clones isolated demonstrated lysis of vac/LMP1 target cells.
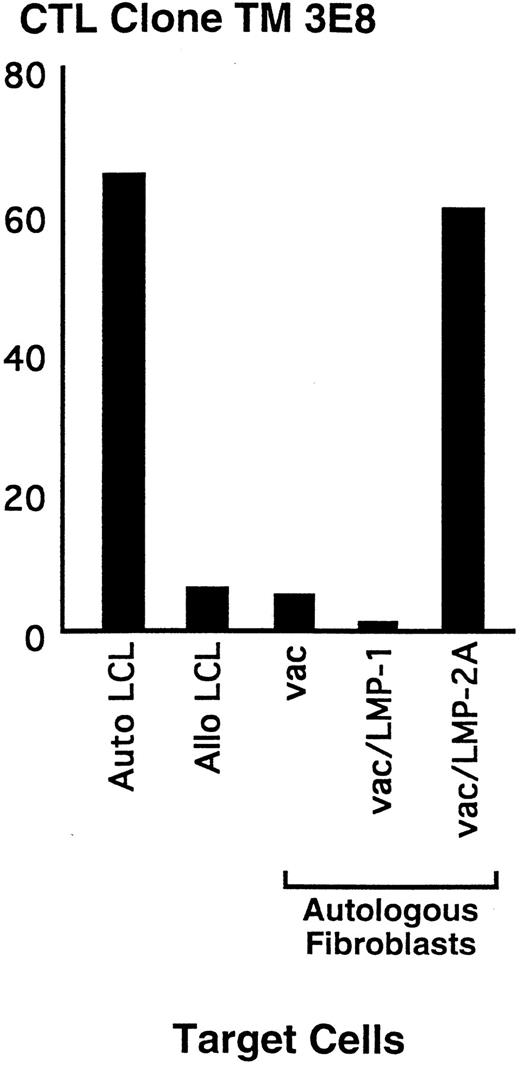
These results demonstrated that LMP2-specific responses could be generated from a HD patient with a dominant response to other EBV proteins and nondetectable responses to LMP2 in the polyclonal EBV-reactive line. However, the majority of clones isolated from such CTL lines recognize EBV proteins other than LMP, and thus would be of little use for treatment of HD. Therefore, we examined an alternative in vitro method that might increase the likelihood of isolating predominantly the clones of interest. To avoid the preferential generation of responses to the more immunodominant EBNA proteins that occurs if LCL are used as stimulator cells, PBMC from a HD patient were stimulated with autologous fibroblasts infected with a vac-recombinant encoding LMP2. After three weekly stimulations with fresh stimulators and autologous feeder cells, responding cells were enriched for CD8+ T cells and cloned by limiting dilution at 0.3 cells per well with autologous LCL as stimulators to selectively expand the LMP2-specific cells generated in the primary culture and not propagate T cells reactive to vaccinia proteins. Using this method, 50% of the clones that specifically lysed autologous LCL were determined to be LMP2-specific with lysis of target cells infected with vac/LMP2, but not with vac/LMP1 or vac alone. A representative clone, 3E8, generated by this method demonstrated 50% lysis of LMP2-expressing targets and autologous LCL at an E:T of 20:1 (Fig 6). These results suggest that LMP responses can be isolated at an increased frequency using this methodology.
Representative LMP2-specific CD8+ CTL isolated from a HD patient. Polyclonal lines were generated by stimulating PBMC with autologous LMP2-expressing cells. Responding cells were cloned by limiting dilution in the presence of autologous LCL. Clones were screened for specific lysis of autologous LCL in a standard CRA. Specificity for the LMP2 protein was determined in a CRA by lysis of autologous fibroblasts infected with vac/LMP2, but not cells infected with vac/LMP1 or vac alone. Effector cells were added to the plates at an E:T of 10:1.
Representative LMP2-specific CD8+ CTL isolated from a HD patient. Polyclonal lines were generated by stimulating PBMC with autologous LMP2-expressing cells. Responding cells were cloned by limiting dilution in the presence of autologous LCL. Clones were screened for specific lysis of autologous LCL in a standard CRA. Specificity for the LMP2 protein was determined in a CRA by lysis of autologous fibroblasts infected with vac/LMP2, but not cells infected with vac/LMP1 or vac alone. Effector cells were added to the plates at an E:T of 10:1.
DISCUSSION
The demonstration that R-S cells in a subset of EBV+ patients with HD express EBV antigens has engendered enthusiasm for pursuing immunotherapeutic approaches in the treatment of HD. The potential efficacy of this strategy has been further supported by studies of EBV-associated lymphoproliferative disorder, in which the adoptive transfer of EBV-specific CTLs has resulted in eradication of tumor.39 However, the malignant cell in EBV lymphoproliferative disease expresses all of the latent EBV proteins, including the proteins that elicit the immunodominant responses.40-42 By contrast, R-S cells in (EBV+) HD express only three of the latent proteins, EBNA 1, LMP1, and LMP2,16,18-20 none of which generate an immunodominant CTL response.24,43 44 Moreover, although EBV-reactive CTLs efficiently lyse EBV-transformed B cells, there is little information regarding the ability of R-S cells to function as target cells for CTL specific for the expressed viral proteins. The studies presented here demonstrate not only that HD patients may indeed have CTL precursors present in the peripheral blood that are capable of recognizing and lysing R-S cells, but also that R-S cells can be lysed by CTL specific for viral proteins.
Studies of HD have been hindered by the difficulty in isolating the R-S cell due to the paucity of the malignant cell in the affected lymph nodes. Therefore, our studies used an in vitro–derived and maintained R-S cell line, HDLM-2. This line has been extensively analyzed and displays the pathognomonic R-S cell morphology, as well as the associated characteristic surface expression of CD15 and CD30.28 Previous investigations have demonstrated Class I expression in HDLM-2 by immunohistochemistry,45 suggesting that it would be appropriate for analyzing recognition and lysis by CD8+ CTL. Our studies using molecular typing to identify the HLA genotype and specific MoAbs to evaluate surface expression demonstrated that HDLM-2 is HLA-A1,2-B8,44, alleles that are common in the population.
Class I expression in R-S cells is an essential component for success of an immunotherapy strategy that targets the tumor cells for lysis by CD8+ CTL. Downregulation of Class I expression as the basis for evasion of the immune response has been demonstrated for several malignancies,34-36 including an EBV-associated malignancy, Burkitt's lymphoma.46 EBV+ Burkitt's cells use multiple mechanisms to escape from immune-mediated elimination, including expression of only EBNA 1, an EBV protein that does not elicit a CD8+ CTL response, and downregulation of several molecules important for cell-mediated lysis, including Class I and ICAM.47-49 In contrast, EBV+ R-S cells isolated from the lymph nodes of HD patients have demonstrated expression of two CTL immunogenic proteins.18-20 Evaluation of Class I expression in R-S cells isolated from in vivo biopsy specimens demonstrated strong Class I expression in 22 of 40 HD patients,23 suggesting the possibility of a similar mechanism of tumor escape from immune surveillence for a proportion of HD patients. However, Class I expression in 24 of 25 HD patients with EBV+ R-S cells was preserved,50 with intact adhesion molecule expression.51 52 Therefore, EBV+ R-S cells appear to retain the potential to be eliminated by adoptively transferred viral-specific CTL. Our studies with HDLM-2 support this supposition, demonstrating that R-S cells can be lysed by CTL in a Class I–restricted manner.
The lysis of R-S cells expressing potentially transforming EBV genes further depends on the ability of the cell to process and present the viral proteins in the context of Class I molecules. Tumor cells can also evade immune surveillance by developing a defect in the processing of proteins that are potential CTL target antigens, which results in failure to present the tumor antigens in the context of Class I on the cell surface.53 Our studies with HDLM-2 have demonstrated that this R-S cell can process endogenously synthesized viral proteins and be lysed by CD8+ CTL clones specific for the viral proteins. The appropriate processing and presentation of viral proteins by HDLM-2, in particularly the LMP1 and LMP2 proteins of EBV, provides further support for pursuing a treatment strategy using the adoptive transfer of EBV-specific CTL in patients with (EBV+) HD.
Expression of nonimmunodominant proteins represents another mechanism by which tumor cells have been shown to evade immune elimination.54,55 EBV+ R-S cells express only three of the latent EBV proteins, EBNA 1, LMP1, and LMP2. Although the latter two proteins have been shown to elicit CD8+ CTL responses,25,44 these responses are weak compared with the immunodominant CTL responses to EBNA 2 and 3 proteins.24 Our studies demonstrate that even when responses are not readily detectable in the reactivity of polyclonal EBV-specific lines, it is possible to isolate CTL clones specific for the relevant proteins. Previously, we have demonstrated that such antigen-specific T-cell clones can be expanded to large numbers in vitro, and following reinfusion, can achieve frequencies in vivo of 1:100 to 1:1,000 of every cell in the peripheral blood.8 Thus, by this technology, it is possible to convert a weak response into an extremely strong response. These findings suggest that although R-S cells may evade immune eradication by expressing only poorly immunogenic EBV proteins, adoptive immunotherapy may provide a means to effectively treat this malignancy.
The essential requisites for specific adoptive T-cell therapy are the identification of target antigens that can elicit an immune response and the technology for isolating and expanding T cells reactive with these antigens. Our previous studies in immunocompromised hosts have demonstrated the feasibility and potential efficacy of adoptive T-cell therapy with CMV-specific CD8+ T-cell clones for the prevention of CMV disease.7,8 The preclinical studies described here demonstrate that the malignant R-S cell is a suitable target for recognition by CD8+ CTL and that LMP-specific CTL can be isolated from HD patients. Recent studies have demonstrated that T cells from patients with HD, as well as other malignancies, may exhibit deficient expression of the ζ chain, a critical component of the T-cell receptor signaling complex.56-58 Although this defect could contribute to the inability of patients with HD to generate an effective response in vivo to the EBV antigens expressed in R-S cells, our results suggest that in vitro activation and expansion may provide a means to overcome the consequences of such T-cell dysfunction. Furthermore, additional obstacles to therapeutic efficacy may exist, such as poor localization of CTL to the region of the lymph node containing R-S cells, selection of antigen-loss variants, or the presence of local factors in lymph nodes affected with HD that can interfere with maintenance of an effective T-cell response.59 60 The efficacy and/or limitations of a T-cell therapy strategy will likely only be elucidated by a clinical trial in which LMP1- and/or LMP2-specific CTL are adoptively transferred as treatment for (EBV+) HD.
ACKNOWLEDGMENT
The authors would like to thank Drs D. Geraghty and T. Spies (FHCRC) for generously providing MoAbs to HLA-A1 and -B8, and Dr M. Kurilla (University of Virginia, Charlottesville, VA) for generously providing the vaccinia recombinants. We would also like to thank J. Factor for preparation of the manuscript.
Supported by a Physician Research Training Award from the American Cancer Society and by grant no. R01 CA63522 from the National Institutes of Health/National Cancer Institute. R.F.A. is a Leukemia Society Scholar.
Address reprint requests to Amy P. Sing, MD, Division of Oncology, Box 356527, University of Washington, Seattle, WA 98195-6527.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal