Abstract
To further elucidate the incidence and potential mechanism of asparaginase-associated lipid abnormalities in children with acute lymphoblastic leukemia (ALL), we serially obtained fasting lipid and lipoprotein studies on 38 of the 43 consecutively diagnosed children with ALL before, during, and after asparaginase therapy. We also evaluated a second population of 30 long-term survivors of childhood ALL; a fasting lipid and lipoprotein profile was obtained once at study entry. The mean peak triglyceride level during asparaginase of 465 mg/dL (standard deviation [SD] 492) was significantly higher (P = .003) than the level of 108 mg/dL (SD 46) before the initiation of asparaginase therapy. Sixty-seven percent of the newly diagnosed patients had fasting triglyceride levels greater than 200 mg/dL during asparaginase therapy; 15 patients (42%) had levels greater than 400 mg/dL, 7 with levels greater than 1,000 mg/dL. The incidence of hypertriglyceridemia did not vary by type of asparaginase or risk status of ALL (defined by white blood cell count and age). None of the 7 patients with triglyceride levels greater than 1,000 mg/dL developed pancreatitis. In contrast, 4 of the 13 patients without triglyceride elevation developed pancreatitis; 3 of the 4 patients had fasting studies at the height of their abdominal pain. Nuclear magnetic resonance analysis of lipid subclasses showed a significant increase in the smaller, denser forms of very low density lipoprotein (VLDL) and negligible chylomicron fraction in a subset of patients with marked triglyceride elevation. Lipoprotein lipase activity was consistently above normative values for all levels of triglyceride and could not be explained by obesity or hyperglycemia. Apolipoprotein B100 levels increased during asparaginase therapy, although the mechanism of this remains unclear. LDL reciprocally decreased with increased VLDL during asparaginase therapy. After asparaginase therapy, triglyceride levels (mean, 73 mg/dL [SD 33]) were significantly lower than levels obtained during asparaginase therapy. Triglyceride levels for survivors did not differ from the normal range or postasparaginase levels in the newly diagnosed patients. These data show a striking temporal association between asparaginase therapy and hypertriglyceridemia. Changes in cholesterol, in contrast, were not temporally related to asparaginase treatment. Cholesterol levels were elevated (<200 mg/dL) in 20% of the patients after asparaginase, which may be due to continued treatment with corticosteroids. The mean cholesterol level of long-term survivors of 177 mg/dL was significantly higher than the norm (P = .045). High-density lipoprotein (HDL) levels were significantly lower than normal at all time periods and for both populations; 25% of survivors had HDL levels less than 35 mg/dL. We conclude that modifications in asparaginase therapy are not necessary. In cases of triglyceride elevation greater than 2,000 mg/dL when the risk of pancreatitis is increased, close clinical monitoring is imperative. Larger studies are needed to determine the incidence of dyslipidemia in long-term survivors of ALL as well as the relationship between lipid abnormalities and other late effects of treatment, notably obesity and cardiomyopathies.
ENHANCED LONG-TERM disease-free survival for children with acute lymphoblastic leukemia (ALL) can be attributed to four decades of clinical research beginning with the development of effective single agents and the principles of combination (multidrug) chemotherapy and dose intensification. Current investigations focus on the development of treatment regimens that maximize efficacy and minimize acute and long-term sequelae.
Asparaginase, an effective drug in the treatment of childhood ALL, has become an important component of most childhood ALL regimens during the remission induction or intensification phases of treatment. The drug depletes the blood of asparagine, a nonessential amino acid on which many cells depend for normal metabolic processes. Whereas normal cells compensate by synthesizing L-asparagine from aspartic acid and glutamine via the enzyme, asparagine synthetase, selected malignant lymphoid cells have low levels of the synthetic enzyme and depend on intracellular pools of L-asparagine for protein synthesis and cell functioning.1,2 Asparaginase treatment is associated with acute side effects that include unpredictable toxicities such as allergy (20%), thromboembolic events (2% to 11%), and severe pancreatitis (4% to 7%).3-6
Asparaginase has been reported to cause abnormalities in lipid metabolism, ranging from hypocholesterolemia and hypotriglyceridemia to hypercholesterolemia and hypertriglyceridemia during asparaginase therapy.7-11 No studies have adequately addressed the mechanism, incidence, or severity of these abnormalities.
In addition to the effects of asparaginase on lipid metabolism, both the diagnosis of ALL, per se, and the use of other chemotherapeutic agents, most notably corticosteroids, have been associated with alterations in lipid synthesis and clearance. Corticosteroids alter lipid and lipoprotein metabolism by increasing hepatic cholesterol synthesis.9 These agents are often used intermittently throughout the course of antileukemic therapy. Several studies have reported a variety of lipid changes in adults and children at the time of diagnosis of ALL, ranging from increased cholesterol9 and triglyceride, as either chylomicrons12 or as very low density lipoprotein (VLDL).10,13 Although the mechanism of these lipid abnormalities is not known, some have suggested that these changes may be specific to hematologic malignancies and may correlate with the quantity of tumor.13 14 The incidence and prognostic importance of these findings have not been well described.
With the inception of an institutional multiagent ALL protocol in 1991 at Children's Hospital/Dana Farber Cancer Institute (Boston, MA), we anecdotally noted that several patients developed gross lipemia and elevated serum triglyceride levels during the intensification phase of ALL treatment. Because of the uncertain importance of these striking laboratory findings in a population known to be at risk for pancreatitis, we determined the incidence, timing, and severity of lipid abnormalities in newly diagnosed patients with childhood ALL. Our findings showed that the majority of studied patients developed elevation in triglyceride levels during an extended course of asparaginase treatment. There was no association between the magnitude of hypertriglyceridemia and clinical pancreatitis. Although the gross abnormalities in lipid metabolism resolved after completion of asparaginase therapy, these findings may have implications for the long-term follow-up of childhood leukemia survivors.
MATERIALS AND METHODS
Patient Selection
From the beginning of the study in August 1993, 43 consecutively diagnosed patients with childhood ALL at our institution were eligible for participation; 38 (88.3%) agreed to participate. Patients were recruited immediately after the diagnosis of ALL was confirmed and, when possible, before the initiation of treatment. All newly diagnosed patients provided written consent before initiating the study according to the policies of the hospitals' institutional review boards.
All 38 patients received multiagent chemotherapy, stratified by risk group (based on white blood cell count, age, and the presence of central nervous system disease). The protocol consisted of a 3-day initial treatment with steroids (detailed below), followed by a 28-day remission induction treatment consisting of vincristine, prednisone, doxorubicin, methotrexate, and intrathecal cytosine arabinoside. After documentation of induction remission, patients received intensification therapy with systemic chemotherapy (vincristine, dexamethasone, methotrexate, 6-mercaptopurine [6-MP], and asparaginase for all patients; doxorubicin added to the treatment of high-risk patients). Central nervous system treatment included periodic intrathecal administration of methotrexate and cytosine arabinoside with or without cranial irradiation, depending on risk group. After the completion of intensification, all patients received maintenance chemotherapy with vincristine, steroids, 6-MP, and methotrexate to complete 24 months of continuous clinical remission. Intrathecal therapy with methotrexate and cytosine arabinoside was administered to all patients every 18 weeks during the maintenance period.
All patients received asparaginase as part of the intensification phase of treatment beginning after completion of central nervous system treatment (week 7 of therapy) and continuing for 31 weeks. Patients were randomized at the time of diagnosis to receive either native Escherichia coli asparaginase at 25,000 IU/m2 every week for 30 doses or the polyethylene glycolated form of E coli asparaginase (PEG asparaginase) at 2,500 IU/m2 every 2 weeks for 15 doses. Patients who developed allergic reactions to native E coli asparaginase were switched to the PEG form; those reacting to PEG asparaginase were switched to Erwinia species asparaginase at 25,000 IU/m2 each week to complete 30 weeks of treatment. Any reactions to Erwinia asparaginase resulted in cessation of asparaginase therapy. For purposes of analysis, fasting lipid levels were analyzed by the type of asparaginase the patient was receiving at the time the sample was obtained.
As noted, all patients received corticosteroids as part of their antileukemia therapy, beginning with a 3-day course at the start of therapy (investigational window). Patients were randomized to receive one of three doses of dexamethasone (6, 18, or 150 mg/m2/d) or prednisolone at 40 mg/m2/d for 3 consecutive days, followed by a 28-day course of prednisolone at 40 mg/m2/d for all patients during remission induction. During the intensification and maintenance phases of treatment, all patients received a 5-day course at the beginning of each 3-week cycle of chemotherapy. Steroid doses varied by risk group assignment: standard-risk patients received 6 mg/m2/d and high-risk patients received 18 mg/m2/d.
A second study population, consisting of the entire cohort of 63 disease-free survivors of our previous ALL treatment protocol (DFCI 87-001), which was conducted between 1987 and 1991, was contacted by mail and invited to participate in the study. Thirty-six patients indicated a willingness to participate, 24 (66.7%) of whom were fully evaluable; 6 additional patients completed all but the fasting blood testing.
The 30 long-term survivors had received E coli asparaginase as part of their original ALL treatment intensification at comparable doses to the newly diagnosed patients and received 20 weekly doses, barring complications. Patients were switched to Erwinia asparaginase if allergic reactions occurred to E coli asparaginase. Patients who reacted to Erwinia species received no further asparaginase.
Family History Questionnaire
Parents of all participating children were asked to complete a family history questionnaire of risk factors for and clinical history of familial lipid abnormalities. This questionnaire (available upon request) was designed for routine use in the Children's Hospital Lipid Clinic. A positive family history, based on the National Cholesterol Education Program definition,15was defined as any patient with a parent whose fasting serum cholesterol was greater than 240 mg/dL or a parent, aunt, uncle, or grandparent with a history of a premature cardiovascular or cerebrovascular event (<55 years of age). Given the relatively young age of the patients' parents, particular attention was given to the grandparents' history of coronary heart disease and dyslipidemia.
Patient Evaluation
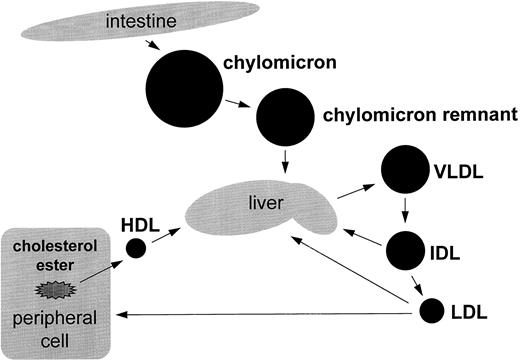
Newly diagnosed patients were prospectively evaluated with serial blood samples, including fasting lipid (triglyceride and cholesterol) and lipoprotein profiles to delineate the potential sites of abnormality in lipid metabolism (Fig 1).
Overview of lipid metabolism. Triglycerides enter the circulation via the exogenous or endogenous pathway. In the exogenous pathway, dietary fats are absorbed in the intestines and packaged into triglyceride-rich particles, the chylomicrons. These large particles lose some of triglyceride and in the process are converted to chylomicron remnant, a step catalyzed by lipoprotein lipase. Chylomicron remnant is removed by specific hepatic receptors. The liver can also synthesize VLDL, a triglyceride-rich lipoprotein that also contains Apo-B100. Triglyceride is removed from VLDL via LPL and is converted first to IDL and then LDL. LDL are principally removed from the circulation by specific hepatic receptors.
Overview of lipid metabolism. Triglycerides enter the circulation via the exogenous or endogenous pathway. In the exogenous pathway, dietary fats are absorbed in the intestines and packaged into triglyceride-rich particles, the chylomicrons. These large particles lose some of triglyceride and in the process are converted to chylomicron remnant, a step catalyzed by lipoprotein lipase. Chylomicron remnant is removed by specific hepatic receptors. The liver can also synthesize VLDL, a triglyceride-rich lipoprotein that also contains Apo-B100. Triglyceride is removed from VLDL via LPL and is converted first to IDL and then LDL. LDL are principally removed from the circulation by specific hepatic receptors.
When feasible, all laboratory studies were obtained at diagnosis, at induction of remission, periodically during asparaginase treatment, and after completion of asparaginase. The study was initially designed to obtain a fasting lipid and lipoprotein profile before and during asparaginase therapy, as well as at the end of the 2 years of leukemia therapy. However, because of the striking temporal association between triglyceride elevation and asparaginase therapy, the study was amended to include a fasting lipid profile approximately 9 weeks after completion of asparaginase therapy. This time point was selected to allow for an adequate wash out of all forms of asparaginase. Blood drawing was timed to correspond to other scheduled laboratory tests or prearranged fasting. Long-term survivors had blood drawn for fasting lipid profiles only at study entry.
In accordance with the working definitions used in the treatment protocol, the diagnosis of clinical (severe) pancreatitis was based on the presence of hyperamylasemia ≥3× normal in the setting of ≥72 hours of abdominal pain, which usually required inpatient hospitalization. Mild/moderate pancreatitis was defined as elevated serum amylase of less than 3× normal and abdominal pain of less than 72 hours in duration. The diagnosis of severe pancreatitis would result in the cessation of asparaginase therapy. In milder cases, asparaginase could be reinstituted after the complete resolution of physical examination findings and amylase elevation.
Lipid Testing
Fasting lipid profile.Heparinized plasma specimens were collected for lipid testing at designated study intervals after an overnight fast and analyzed on the same day. Cholesterol and triglyceride levels were determined enzymatically on the Hitachi 911 autoanalyzer (Boehringer Mannheim Diagnostics, Indianapolis, IN). Triglyceride measurement was corrected for endogenous glycerol. High-density lipoprotein (HDL) cholesterol was determined after precipitation of the apolipoprotein B (Apo-B) containing particles by magnesium chloride and dextran sulfate (molecular weight, 50,000), as previously described.16 LDL cholesterol was estimated using the Friedewald calculation when the serum triglyceride level was less than 400 mg/dL.17 Our lipid laboratory is certified by the National Heart, Lung and Blood Institute and the Centers for Disease Control and Prevention Lipid Standardization Program.
Results were categorized according to hospital laboratory norms. Normal triglyceride was defined as a serum concentration of less than 110 mg/dL, which included up to the 95th percentile for males and females in the first two decades of life (112 mg/dL ± standard error of 1.27).18 Triglyceride levels above the 95th percentile, expressed in mg/dL, were classified as mild, 110 to 200; moderate, 200 to 400; severe, greater than 400 to 1,000; and extreme, greater than 1,000. Cholesterol levels were categorized as desired, less than 170 mg/dL; borderline, 170 to 199; and high, ≥200. A level of 200 mg/dL defines the 95th percentile for both genders in the first two decades of life.15 18
Apo-AI and Apo-B100.Apo-AI and Apo-B100 were determined by nephelometric immunoassay techniques using the Behring BN-100 analyzer (Behring Diagnostics Inc, Westwood, MA). The Apo assays were standardized by the International Federation of Clinical Chemistry/World Health Organization's newly approved calibrators. To date, there are no large, population-based standards for interpretations of Apo-B100 and Apo-AI levels. In adult studies, men tend to have higher Apo-B100 levels than women; the converse is true for Apo-AI. Apo-B100 levels increase with elevated cholesterol and LDL, whereas Apo-AI levels reflect the HDL fraction.19 Although there are no comparable population-based studies in the pediatric population, Rifai et al20 and Marcovina et al21 22 recently reported frequency distribution for apolipoproteins in adolescents using the new calibrators.
Lipoprotein subclass determination: nuclear magnetic resonance (NMR).Plasma lipoprotein subclass concentrations were determined by proton NMR spectroscopy, as previously described.23 24 The method is based on the observation that each lipoprotein particle in plasma within a given size range broadcasts its own, characteristic lipid NMR signal, the intensity of which is proportional to its concentration. Using computer line shape analysis of the resultant plasma lipid signal, which is the linear sum of the signals from the various lipoprotein subclasses, it is possible to simultaneously derive the concentrations of each of 16 lipoprotein subspecies (chylomicrons, 6 VLDL, 4 LDL, and 5 HDL subclasses). Concentrations of chylomicron and VLDL subspecies are expressed in units of triglyceride (in milligrams per deciliter) and those of LDL and HDL subspecies in units of cholesterol (in milligrams per deciliter).
Lipoprotein lipase (LPL).Blood samples were collected for the determination of LPL mass and catalytic activity after an overnight fast. The blood samples were obtained 10 minutes after an intravenous injection of heparin was administered (60 U/kg body mass). Plasma was immediately separated after centrifugation at 3,000g for 10 minutes at 4°C. LPL mass in preheparin and postheparin plasma samples was determined by an enzyme-linked immunosorbent assay assay25 using a monoclonal antibody (5D2) that recognizes the epitope located at residue 400 of human LPL.26 For the LPL and hepatic lipase (HL) catalytic activity assays, the plasma samples were diluted 100-fold before assay to eliminate any effect of endogenous substrate. LPL and HL lipolytic activity were measured using a radiolabeled tri[1-14C] oleate emulsion as previously described.27 Results of LPL and HL activity were compared with a corrected mean for each (LPL, 214 ± 86; HL, 179 ± 91).
Statistical Considerations
One-sample t-tests were used to compare the mean lipid levels of study patients with published pediatric normal values. Paired t-tests were used to compare patients' lipid levels during the three time periods of interest. Analyses were based on peak lipid values for each patient during a given time period. Comparisons to available pediatric norms were based on all evaluable patients. Comparisons between time periods were restricted to only those patients with complete serial data to more accurately estimate the effect of asparaginase therapy. No adjustments were made for associations between binary and categorical variables, and the exact Wilcoxon test was used to compare ordered groups. Exact methods were used to compute confidence intervals.
RESULTS
Patient Characteristics
Thirty-eight patients with newly diagnosed childhood ALL and 30 long-term disease-free survivors of ALL treatment were included in our analysis (Table 1). The median age of the newly diagnosed patients was 4.2 years (range, 1.6 to 16.2 years); 66% of the patients were male. In the long-term survivor group, the median age was 11.1 years (range, 4.6 to 21.2 years); 47% of the patients were male. Laboratory data were available on 24 of the 30 long-term survivors. The majority of patients received native E coli asparaginase; 8 newly diagnosed patients and 12 long-term patients received more than one type of asparaginase (ie, native E coli species, Erwinia species, or PEG species). In the newly diagnosed patients, the type of asparaginase (native E coliv PEG asparaginase) was not a significant predictor of elevated triglyceride levels during asparaginase therapy (P > .39 for all hypertriglyceridemic values). Newly diagnosed patients began asparaginase at a median time of 7.7 weeks from diagnosis. The median duration of asparaginase for the 36 patients who have completed their asparaginase therapy was 6.7 months (range, 1.4 to 10.0 months). The proportion of patients with elevated triglyceride levels during asparaginase did not significantly differ by risk status (standard-risk v high-risk ALL, based on age and white blood cell count at diagnosis; P = .09, exact Wilcoxon), although the presumed disease burden and the steroid dose during maintenance chemotherapy differed between the two groups. Family history questionnaires showed a 5% (n = 2) prevalence of premature coronary artery disease in the grandparents of our newly diagnosed patients, which is consistent with previously reported population data.28
Patient and Treatment Characteristics
| . | Newly Diagnosed . | Long-Term Survivors . |
|---|---|---|
| N | 38 | 30 |
| Demographics | ||
| Males | 25 (66) | 14 (47) |
| Females | 13 (34) | 16 (53) |
| Median age (yr) | 4.2 | 11.1 |
| Age range (yr) | 1.6-16.2 | 4.6-21.2 |
| Type of asparaginase received | ||
| E coli | 28 (74) | 30 (100) |
| PEG | 17 (45) | 7 (23) |
| Erwinia | 1 (3) | 9 (30) |
| . | Newly Diagnosed . | Long-Term Survivors . |
|---|---|---|
| N | 38 | 30 |
| Demographics | ||
| Males | 25 (66) | 14 (47) |
| Females | 13 (34) | 16 (53) |
| Median age (yr) | 4.2 | 11.1 |
| Age range (yr) | 1.6-16.2 | 4.6-21.2 |
| Type of asparaginase received | ||
| E coli | 28 (74) | 30 (100) |
| PEG | 17 (45) | 7 (23) |
| Erwinia | 1 (3) | 9 (30) |
Percentages are in parentheses.
Four of the 38 newly diagnosed patients (10.5%) developed severe pancreatitis, resulting in the early cessation of asparaginase therapy (median, 18 weeks; range, 7 to 29 weeks). One of the 4 patients had had an episode of mild pancreatitis earlier in the treatment course, but had resumed asparaginase therapy after the resolution of symptoms and amylase elevation. Of note, none of the patients with severe pancreatitis had measured fasting triglyceride levels greater than 400 mg/dL at any point during their treatment course. Three of the 4 patients had triglycerides measured during the height of their pain. In addition, none of the 7 newly diagnosed patients with extreme elevation in fasting triglycerides (>1,000 mg/dL) developed pancreatitis. However, of note, only 1 of the 7 had a triglyceride level of greater than 2,000 mg/dL, which is sufficient to cause triglyceride-induced pancreatitis.29
Fasting Lipid Profiles
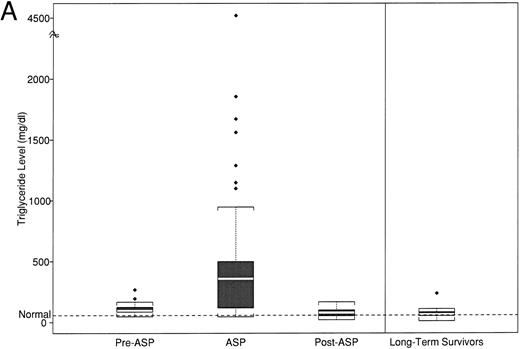
Triglyceride.The median peak triglyceride, calculated from 40 samples on 27 patients, obtained at any point before initiation of asparaginase therapy (ie, diagnosis, remission, and preasparaginase therapy), was 95 mg/dL (range, 48 to 268 mg/dL; Table 2 and Fig 2A). There was no difference in the median peak triglyceride levels between the 16 patients with measurements at the time of diagnosis before initiation of ALL therapy (median, 97; range, 80 to 195) compared with the 11 obtained after the initiation of antileukemia treatment, but before the start of asparaginase therapy (median, 108; range, 48 to 268).
Peak Lipid and Lipoprotein Levels by Timepoint of Asparaginase Therapy
| . | Pre . | During . | Post . | LT . | ||||
|---|---|---|---|---|---|---|---|---|
| . | Mean (SD) . | Median (range) . | Mean (SD) . | Median (range) . | Mean (SD) . | Median (range) . | Mean (SD) . | Median (range) . |
| Triglyceride | 109 (45)* | 95 (48-268) | 589 (830)* | 359 (46-4520) | 79 (37)* | 79 (21-168) | 71 (44) | 63 (11-238) |
| Cholesterol | 171 (45) | 166 (83-263) | 205 (77)* | 193 (87-426) | 163 (38) | 165 (85-234) | 176 (39)† | 172 (118-278) |
| LDL | 119 (40) | 120 (50-213) | 96 (57)* | 88 (24-328) | 104 (32)* | 108 (54-163) | 118 (36) | 115 (70-221) |
| HDL | 34 (13)* | 35 (15-71) | 37 (15)* | 32 (11-72) | 47 (12)† | 46 (21-78) | 45 (13)* | 44 (24-82) |
| Apo-A1 | 139 (32) | 144 (108-196) | 123 (32) | 126 (52-164) | 128 (21) | 122 (105-181) | 136 (23) | 132 (105-198) |
| Apo-B100 | 122 (27) | 121 (88-183) | 153 (95) | 135 (54-392) | 79 (20) | 85 (47-105) | 82 (22) | 76 (44-142) |
| . | Pre . | During . | Post . | LT . | ||||
|---|---|---|---|---|---|---|---|---|
| . | Mean (SD) . | Median (range) . | Mean (SD) . | Median (range) . | Mean (SD) . | Median (range) . | Mean (SD) . | Median (range) . |
| Triglyceride | 109 (45)* | 95 (48-268) | 589 (830)* | 359 (46-4520) | 79 (37)* | 79 (21-168) | 71 (44) | 63 (11-238) |
| Cholesterol | 171 (45) | 166 (83-263) | 205 (77)* | 193 (87-426) | 163 (38) | 165 (85-234) | 176 (39)† | 172 (118-278) |
| LDL | 119 (40) | 120 (50-213) | 96 (57)* | 88 (24-328) | 104 (32)* | 108 (54-163) | 118 (36) | 115 (70-221) |
| HDL | 34 (13)* | 35 (15-71) | 37 (15)* | 32 (11-72) | 47 (12)† | 46 (21-78) | 45 (13)* | 44 (24-82) |
| Apo-A1 | 139 (32) | 144 (108-196) | 123 (32) | 126 (52-164) | 128 (21) | 122 (105-181) | 136 (23) | 132 (105-198) |
| Apo-B100 | 122 (27) | 121 (88-183) | 153 (95) | 135 (54-392) | 79 (20) | 85 (47-105) | 82 (22) | 76 (44-142) |
All values are expressed in milligrams per deciliter. Statistical comparisons are based on comparisons with published general population norms. Population norms for Apo-A1 and Apo-B100 are not available for children of all ages.
Abbreviations: Pre, before initiation of asparaginase therapy; During, during 30-week asparaginase therapy; Post, after completion of asparaginase therapy; LT, long-term survivors.
P < .01.
P < .05.
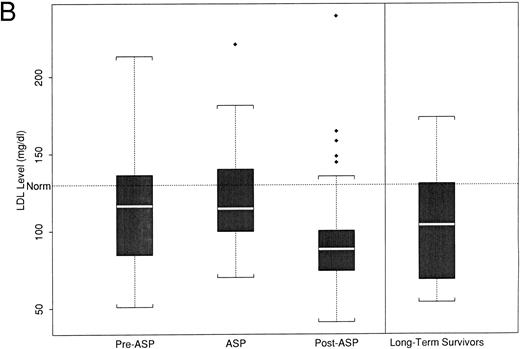
(A) Peak fasting triglyceride levels by time point of asparaginase therapy. (B) Peak calculated LDL levels by time point of asparaginase therapy. The boxplot distribution of fasting serum triglyceride (A) and calculated LDL (B) represent peak levels (in milligrams per deciliter) from serially obtained fasting lipid profiles on 38 newly diagnosed children with ALL before, during, and after asparaginase therapy. The plots also contain results from a one-time sample of 24 long-term survivors of childhood ALL. The boxplot describes the behavior of the measurements in the middle and ends of the distribution. The two ends of the box represent the first and third quartiles. The white line inside the box is the median. The tails of the distribution are illustrated by the whiskers. The extreme values beyond the whiskers, denoted by diamond-shaped points, represent the outliers. The horizontal dashed line represents the age-based normative value.
(A) Peak fasting triglyceride levels by time point of asparaginase therapy. (B) Peak calculated LDL levels by time point of asparaginase therapy. The boxplot distribution of fasting serum triglyceride (A) and calculated LDL (B) represent peak levels (in milligrams per deciliter) from serially obtained fasting lipid profiles on 38 newly diagnosed children with ALL before, during, and after asparaginase therapy. The plots also contain results from a one-time sample of 24 long-term survivors of childhood ALL. The boxplot describes the behavior of the measurements in the middle and ends of the distribution. The two ends of the box represent the first and third quartiles. The white line inside the box is the median. The tails of the distribution are illustrated by the whiskers. The extreme values beyond the whiskers, denoted by diamond-shaped points, represent the outliers. The horizontal dashed line represents the age-based normative value.
In contrast, 67% (95% confidence interval [CI], 49% to 81%) of the patients developed marked elevation in triglycerides (>200 mg/dL) while on asparaginase therapy; 15 patients (42%; 95% CI, 25% to 59%) had levels exceeding 400 mg/dL, 7 of whom had levels greater than 1,000 mg/dL (19%; 95% CI, 8% to 36%). The median peak triglyceride level was 359 mg/dL (range, 46 to 4,520 mg/dL). The mean peak triglyceride level during asparaginase treatment in the 21-patient paired analysis (Table 3) was significantly different than the preasparaginase mean levels (465 mg/dL [SD 492] v 108 mg/dL [SD 46]; P = .003, paired t-test). There was no correlation between the magnitude of triglyceride elevation during asparaginase therapy with baseline levels before asparaginase treatment (r = .05), although the variance within the samples was large.
Peak Values of Lipid and Lipoprotein by Timepoint of Asparaginase Therapy for Patients With Complete Serial Data
| . | N . | Mean (SD) . | ||
|---|---|---|---|---|
| . | . | Pre . | During . | Post . |
| Triglyceride*†‡ | 21 | 108 (46) | 465 (492) | 73 (33) |
| Cholesterol3-151 | 21 | 172 (40) | 205 (73) | 161 (41) |
| LDL | 20 | 121 (33) | 97 (49) | 97 (29) |
| HDL*† | 21 | 36 (13) | 37 (15) | 46 (11) |
| Apo-A1 | 7 | 133 (33) | 124 (36) | 128 (28) |
| Apo-B100†‡ | 7 | 122 (31) | 176 (115) | 78 (26) |
| . | N . | Mean (SD) . | ||
|---|---|---|---|---|
| . | . | Pre . | During . | Post . |
| Triglyceride*†‡ | 21 | 108 (46) | 465 (492) | 73 (33) |
| Cholesterol3-151 | 21 | 172 (40) | 205 (73) | 161 (41) |
| LDL | 20 | 121 (33) | 97 (49) | 97 (29) |
| HDL*† | 21 | 36 (13) | 37 (15) | 46 (11) |
| Apo-A1 | 7 | 133 (33) | 124 (36) | 128 (28) |
| Apo-B100†‡ | 7 | 122 (31) | 176 (115) | 78 (26) |
All values expressed in milligrams per deciliter. Significant differences noted at P < .05.
Abbreviations: Pre, before initiation of asparaginase; During, during 30-week asparaginase therapy; Post, after completion of asparaginase therapy.
Significant difference between Pre and During levels.
Significant difference between During and Post levels.
Significant difference between Pre and Post levels.
After the completion of the 30-week asparaginase therapy, triglyceride levels decreased to a median of 79 mg/dL (range, 21 to 168 mg/dL). Given the heterogeneity in the timing of these samples, we cannot calculate a time to normalization. The mean postasparaginase triglyceride level in the 21-patient paired analysis of 73 mg/dL (33) was significantly lower than mean levels before (P = .006) or during asparaginase therapy (P = .001). However, mean triglyceride levels were significantly higher than normal levels at all three phases (before and during, P ≤ .001; after, P = .002).
The mean triglyceride level for the 24 survivors, obtained after at least 2 years off all antileukemia treatment, was 71 mg/dL (44), which was not statistically different from the normal range or from the mean peak level of 73 mg/dL (33), which was obtained from the 21 newly diagnosed patients at the end of asparaginase therapy.
Cholesterol.Sixteen patients underwent fasting lipid profiles at diagnosis, before the initiation of any therapy. The mean cholesterol level of this group was 155 mg/dL (32), which did not differ from the normal range. However, during ALL therapy, but before asparaginase treatment, 30% (95% CI, 10% to 38%) of the patients had cholesterol levels greater than 200 mg/dL. The median peak cholesterol level was 166 mg/dL (range, 83 to 263 mg/dL). The increased proportion of patients with elevated cholesterol before asparaginase therapy may have been due to use of corticosteroids during induction therapy.
During asparaginase therapy, the median peak cholesterol level increased to 193 mg/dL (range, 87 to 426 mg/dL) and was not statistically different from the preasparaginase levels. After asparaginase therapy, cholesterol levels decreased to a median of 165 mg/dL (range, 85 to 234 mg/dL), with the proportion of patients with high serum cholesterol decreasing from 47% (95% CI, 30% to 65%) during asparaginase to 21% (95% CI, 9% to 38%) after asparaginase. The mean serum cholesterol level after asparaginase (161 mg/dL [41]) was significantly lower in the 21-patient paired analysis than the mean level on asparaginase (205 mg/dL [73]; P = .009). Based on population norms defining the 95th percentile, we would expect that only 5% of patients would have cholesterol levels in the high range.15 This persistent mild hypercholesterolemia in the postasparaginase period may be due to continued use of corticosteroids in the maintenance phase of chemotherapy (Table 2). Additionally, the mean cholesterol level during asparaginase therapy was significantly higher than normal (P = .01), but preasparaginase and postasparaginase levels were not significantly different from the norm. The mean cholesterol level of survivors of 176 mg/dL (39) was significantly higher than the norm (P = .045). Prior serum cholesterol levels were not available as a basis of comparison for these patients.
LDL cholesterol.Mean LDL levels decreased with the initiation of asparaginase therapy (Fig 2B). Preasparaginase levels were not significantly different from normal (120 mg/dL [40]; P = .18), whereas LDL levels obtained during asparaginase therapy decreased to 97 mg/dL (49), which was significantly lower than normal (P = .002). These levels remained lower than the normal postasparaginase levels (mean LDL, 97 [29]; P = .001). The LDL levels for long-term survivors were not statistically different from normal (mean, 118 [36]; P = .11).
HDL cholesterol.HDL levels changed very little with the initiation of asparaginase; the median levels before asparaginase of 35 mg/dL (range, 15 to 71 mg/dL) increased to 37 mg/dL (range, 11 to 72 mg/dL) during asparaginase. The mean levels in the 21 patients were not significantly different in the before asparaginase and during asparaginase time periods (before, 36 mg/dL [13]; during, 37 mg/dL [15]; P = .80). After completion of asparaginase therapy, the mean HDL value increased to 46 mg/dL (11), which was significantly higher than both the preasparaginase mean value (P = .013) and the mean value during asparaginase (P = .019). Of note, mean HDL levels at all time points were significantly lower than normal (before and during, P < .001; after, P < .02). This pattern was also noted in the survivors, in whom the mean HDL level was 45 mg/dL (±13). This was significantly lower than the normal range (P = .009), but was comparable to levels measured at the end of asparaginase therapy in the newly diagnosed patients (Table 2). Of note, 6 of the 24 long-term patients had HDL levels less than 35 mg/dL, which has been shown to be an independent risk factor for coronary heart disease.15
Apo-AI and Apo-B100
Apo-AI and Apo-B100 levels were obtained to further characterize the composition of lipoproteins (HDL, LDL, and VLDL). Apo-AI and Apo-B100 analyses were performed on a subset of patients including 9 patients before asparaginase, 19 patients during asparaginase, and 15 patients after asparaginase (Table 2). Serial samples, obtained at all three time points, were available for 7 patients (Table 3). The results from the paired samples were the basis of statistical comparison (via paired t-tests). In the absence of general population norms for all pediatric ages, no comparisons were made between study samples and normative data (Table 2). The mean Apo-AI level before asparaginase was 139 mg/dL (32), which decreased slightly to 123 mg/dL (32) during asparaginase therapy. After asparaginase, mean Apo-AI levels increased to 128 mg/dL (21). None of the variation in these levels was statistically significant by phase of treatment (before and during, P = .70; during and after, P = .80; and before and after, P = .65). The long-term survivors had a mean Apo-AI level of 136 (23).
Mean Apo-B100 levels increased from 122 mg/dL (27) before asparaginase to 153 mg/dL (95) during asparaginase, although in paired analysis, this was not statistically different from the preasparaginase level. After asparaginase, the mean Apo-B100 level decreased to 79 mg/dL (20), which was significantly lower than levels before asparaginase (P = .05) and during asparaginase (P = .01). The mean Apo-B100 level for the long-term survivors was 82 mg/dL (22), which did not significantly differ from the level obtained after asparaginase in the newly diagnosed patients.
Lipoprotein Subclass Analysis
NMR spectroscopy was performed on a subset of newly diagnosed patients to further characterize the changes in lipid subfractions during asparaginase therapy. Four patients with severe elevation (>400 mg/dL) in serum triglyceride (ie, affected) were compared with 3 patients who had no significant change in triglyceride levels (ie, unaffected). In the affected group, results obtained before asparaginase were statistically compared with those obtained during asparaginase (Table 4). In the affected group, despite the increase in triglyceride during asparaginase therapy, only slight traces of triglyceride were present in the chylomicron fraction with no significant change in that compartment from the results before asparaginase (P = .52). In contrast, in the affected group, there was a marked shift in the VLDL and LDL fractions during asparaginase; a similar shift was not noted in the unaffected group. The larger, more buoyant forms of VLDL (VLDL5,6 ) increased from 30.5 to 396.3 mg/dL, although due to the considerable variance within the small sample, this was not statistically significant (P = .14). The increase in buoyant VLDL is consistent with either the presence of increased chylomicron remnant or increased endogenous synthesis of VLDL. However, the significant increase in the amount of smaller, denser VLDL (VLDL1-4, P = .005) provides more conclusive evidence of endogenous synthesis. The LDL fraction shift during asparaginase was also to the smaller, denser fractions (LDL1,2 , P = .03), with a significant reduction in larger, more buoyant fractions (LDL3,4 , P = .02). Both the overall increase in the LDL fraction during asparaginase as well as the shift to denser, more atherogenic forms of LDL are noteworthy, given the inability to accurately estimate the amount of LDL using the Friedewald calculation in the presence of very high levels of triglyceride (>400 mg/dL). There were no comparable shifts noted in the HDL fraction in either group. NMR analysis was not performed on samples from long-term survivors.
Lipid Subclass Analysis for Selected Patients With and Without Elevated Serum Triglyceride
| Subclass . | Unaffected . | Affected . | ||
|---|---|---|---|---|
| (particle diameter) . | (TRI < 400 mg/dL) . | (TRI > 400 mg/dL) . | ||
| . | (N = 3; mean [SD]) . | (N = 4; mean [SD]) . | ||
| . | Pre . | During . | Pre . | During . |
| Chylomicron (>160 nm) | 1.5 (1.4) | 1.1 (1.3) | 4.7 (4.6) | 5.6 (6.7) |
| VLDL5,6 (60-160 nm) | 18.5 (5.7) | 20.3 (17.2) | 30.5 (27.9) | 396.3 (343.5) |
| VLDL1-4 (30-60 nm) | 47.0 (5.9) | 27.4 (9.4) | 70.2 (33.0) | 222.0 (52.2)4-150 |
| LDL3,4 (21.3-27 nm) | 38.8 (21.6) | 53.4 (42.2) | 66.8 (28.7) | 1.7 (3.5)4-151 |
| LDL1,2 (18.3-21.3 nm) | 62.2 (33.8) | 41.5 (23.6) | 80.0 (52.2) | 230.2 (66.1)4-151 |
| HDLTOTAL (7.3-13 nm) | 26.9 (10.8) | 32.6 (15.6) | 42.1 (19.5) | 48.2 (17.7) |
| Subclass . | Unaffected . | Affected . | ||
|---|---|---|---|---|
| (particle diameter) . | (TRI < 400 mg/dL) . | (TRI > 400 mg/dL) . | ||
| . | (N = 3; mean [SD]) . | (N = 4; mean [SD]) . | ||
| . | Pre . | During . | Pre . | During . |
| Chylomicron (>160 nm) | 1.5 (1.4) | 1.1 (1.3) | 4.7 (4.6) | 5.6 (6.7) |
| VLDL5,6 (60-160 nm) | 18.5 (5.7) | 20.3 (17.2) | 30.5 (27.9) | 396.3 (343.5) |
| VLDL1-4 (30-60 nm) | 47.0 (5.9) | 27.4 (9.4) | 70.2 (33.0) | 222.0 (52.2)4-150 |
| LDL3,4 (21.3-27 nm) | 38.8 (21.6) | 53.4 (42.2) | 66.8 (28.7) | 1.7 (3.5)4-151 |
| LDL1,2 (18.3-21.3 nm) | 62.2 (33.8) | 41.5 (23.6) | 80.0 (52.2) | 230.2 (66.1)4-151 |
| HDLTOTAL (7.3-13 nm) | 26.9 (10.8) | 32.6 (15.6) | 42.1 (19.5) | 48.2 (17.7) |
Chylomicron and VLDL subclass concentrations are expressed in milligrams per deciliter of triglyceride; LDL and HDL subclass concentrations are expressed in milligrams per deciliter of cholesterol.
Abbreviation: TRI, triglyceride.
P = .005.
P < .05.
LPL
Postheparin LPL analyses were performed on samples obtained from a subset of 12 newly diagnosed patients to determine whether the observed elevation in triglycerides could be due to decreased clearance of chylomicron triglyceride via lipoprotein lipase. Only 1 of 7 patients with LPL analysis during asparaginase had LPL levels less than the reported normative mean. This patient's fasting triglyceride level at the time was 188 mg/dL. We detected no difference between LPL levels in those patients with triglyceride levels ≥400 mg/dL and those with levels less than 400 mg/dL (P = .57, exact Wilcoxon rank sum test). Furthermore, for the 5 patients with triglyceride levels ≥400 mg/dL, there was no difference between the mean LPL level and normative levels (P = .07). The LPL levels obtained for this subset of patients could not be explained by either aberrant growth patterns (ie, obesity) or hyperglycemia. Calculated z-scores from serial height and weight measurements obtained during treatment and compared with gender and age-based norms show that patients were within 1 standard deviation (SD) of the norm (z-score = 1.00) for weight and weight for height at the time of the LPL assessment (data not shown). This pattern was also preserved throughout treatment. Moreover, none of the patients had a fasting glucose level greater than 140 mg/dL during asparaginase therapy (median, 78 mg/dL; range, 55 to 118 mg/dL). Only 1 patient had an elevated fasting serum glucose level of 170 mg/dL at the time of diagnosis, which subsequently normalized in repeat testing.
DISCUSSION
These data show the striking temporal association between asparaginase therapy and hypertriglyceridemia in newly diagnosed children with ALL. Our observed 67% incidence of hypertriglyceridemia, 19% with levels greater than 1,000 mg/dL (Fig 2A), indicates that this abnormality is more common than previously reported.9 11 One possible explanation of this apparent increased incidence is that hypertriglyceridemia, ranging from 200 mg/dL to 1,000 mg/dL, may be undetected in the absence of grossly lipemic serum or other clinical correlates.
The physiology of normal lipid metabolism is well characterized at a molecular level. Triglycerides enter the circulation by two distinct pathways — exogenously via the diet and endogenously via hepatic synthesis.30 The highlights of these pathways are summarized in Fig 1. In the fed state, triglycerides from the diet are absorbed in the intestine, packaged as chylomicrons, and carried with Apo-B48. This protein is genetically related to Apo-B100 and is the amino-terminus 48% of B100. Chylomicrons lose triglyceride in the conversion to chylomicron remnant, a step catalyzed by LPL. Chylomicron remnants are removed from the circulation by specific hepatic receptors. In the endogenous pathway, the liver can synthesize and secrete into the circulation the triglyceride-rich particles, VLDL, which contain Apo-B100. VLDL loses triglyceride through the action of LPL and in the process is converted initially to intermediate density lipoprotein (IDL) and then to LDL. LDL is removed from the circulation by specific hepatic receptors or via the scavenger pathway in cells such as macrophages. Two-thirds of LDL is normally removed by LDL receptors, and the remainder is removed by the scavenger cell system. Apo-B100 is the major protein in each of these lipoproteins. One Apo-B100 is present in each LDL, IDL, and VLDL particle. The principal proteins in HDL are Apo-AI and Apo-AII, which comprise approximately 50% of HDL mass. HDL is secreted from liver or intestines as nascent particles consisting primarily of phospholipids and Apo-AI. Through the extracellular addition of surface components of triglyceride-rich particles, such as phospholipids, cholesterol, and certain apolipoproteins, HDL is converted from a disk-shaped to more spherical particle. HDL particles deliver cholesteryl esters to the liver directly or after transfer to Apo-B100 containing lipoprotein. This important pathway is the reverse cholesterol transport mechanism by which cellular and lipoprotein cholesterol is delivered back to the liver for reuse or disposal. Because of the complexity of this metabolic pathway, we serially obtained a diverse panel of tests to elucidate the mechanism of asparaginase-induced hypertriglyceridemia.
Our data strongly suggest that the observed elevation in fasting triglyceride levels in our patients during asparaginase therapy was due to an increase in endogenous synthesis of VLDL. Lipid subfractionation (via NMR) showed only trace chylomicron and a statistically significant increase in the smaller, more dense VLDL1-4. The documented increase in larger, more buoyant forms of VLDL (VLDL5,6 ) may also reflect increased VLDL synthesis, although we cannot definitively rule out a delay in the removal of chylomicron remnant via specific hepatic receptors. In addition, we detected an increase in Apo-B100 levels during asparaginase therapy, suggestive of an overproduction of VLDL particles. The mechanism of increased Apo-B100 synthesis during asparaginase therapy is not clear. Further research is needed to elucidate this mechanism at a molecular level.
Changes in serum cholesterol levels were not temporally related to asparaginase therapy. Instead, these changes are consistent with the known association between hypercholesterolemia and corticosteroids. As expected, we observed a reciprocal decrease in LDL cholesterol as VLDL increased during asparaginase therapy. The shift we detected from larger, more buoyant LDL to small, more dense LDL in a subset of patients with hypertriglyceridemia merits further study, particularly because the denser fractions have been implicated in atherogenesis.31
Our data on HDL in both the newly diagnosed and long-term survivor groups shows important trends that also merit further investigation. Before the initiation of asparaginase therapy, both Apo-AI and HDL levels were below normal, with Apo-AI levels approximating the norm more than HDL levels did. Although the precise mechanism of these findings is not known, diminished HDL can be the result of either decreased formation or increased removal from the circulation via specific hepatic receptors. The latter explanation is favored by some investigators who suggested that low HDL concentrations are indicative of active cell proliferation.32 Dessi et al32 reported in a study of 57 children with malignancies statistically lower HDL levels in all study children when compared with age-matched controls, with the most profound decrease noted in children with hematologic malignancies (n = 32). However, in our study, during and after asparaginase, median Apo-AI levels decreased, whereas HDL levels increased (Table 2). This finding suggests an important structural change in the HDL particles from high density to a lower density that is reflective of an altered ratio of lipid to protein. Specifically, the HDL-cholesterol particle before asparaginase was protein-rich, shifting over time to more lipid-rich. One hypothesis would be that this reflects the effect of asparaginase on diminishing protein synthesis, but the precise mechanism requires further elucidation. Given the heterogeneity of HDL in composition and the relatively small sample size, we were unable to characterize the HDL-cholesterol particles by subfractionation in our newly diagnosed patients.
Perhaps of greater concern is the finding that our long-term survivors have persistently low Apo-AI and HDL levels, despite resolution of their underlying disease. As noted, 25% of the patients had HDL levels less than 35 mg/dL. This subset of patients did not differ from the other long-term survivors with respect to growth abnormalities (ie, obesity). These long-term changes in HDL are consistent with those recently reported by Talvensaari et al.33 In their review of 50 long-term survivors of childhood cancer, 28 of whom had ALL, HDL levels were statistically lower than age- and sex-matched controls — even after adjustment for relative weight.33 These findings may represent increased risk of coronary artery disease and merit further study.
In summary, the clinical implications of our findings are twofold. First, although the incidence of hypertriglyceridemia of 67% in our study was appreciably higher than previously reported, we detected no association between hypertriglyceridemia and acute asparaginase toxicities, notably pancreatitis. These findings suggest that modifications of asparaginase therapy are not necessary in the setting of hypertriglyceridemia, with two important caveats. In the rare child who develops hypertriglyceridemia greater than 2,000 mg/dL, the risk of pancreatitis may be enhanced due to severe chylomicronemia. Moreover, in chylomicron-induced pancreatitis, serum amylase levels may be normal and abdominal pain may be the only marker of pancreatitis. For such patients, close monitoring of clinical signs and symptoms is imperative. Additionally, although our study lacked the statistical power to detect the likely association between triglyceride levels before and during asparaginase therapy, we suspect that a subset of patients with slightly elevated triglyceride levels before the initiation of asparaginase could be more prone to exaggerated elevation of triglyceride in response to the stressor of asparaginase therapy. Once that stressor is removed with the completion of asparaginase therapy, triglyceride levels normalize.
Clarification of this pattern through larger studies would aid oncologists balancing the important antileukemic effect of asparaginase with potential for increased toxicity in this subset of patients. Further exploration of dietary modification and/or the use of agents such as gemfibrozil to modulate triglyceride levels during asparaginase therapy are warranted.
Secondly, our findings suggest the need for larger studies of long-term survivors of ALL to determine the true incidence of dyslipidemia (ie, low HDL and composition of LDL) in this population and the association, if any, with intensive asparaginase therapy and intermittent use of steroids during initial treatment. Moreover, the relationship between lipid abnormalities and other emerging late effects of treatment, notably obesity and cardiomyopathies, warrants further study. As the population of survivors of ALL increases, more information is needed to guide the long-term care of this complex group of patients.
ACKNOWLEDGMENT
The authors gratefully acknowledge the participation of the physicians, nurses, and laboratory personnel at Children's Hospital and the Dana Farber Cancer Institute's Jimmy Fund Clinic. We also are indebted to D. Neuberg for her critical review of the statistical design and analysis.
Supported in part by the National Institutes of Health Grants No. CA68484 and DK02456, the Food and Drug Administration Grant No. FD-R 000199, and research funds from Rhône-Poulenc Rorer. S.K.P. is supported by a Career Development Award from the American Society of Clinical Oncology. S.X.S. is supported by a Clinical Investigator Development Award, National Institute of Child Health and Human Development (K08HD01105-01).
Address reprint requests to Susan K. Parsons, MD, MRP, Department of Pediatric Oncology, Dana Farber Cancer Institute, 44 Binney St, Boston, MA 02115.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal