Abstract
Thrombopoietin (Tpo) is a major regulator of megakaryopoiesis both in vivo and in vitro. Tpo initiates its biologic effects by binding to the Mpl receptor, which is a member of the hematopoietin receptor family. To define the Tpo binding characteristics of the Mpl receptor, we iodinated purified 70-kD recombinant human Tpo using the Bolton-Hunter reagent. Autoradiographic analysis of 125I-Tpo binding to normal human marrow mononuclear cells showed many grains specifically associated with megakaryocytes; there were no grains specifically associated with myeloblasts or erythroblasts. Equilibrium binding experiments with 125I-Tpo and normal human platelets showed a single class of high-affinity receptors (kd, 190 pmol/L) with approximately 30 Mpl receptors per platelet. Affinity cross-linking with 125I-Tpo showed that the Mpl receptor on platelets is of molecular weight ∼98 kD. Despite their sequence similarity, erythropoietin and Tpo did not cross-compete for binding to BaF3 cells engineered to coexpress Mpl receptor and erythropoietin receptor. Progeny of normal human burst-forming units-erythroid (BFU-E) contained Mpl receptor mRNA, and flow cytometric analysis showed the presence of Mpl receptor protein on the surface of these cells. These data indicate that display of the Mpl receptor is not limited to the megakaryocytic lineage, but also includes progeny of BFU-E. Like receptors for other hematopoietic cytokines, the binding affinity of the Mpl receptor for Tpo is high, with relatively few receptors displayed per cell. These results suggest that the effects of Tpo to speed red blood cell recovery after myelosuppressive therapy in vivo and to enhance colony-forming unit-erythroid generation in vitro may be mediated by direct interaction of Tpo and erythroid progenitor cells.
THROMBOPOIETIN (Tpo), the ligand for the Mpl receptor, plays a major role in the regulation of megakaryopoiesis.1 Tpo promotes both the proliferation of colony-forming units-megakaryocyte (CFU-Meg) and the cytoplasmic and nuclear maturation of megakaryocytes in vitro.2-6 Tpo also exerts a direct effect on platelets; exposure of platelets to Tpo in vitro potentiates the effects of platelet aggregatory agents.7,8 Experiments using highly enriched populations of hematopoietic stem cells that are capable of reconstituting hematopoiesis long-term in lethally irradiated hosts suggest that Tpo can act directly on these primitive cells as well.9 10
Studies of knock-out mice engineered to lack either Mpl receptor or Tpo confirm the importance of Tpo in the regulation of megakaryopoiesis.11-13 These mice have platelet counts that are approximately 6% to 15% of normal as well as equally reduced numbers of megakaryocytes in the marrow. Conversely, preclinical studies show that treatment with Tpo increases the platelet count 5- to 10-fold in normal animals and speeds platelet recovery after chemoradiotherapy.8 14-17
Tpo and erythropoietin (Epo) share a number of structural features. The N-terminal domain of Tpo has 22% identical and 25% conservative substitutions in comparison to the amino acid sequence of Epo, and the location of 3 of the 4 cysteine residues are conserved between these two molecules.18 The Mpl receptor and the Epo receptor, both members of the hematopoietin receptor superfamily, share 26% amino acid homology.19 There is a degree of functional overlap between these two cytokines as well. Tpo and Epo display synergistic effects on megakaryopoiesis (stimulation of CFU-Meg colony growth)2 and on erythropoiesis (generation of colony-forming unit-erythroid [CFU-E] and growth of burst-forming unit-erythroid [BFU-E] progeny)20-22 in vitro. Tpo amplifies the production of BFU-E from cellular populations containing long-term culture-initiating cells.23 Moreover, Tpo can stimulate BFU-E colony growth from liver cells of fetal mice engineered to lack the Epo receptor.24 Although treatment with Tpo does not increase the hematocrit of normal mice, it does increase reticulocyte production and accelerate red blood cell recovery in myelosuppressed mice, likely by synergizing with the high level of endogenous Epo found in these mice.20
Binding of Tpo to the Mpl receptor triggers a number of events, including tyrosine phosphorylation of the receptor and activation of JAK2, TYK2, and Shc as well as dimerization and nuclear translocation of STAT proteins.25-31 Although the intracellular events that follow Tpo binding to the Mpl receptor have been the focus of intense investigation, less is known about the cellular distribution and Tpo binding characteristics of the cell surface Mpl receptor. Mpl receptor mRNA has been identified in normal human CD34+ cells, in megakaryocytic cell lines, and in blasts obtained from a portion of patients with acute myelogenous leukemia.32-35 The majority of myeloid and erythroid cell lines studied have been reported to lack Mpl receptor mRNA. However, Mpl receptor mRNA has been found in murine fetal liver BFU-E progeny.24 Flow cytometric analysis and Western blotting with an anti-Mpl receptor monoclonal antibody (MoAb) have documented antigenic display of the Mpl receptor protein on human megakaryocytic cell lines and on platelets35; and platelets can bind and metabolize Tpo.36 37
In the present study, we sought to more fully define the Tpo binding characteristics and cellular distribution of the functional Mpl receptor on normal human hematopoietic cells. We also investigated whether the overlapping biologic effects of Tpo and Epo could be explained in part by cross-competition for receptor binding and whether BFU-E and their progeny exhibit Mpl receptors. The results showed that normal human platelets display a low number of high-affinity Mpl receptors and that Tpo and Epo synergy cannot be explained by cross-competition for receptor binding to cells that coexpress functional receptors for both cytokines. Normal human BFU-E progeny contain Mpl receptor mRNA and display the Mpl receptor on the cell surface, suggesting that the effects of Tpo on erythropoiesis may be direct.
MATERIALS AND METHODS
Normal hematopoietic cells and cell lines.Human marrow cells were obtained by aspiration from the posterior iliac crest of healthy adults after obtaining informed consent. These studies were approved by the Human Subjects Division of the University of Washington (Seattle, WA). Marrow mononuclear cells were prepared by density centrifugation using Ficoll-Hypaque (Organon Teknika Corp, Durham, NC). Marrow mononuclear cells were used for autoradiographic analysis of 125I-Tpo binding.
To obtain BFU-E and colony-forming unit–granulocyte-macrophage (CFU-GM) progeny, the mononuclear cells were cultured at a concentration of 1 to 2 × 105 cells/mL in 1.4% methylcellulose (Dow, Midland, MI) in Iscove's modified Dulbecco's medium (IMDM; GIBCO, Grand Island, NY) supplemented with 30% heat-inactivated fetal calf serum (FCS; Hyclone, Logan, UT), 1% bovine serum albumin (Reheis, Phoenix, AZ), 2 U/mL recombinant human Epo,38 and 50 ng/mL recombinant human stem cell factor (a gift from Dr K. Langley, Amgen, Inc, Thousand Oaks, CA). For CFU-GM progeny, the cultures also included 2 ng/mL recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF; a gift from Dr K. Kaushansky, University of Washington). After 5 to 14 days of incubation at 37°C, in a humidified atmosphere containing 5% CO2 , BFU-E or CFU-GM progeny were plucked under the inverted microscope as previously described.39 To further define the proliferative potential and cellular composition of the population of cells thus obtained, a portion of these cells were replated in CFU-E colony assays39 in the presence of Epo (2 U/mL) plus recombinant human interleukin-3 (IL-3; 1 ng/mL; Genzyme Corp, Boston, MA). The BFU-E progeny were also labeled with phycoerythrin (PE)-conjugated anti-glycophorin A antibody (Dako, Carpinteria, CA), as previously described,22 and analyzed by fluorescence microscopy (Zeiss Universal; Zeiss, Thornwood, NY).
Peripheral blood from healthy adult volunteers was drawn into a syringe containing 15% acid-citrate-dextrose anticoagulant. Platelets were isolated by centrifuging the blood at 250g for 10 minutes at room temperature. An aliquot of each platelet preparation was sent to the clinical laboratory at the University of Washington Medical Center, where the number and purity of the platelets were quantitated using a Abbott Cell-Dyn 3500 (Santa Clara, CA). All of the platelet preparations used for Scatchard analysis of 125I-Tpo binding or affinity cross-linking contained more than 99% platelets and less than 1% white blood cells. To assess the activation status of the platelets thus isolated, display of CD62P (P-selectin) on the platelet surface was examined.40 The platelets were incubated with biotinylated anti-CD61 MoAb (20 μg/mL; Southern Biotechnology Associates, Birmingham, AL) for 30 minutes at 4°C and then with a mixture of avidin-PE (1:100 final dilution; Jackson ImmunoResearch Laboratories, West Grove, PA) plus either anti-CD62P MoAb directly conjugated to fluorescein isothiocyanate (FITC; 1:10 final dilution; PharMingen, San Diego, CA) or control mouse IgG1κ directly conjugated to FITC (1:10 final dilution; PharMingen) for 30 minutes at 4°C and analyzed with Coulter Epics Elite flow cytometer (Coulter, Miami, FL). Of the CD61+ cells, 8.5% displayed CD62P on the cell surface, which is within the range previously reported for normal human platelets.41
The HEL and K562 human erythroleukemia cell lines were maintained in IMDM supplemented with 10% FCS. The growth factor-dependent murine lymphoblastoid cell line BaF3 engineered to express human Mpl receptor (BaF3-MplR)14 was maintained in IMDM supplemented with 10% FCS and 100 U/mL recombinant human Tpo (a gift from Dr D. Foster, ZymoGenetics Inc, Seattle, WA). BaF3 cells that coexpressed the mouse Epo receptor and human Mpl receptor were generated by electroporation of BaF-MplR cells with a plasmid vector that expresses the Epo receptor (pXM/mEpoR; kindly provided by Dr A. D'Andrea, Dana-Farber Cancer Institute, Boston, MA) and selection in 2 U/mL Epo. A cell line that proliferates in the presence of either human Epo or human Tpo was obtained by limiting dilution.
Binding experiments with 125I-Tpo.Purified recombinant human Tpo, expressed in BHK cells, was iodinated using the Bolton-Hunter reagent (Dupont/NEN, Boston, MA). Briefly, Tpo was incubated with the Bolton-Hunter reagent in 100 mmol/L phosphate buffer (pH 8.5) for 16 hours at 4°C and then passed over a Sephadex G 75-120 column previously equilibrated with 100 mmol/L phosphate-buffered saline (pH 7.4) containing 0.5% gelatin (Sigma Chemical Co, St Louis, MO). The radiologic specific activity of 125I-Tpo was determined by self-displacement analysis42 and was generally approximately 1,000 Ci/mmol. Parallel displacement curves were obtained, showing that 125I-Tpo and unlabeled Tpo exhibited similar receptor binding affinity.
Autoradiographic analysis of 125I-Tpo binding to normal human marrow mononuclear cells and to BaF3-MplR cells was performed by incubating 125I-Tpo (0.6 nmol/L) with the cells in binding buffer for 1 hour at 37°C.39 The binding buffer consisted of RPMI 1640 (MA Bioproducts, Walkersville, MD) supplemented with 50 mmol/L HEPES (pH 7.4), 0.5% gelatin, 0.1% azide, and 10 μg/mL cytochalasin B. Parallel samples were incubated with 125I-Tpo plus a 100-fold excess of unlabeled Tpo. At the conclusion of the incubation, the cell suspension was gently layered on percoll (density, 1.028 g/mL) and centrifuged for 1 minute at 4°C in a Beckman Microfuge 11 (Beckman, Palo Alto, CA) to separate cell-associated 125I-Tpo from free 125I-Tpo. Cytopreparations were made using a Shandon Southern Cytospin (Shandon Co, Pittsburgh, PA). The samples were exposed to photographic emulsion for 3 weeks at 4°C and developed as previously described.39 43 Grain counts were performed on approximately 30 cells in each sample. Specific binding was defined as the number of grains overlaying the cells in the presence of 125I-Tpo minus the number of grains overlaying the cells in the presence of 125I-Tpo plus unlabeled Tpo.
To determine whether 125I-Tpo and 125I-Epo could cross compete for receptor binding, BaF3 cells coexpressing functional Mpl and Epo receptors (1.2 × 106 cells) were incubated with 125I-Tpo (0.3 nmol/L) in the presence of a 100-fold molar excess of unlabeled Tpo or unlabeled recombinant human Epo for 1 hour at 37°C. In parallel, the BaF3-MplR-EpoR cells were incubated with 125I-Epo (0.3 nmol/L; Amersham, Arlington Heights, IL) with or without a 100-fold excess of unlabeled Tpo or unlabeled Epo for 1 hour at 37°C. The binding experiments were performed in triplicate. At the conclusion of the incubation, the cells were sedimented through phthalate oil as previously described39 and both cell-associated and free radiolabeled ligand were quantitated using a Packard Cobra II Gamma Counter (Packard Instrument Co, Downers Grove, IL).
To quantitate the binding affinity and number of Mpl receptors per cell, the cells were incubated in 125I-Tpo (30 pmol/L to 1.2 nmol/L) with or without a 100-fold excess of unlabeled Tpo in binding buffer that contained azide and cytochalasin B to inhibit internalization of the ligand for 1 hour at 37°C.39,44 For binding experiments with platelets, the binding buffer also contained 15% acid-citrate-dextrose to prevent platelet clumping. In one experiment, 125I-Tpo binding experiments with platelets were conducted overnight at 4°C and for 1 hour at 37°C in parallel. To determine whether partial receptor occupancy by Tpo acquired in vivo would alter the number of Mpl receptors detected by Scatchard analysis of 125I-Tpo binding, samples of platelets were incubated in IMDM supplemented with 10% FCS and 10% acid-citrate-dextrose for 90 minutes at 37°C to permit internalization and degradation of surface bound Tpo before quantitation of Mpl receptor number. All binding experiments were performed in triplicate, and the data were analyzed using the Ligand program.45
Affinity cross-linking.Normal human platelets (1 × 109) or BaF3-MplR cells (2 ×107) were incubated with 125I-Tpo (0.6 nmol/L) with or without excess unlabeled Tpo in binding buffer for 1 hour at 37°C and then cross-linked with 1 mmol/L bis (sulfosuccinimidyl suberate) (BS3; Pierce Chemical Co, Rockford, IL) for 30 minutes at 22°C, as previously described.44 A parallel experiment was performed in the absence of BS3. The cells were lysed in 1% Triton X-100, 20 mmol/L Tris-HCl (pH 8.0), 150 mmol/L NaCl, 10 mmol/L EDTA, 10% glycerol, 1 mmol/L phenylmethyl sulfonyl fluoride, 10 μg/mL leupeptin, 0.5 mg/mL aprotinin, and 10 μg/mL pepstatin. For some experiments, the cell lysates were directly suspended in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer containing dithiothreitol, boiled for 3 minutes, and analyzed on a 6% polyacrylamide gel. In experiments designed to determine whether the cross-linked complexes contained Mpl receptor, the cell lysates were immunoprecipitated with affinity-purified anti-Mpl receptor polyclonal antibody (ZymoGenetics, Inc) or with control rabbit IgG and analyzed by SDS-PAGE. Molecular weight markers (range, 14 to 340 kD [Boehringer Mannheim, Indianapolis, IN] or 49.5 to 205 kD [BioRad, Hercules, CA]) were used.
Reverse transcription-polymerase chain reaction (RT-PCR) analysis of Mpl receptor mRNA.Total cellular RNA was prepared from HEL and from K562 cells using the RNAgents Total RNA Isolation System (Promega, Madison, WI). Whole cell lysates from CD34+ cells isolated from human bone marrow and from day-5, day-7, and day-10 colonies derived from BFU-E progeny and from day-12 CFU-GM progeny were made by lysing the cells in a buffer containing 0.8% Igepal (Sigma), 5 mmol/L dithiothreitol (BioRad), and 30 U RNAisin (Promega). cDNA was made from HEL and K562 cell RNA, and from lysates from 5 × 104 CD34+ marrow cells and from lysates from BFU-E progeny using the Superscript Preamplification System (GIBCO-BRL, Gaithersburg, MD). Oligo dT primers were used to prime first-strand synthesis for all reactions. PCR amplification was performed using Pfu polymerase (Stratgene, La Jolla, CA) with an Ericomp Thermocycler (Ericomp Inc, San Diego, CA). To increase the sensitivity of detection, 5 μL of the product obtained after an initial 30 cycles of PCR was used in a second PCR amplification step using the same conditions. Amplification of the membrane-proximal extracellular region and the transmembrane domain of human mpl was performed using a sense primer with the sequence 5′-GACCCTGGAGCTGCGCCCGCGATCTCGCTA-3′ and an antisense primer with the sequence 5′-TCGAGTCAATGCCTCAGTCTCCTGTAGTGC-3′. The primers used for amplification of mpl were selected to span the boundary between exons 9 and 10 and result in a 238-bp PCR product from mRNA and a 490-bp PCR product from genomic DNA. Amplification of GAPDH cDNA was performed using a sense primer with the sequence 5′-CCATGGAGAAGGCTGGGG-3′ and an antisense primer with the sequence 5′-CAAAGTTGTCATGGATGACC-3′. Full-length human mpl in the expression vector PHZ-1 (ZymoGenetics, Inc) was used as a control for detection of the human mpl sequence.46 Amplified PCR products were analyzed by gel electrophoresis.
Flow cytometric analysis of Mpl receptor display on BFU-E and CFU-GM progeny.Colonies containing progeny of normal human BFU-E and CFU-GM were plucked as described above and were incubated with affinity-purified rabbit anti-Mpl receptor antibody (50 μg/mL; ZymoGenetics, Inc) or with purified control rabbit IgG (50 μg/mL; Jackson ImmunoResearch Laboratories) and then with mouse monoclonal anti-CD16 antibody to block nonspecific Fc receptors (1:10 final dilution; Dako) and FITC-conjugated goat antirabbit IgG (1:20 final dilution; Southern Biotechnology Associates) and were analyzed by flow cytometry. To determine whether the population of BFU-E progeny contained megakaryocytes, the cells were labeled as previously described22 with the Tab anti-CD41 MoAb47 (a gift from Dr R. McEver, University of Oklahoma, Oklahoma City, OK) or with mouse IgG1 control antibody (Caltag Laboratories, South San Francisco, CA) and then with FITC-conjugated goat antimouse IgG1 (American Quaalex, La Miranda, CA). All incubations were for 30 minutes at 4°C. HEL cells were used as a positive control for CD41 expression.
RESULTS
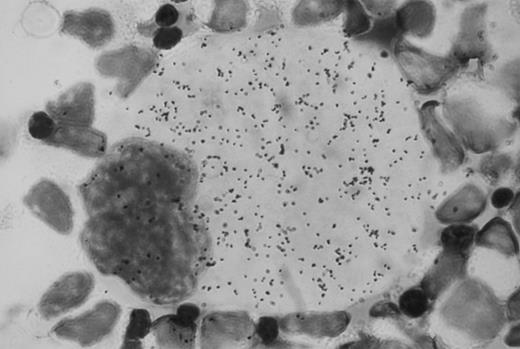
Autoradiographic analysis of 125I-Tpo binding to normal human bone marrow cells showed many grains associated with megakaryocytes (Fig 1). These grains were absent when the cells were incubated with 125I-Tpo in the presence of excess unlabeled Tpo. Grain counts showed that there were an average of approximately 1,300 grains per megakaryocyte in the presence of 125I-Tpo, and 200 grains per megakaryocyte in the presence of 125I-Tpo plus excess unlabeled Tpo. Thus, there were approximately 1,100 specifically bound grains per megakaryocyte. There was no detectable specific binding of 125I-Tpo to morphologically recognizable erythroblasts or myeloblasts. Parallel autoradiographic analysis of 125I-Tpo binding to the murine lymphoblastoid cell line BaF3 transfected with human Mpl receptor (BaF3-MplR cells) showed an average of 10 specifically bound grains per cell.
Autoradiographic analysis of 125I-Tpo binding to normal human bone marrow mononuclear cells. Original magnification × 100.
Autoradiographic analysis of 125I-Tpo binding to normal human bone marrow mononuclear cells. Original magnification × 100.
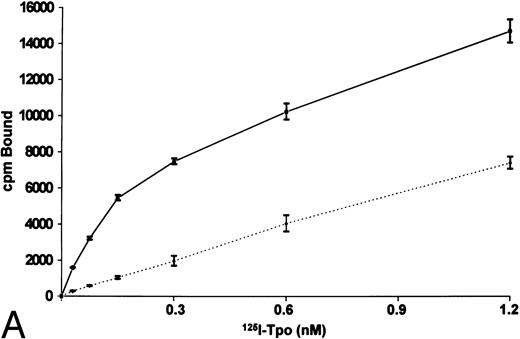
Platelets obtained from normal human peripheral blood displayed a single class of high-affinity Mpl receptors, with a kd of 100 to 200 pmol/L (Fig 2). In this experiment, there were 25 receptors per platelet, with a binding affinity of approximately 110 pmol/L. In four additional experiments, the number of receptors ranged from 20 to 40 per platelet. When all five experiments were analyzed together using the ligand program,45 the kd was 190 pmol/L and the number of receptors per platelet was 30 ± 4 (mean ± SEM). The kd was 200 pmol/L when the equilibrium binding experiment was performed at 4°C, similar to results obtained at 37°C. When the platelets were preincubated for 90 minutes at 37°C to permit internalization and metabolism of Tpo36 acquired in vivo, before performing the equilibrium binding experiments, both the number of Mpl receptors detected (30 receptors per platelet) and the binding affinity were similar to results of a parallel experiment performed without preincubation of the platelets. This result suggests that partial Mpl receptor occupancy with Tpo is not altering the results of Scatchard analysis of Mpl receptor display on normal human platelets. BaF3-MplR cells exhibit a Tpo binding affinity of approximately 120 pmol/L, similar to that of the native receptor found in human platelets, and 480 ± 100 receptors per cell (mean ± SEM of 4 experiments, data not shown). No binding of 125I-Tpo to human marrow mononuclear cells was detected (N = 3 experiments). Because megakaryocytes comprise only a small portion of the heterogeneous population of marrow mononuclear cells (<0.5%), these data reinforce the results of autoradiography.
Binding of 125I-Tpo to normal human platelets. The platelets (1.0 × 108) were incubated with 125I-Tpo (30 pmol/L to 1.2 nmol/L) without (——) or with (⋅⋅⋅) or a 100-fold excess of unlabeled Tpo for 1 hour at 37°C (A). The results represent the mean of triplicate determinations. The data were analyzed using the ligand program (B). Four additional independent experiments gave similar results.
Binding of 125I-Tpo to normal human platelets. The platelets (1.0 × 108) were incubated with 125I-Tpo (30 pmol/L to 1.2 nmol/L) without (——) or with (⋅⋅⋅) or a 100-fold excess of unlabeled Tpo for 1 hour at 37°C (A). The results represent the mean of triplicate determinations. The data were analyzed using the ligand program (B). Four additional independent experiments gave similar results.
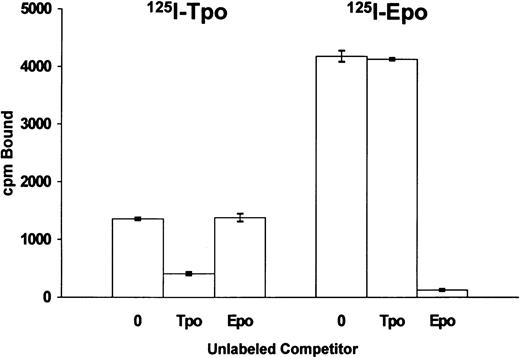
Because of the structural similarity of Tpo and Epo, as well as the partial functional overlap between these two cytokines, we investigated whether Tpo and Epo could cross-compete for binding to BaF3 cells that coexpress functional human Mpl receptor and functional human Epo receptor (Fig 3). The BaF3-MplR-EpoR cells exhibit a proliferative response to both Tpo and Epo (data not shown). 125I-Tpo binding is specifically competed by unlabeled Tpo but not by unlabeled Epo (Fig 3). Similarly, 125I-Epo binding is inhibited by unlabeled Epo but not by an equivalent molar quantity of unlabeled Tpo. These results indicate that Tpo and Epo do not cross-compete for receptor binding to the BaF3-MplR-EpoR cells. Similar results were obtained in one experiment using HEL cells that coexpress native Mpl receptor and Epo receptor but do not exhibit a proliferative response to these cytokines.
Binding of 125I-Tpo or 125I-Epo to BaF3-MplR-EpoR cells. The cells (1.2 × 106) were incubated with 125I-Tpo (0.3 nmol/L) or 125I-Epo (0.3 nmol/L) in the presence or absence of a 100-fold excess of unlabeled Tpo or Epo for 1 hour at 37°C. The data represent the mean ± SEM of triplicate determinations from one of three similar experiments.
Binding of 125I-Tpo or 125I-Epo to BaF3-MplR-EpoR cells. The cells (1.2 × 106) were incubated with 125I-Tpo (0.3 nmol/L) or 125I-Epo (0.3 nmol/L) in the presence or absence of a 100-fold excess of unlabeled Tpo or Epo for 1 hour at 37°C. The data represent the mean ± SEM of triplicate determinations from one of three similar experiments.
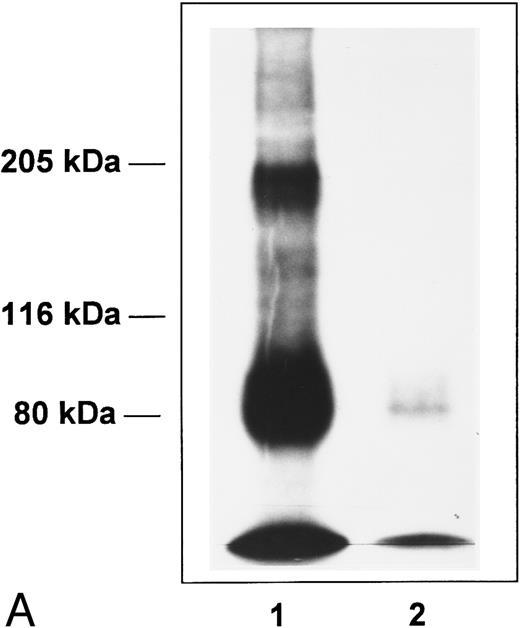
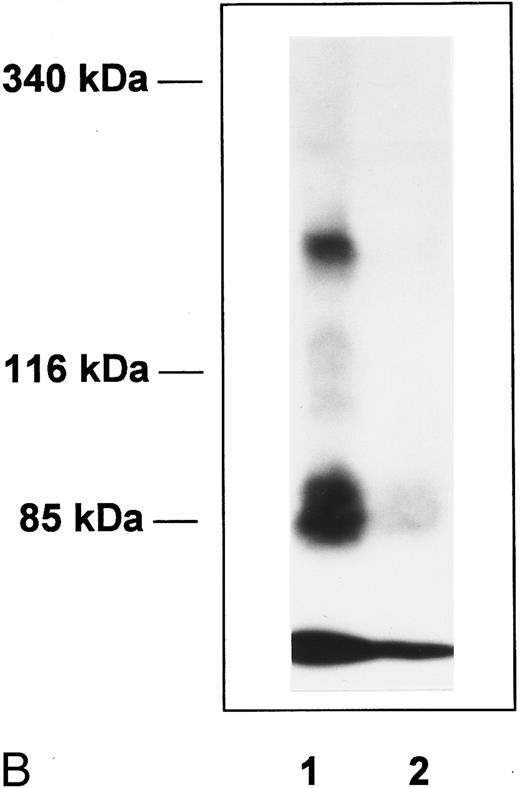
Affinity cross-linking of 125I-Tpo to its receptor on BaF3-MplR cells (Fig 4A) or on normal human platelets (Fig 4B) showed a prominent cross-linked complex. This radiolabeled complex was not seen when affinity cross-linking was performed in the presence of excess unlabeled Tpo or when the cross-linker was omitted. Immunoprecipitation with an affinity-purified polyclonal anti-Mpl receptor antibody confirmed that the cross-linked complex contained Mpl receptor protein (data not shown). After subtracting the contribution of the BHK-expressed 125I-Tpo in the cross-linked complex, the molecular weight of the functional form of Mpl receptor on normal human platelets is estimated to be 98 ± 2 kD (mean ± SEM of 6 experiments). No dimers of the 125I-Tpo-Mpl receptor complex were evident in this or in five independent cross-linking experiments with normal human platelets (Fig 4).
Affinity cross-linking with 125I-Tpo. BaF3-MplR cells (A) or normal human platelets (B) were incubated with 125I-Tpo (0.6 nmol/L) in the presence or absence of a 100-fold excess of unlabeled Tpo and cross-linked with 1 mmol/L BS3. Cell lysates were analyzed by SDS-PAGE and autoradiography. The upper band represents the major 125I-Tpo-Mpl receptor complex and the lower band is free 125I-Tpo. Lane 1, 125I-Tpo; lane 2, 125I-Tpo plus unlabeled Tpo. Molecular weight markers are indicated.
Affinity cross-linking with 125I-Tpo. BaF3-MplR cells (A) or normal human platelets (B) were incubated with 125I-Tpo (0.6 nmol/L) in the presence or absence of a 100-fold excess of unlabeled Tpo and cross-linked with 1 mmol/L BS3. Cell lysates were analyzed by SDS-PAGE and autoradiography. The upper band represents the major 125I-Tpo-Mpl receptor complex and the lower band is free 125I-Tpo. Lane 1, 125I-Tpo; lane 2, 125I-Tpo plus unlabeled Tpo. Molecular weight markers are indicated.
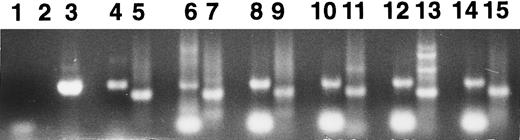
To investigate Mpl receptor display during differentiation of normal human erythroid progenitor cells, progeny of BFU-E plucked after 5 to 10 days of culture and progeny of CFU-GM plucked after 12 days of culture were analyzed for Mpl receptor mRNA by RT-PCR, with CD34+ human marrow mononuclear cells, HEL cells, K562 cells, and cloned human Mpl cDNA serving as positive controls. The day-5, -7, and -10 BFU-E progeny contained Mpl receptor mRNA (Fig 5); the day-12 CFU-GM progeny also contained Mpl receptor mRNA (data not shown). The day-5 and day-7 BFU-E progeny were mainly large pale early erythroblasts, whereas the day-10 BFU-E progeny included a population of smaller well-hemoglobinized late erythroblasts. More than 80% of the day-10 BFU-E progeny expressed glycophorin A. Replating experiments showed that the day-5 BFU-E progeny contained approximately 33% CFU-E, whereas the day-7 BFU-E progeny contained approximately 27% CFU-E and the day-10 BFU-E progeny contained approximately 2% CFU-E. Two additional experiments gave similar results. Direct binding experiments failed to show reproducible 125I-Tpo binding to progeny of peripheral blood BFU-E plucked at day 7 through day 12 (N = 10 experiments, data not shown).
RT-PCR analysis of Mpl receptor mRNA. RT-PCR was performed to detect the presence of c-mpl or GAPDH mRNA in HEL cells (lanes 4 and 5), K562 cells (lanes 6 and 7), CD34+ human marrow cells (lanes 8 and 9), and colonies derived from day-5 (lanes 10 and 11), day-7 (lanes 12 and 13), and day-10 (lanes 14 and 15) BFU-E progeny. The primers used for amplification were specific for the extracellular domain of Mpl (lanes 3, 4, 6, 8, 10, 12, and 14) or for GAPDH (lanes 2, 5, 7, 9, 11, 13, and 15). Cloned human mpl cDNA was used as a positive control (lane 3). Negative controls were performed using no input cDNA (lanes 1 and 2).
RT-PCR analysis of Mpl receptor mRNA. RT-PCR was performed to detect the presence of c-mpl or GAPDH mRNA in HEL cells (lanes 4 and 5), K562 cells (lanes 6 and 7), CD34+ human marrow cells (lanes 8 and 9), and colonies derived from day-5 (lanes 10 and 11), day-7 (lanes 12 and 13), and day-10 (lanes 14 and 15) BFU-E progeny. The primers used for amplification were specific for the extracellular domain of Mpl (lanes 3, 4, 6, 8, 10, 12, and 14) or for GAPDH (lanes 2, 5, 7, 9, 11, 13, and 15). Cloned human mpl cDNA was used as a positive control (lane 3). Negative controls were performed using no input cDNA (lanes 1 and 2).
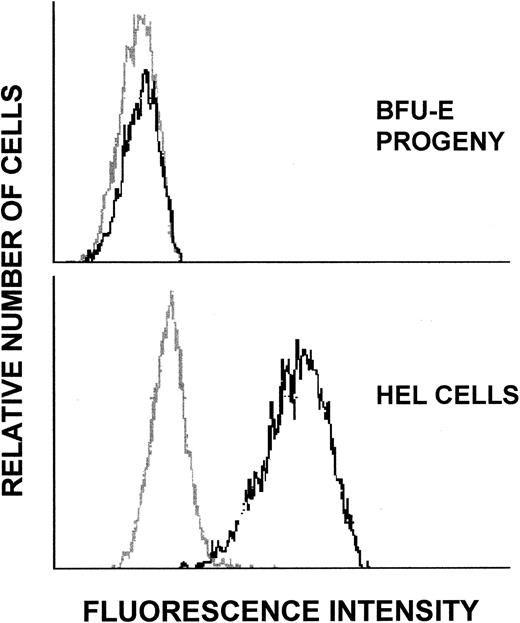
To determine whether the Mpl receptor protein was displayed on the surface of BFU-E and CFU-GM progeny, the cells were labeled with an affinity-purified rabbit anti-Mpl receptor antibody or with purified control rabbit IgG and analyzed by flow cytometry (Fig 6). Cell surface expression of Mpl receptor protein was evident on the BFU-E progeny but not on the CFU-GM progeny. Three additional independent experiments with plucked BFU-E progeny gave similar results. These findings were verified by direct examination of the labeled cells by fluorescence microscopy. The majority of cells obtained from BFU-E progeny showed faint positivity with the anti-Mpl receptor antibody but not with the control antibody. The BFU-E progeny did not express the megakaryocytic marker CD41 (glycoprotein Iib/IIIa; Fig 7), suggesting that the finding of Mpl receptor expression was not due to the presence of megakaryocytes or platelets in this population of cells.
Flow cytometric analysis of Mpl receptor display on progeny of BFU-E and CFU-GM. Day-11 BFU-E and CFU-GM progeny labeled with control rabbit IgG (gray profile) or with affinity-purified rabbit anti-Mpl receptor antibody (dark profile) were analyzed by flow cytometry.
Flow cytometric analysis of Mpl receptor display on progeny of BFU-E and CFU-GM. Day-11 BFU-E and CFU-GM progeny labeled with control rabbit IgG (gray profile) or with affinity-purified rabbit anti-Mpl receptor antibody (dark profile) were analyzed by flow cytometry.
Flow cytometric analysis of CD41 expression on progeny of BFU-E and on HEL cells. Day-11 BFU-E progeny and HEL cells labeled with control mouse IgG1 antibody (gray profile) or with the Tab anti-CD41 MoAb (dark profile) were analyzed by flow cytometry.
Flow cytometric analysis of CD41 expression on progeny of BFU-E and on HEL cells. Day-11 BFU-E progeny and HEL cells labeled with control mouse IgG1 antibody (gray profile) or with the Tab anti-CD41 MoAb (dark profile) were analyzed by flow cytometry.
DISCUSSION
Our objective was to more fully define the cellular distribution and binding characteristics of the Mpl receptor on normal human hematopoietic cells. Among morphologically recognizable cells in the bone marrow, only megakaryocytes display 125I-Tpo binding that is detectable by autoradiography. Myeloid and erythroid precursor cells lacked detectable 125I-Tpo binding. This contrasts with findings with other cytokines that can stimulate megakaryopoiesis in vitro, including IL-3, Epo, and stem cell factor. 125I-Epo binding to both normal human megakaryocytes and erythroid precursor cells can be readily detected.48 Likewise, 125I-stem cell factor binds not only to megakaryocytes but also to both myeloid and erythroid precursor cells.49 The Mpl receptor shows a more restricted cellular distribution among morphologically identified hematopoietic precursor cells in normal human bone marrow than the receptors for these other cytokines.
Normal human platelets bind 125I-Tpo with high affinity. The binding affinity for Tpo, approximately 100 to 200 pmol/L, is similar to that found for other hematopoietic cytokines, including Epo, stem cell factor, IL-3, and GM-CSF. Murine platelets have been reported to bind 125I-murine Tpo with an affinity of 560 pmol/L and to display 220 Mpl receptors per platelet.37 However, the equilibrium binding experiments with the murine platelets in the latter study were performed under conditions that permitted internalization of the 125I-Tpo.37 Thus differences in species and in experimental techniques could contribute to these differing results. Tpo circulates in normal human plasma at a concentration of approximately 85 pg/mL.50 Tpo half-life is prolonged in Mpl receptor knock-out mice,37 reinforcing an earlier suggestion that platelets may regulate the level of Tpo in the circulation by binding and metabolizing the cytokine.3,36 The present results suggest that platelets would exhibit 1% to 2% receptor occupancy at the level of Tpo normally found in the circulation. Because a rich assortment of 125I-Tpo grains was seen on megakaryocytes, these cells may also contribute substantially to the metabolism of Tpo. Although we have not been able to obtain sufficient numbers of purified normal human megakaryocytes for Scatchard analysis of Mpl receptor number and binding affinity, autoradiographic analysis of 125I-Tpo binding showed that megakaryocytes display approximately 100-fold more grains per cell than do BaF3-MplR cells studied in parallel. The BaF3-MplR cells exhibit approximately 480 Mpl receptors per cell. Megakaryocyte metabolism of Tpo may explain the observation that circulating Tpo levels are not elevated in NF-E2 knock-out mice that fail to produce platelets51 or in patients with idiopathic thrombocytopenic purpura.52
The Mpl receptor protein is found on the surface of at least a portion of normal human BFU-E isolated from peripheral blood or bone marrow.22 Debili et al35 used the M1 anti-Mpl receptor MoAb to examine receptor display on normal human bone marrow cells and concluded that Mpl receptor display is limited to late megakaryocytic progenitor cells, megakaryocytes, and platelets. However, the CD34+ M1+ fraction of marrow cells also contained late erythroid colony-forming cells that gave rise to erythroblasts after 8 days in culture,35 consistent with the present finding of Mpl receptor mRNA and cell surface expression of Mpl receptor protein in progeny of normal human BFU-E and with the report of Mpl receptor mRNA in murine fetal liver BFU-E progeny.24
Tpo acts synergistically with Epo to increase CFU-E generation in liquid culture20 and expands both BFU-E and CFU-E numbers in myelosuppressed mice in vivo.20,53 Additionally, Tpo acts directly on progeny of BFU-E plucked after 5 to 6 days of culture to promote erythroid colony growth.21 Taken together with the cellular distribution of the Mpl receptor, these data suggest that the effects of Tpo on erythropoiesis are mediated by Tpo binding to erythroid progenitor cells. Tpo can also act in synergy with stem cell factor to promote CFU-GM colony growth.54 The observations that the number of hematopoietic progenitor cells from multiple lineages are reduced in Mpl receptor knock-out mice12,55 and that Tpo can increase the fraction of primitive hematopoietic stem cells entering the cell cycle10 also suggest that Tpo may act on hematopoietic cells at an earlier stage than previously appreciated.
A multitude of isoforms of human and mouse Mpl receptor has been identified. The Mpl P and Mpl K isoforms of the human Mpl receptor are identical in the extracellular domain but diverge in the intracellular domain after a common 9 amino acid juxtamembrane sequence.19 Both transmembrane and soluble forms of murine Mpl receptor have been found.56,57 A naturally occurring isoform of the murine Mpl receptor that contains a 180-bp deletion in the extracellular domain and fails to bind the 70-kD form of Tpo has also been described.58 The functional significance of these isoforms is unknown at present. Although flow cytometric analysis showed the presence of Mpl receptor protein on BFU-E progeny, 125I-Tpo binding to these cells could not be demonstrated. This finding could reflect the low number of Mpl receptors displayed per cell or the presence of an alternative isoform of Mpl receptor on BFU-E progeny. Binding of Tpo triggers tyrosine phosphorylation of a ∼95-kD form of murine Mpl receptor.25 The present studies of affinity cross-linking to normal human platelets show that this form of the Mpl receptor is also capable of binding Tpo.
Despite the degree of both structural and functional overlap between Tpo and Epo, these cytokines did not cross-compete for binding to cells engineered to express functional forms of the Mpl receptor and the Epo receptor. This suggests that there is not a shared receptor subunit that is present in limiting quantity, analogous to the common chain of the IL-3 receptor, GM-CSF receptor, and IL-5 receptor,59 that might explain some of the functional overlap between Tpo and Epo. Whether there is intracellular cross talk between the Tpo and Epo receptors, as has recently been shown for the Epo and c-kit receptors,60 remains to be determined. Because Tpo can promote BFU-E colony growth from liver cells of fetal mice that lack the Epo receptor, the presence of the Epo receptor is not an absolute requirement for Tpo-induced signal transduction.24
ACKNOWLEDGMENT
We thank Drs C. Hart and D. Foster for providing Tpo and anti-Mpl receptor antibody, Dr K. Langley for providing stem cell factor, and Z. Sisk and A. Dimaunahan for manuscript preparation.
Supported by National Institutes of Health (NIH) Grants No. DK44194, CA31615, and DK49855 and by an American Cancer Society Faculty Research Award to V.C.B. A portion of this work was conducted in the Clinical Research Facility at the University of Washington, supported by NIH/National Center for Research Resources Grant No. M01 RR00037.
Address reprint requests to Virginia C. Broudy, MD, University of Washington, Division of Hematology, Box 357710, Seattle, WA 98195.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal