Abstract
The macrophage colony-stimulating factor receptor and several other hematopoietic growth factor receptors induce the tyrosine phosphorylation of a 145- to 150-kD protein in murine cells. We have previously cloned a cDNA for the murine 150-kD protein, SHIP, and found that it encodes a unique signaling intermediate that binds the SHC PTB domain through at least one tyrosine phosphorylated (NPXY) site in the carboxyl-terminal region. SHIP also contains several potential SH3 domain-binding sites, an SH2 domain for binding other tyrosine phosphorylated proteins, and an enzymatic activity that removes the phosphate from the 5 position of phosphatidylinositol 3,4,5-phosphate or from inositol 1,3,4,5-phosphate. SHIP has a negative effect on cell growth and therefore loss or modification may have profound effects on hematopoietic cell development. In this study, we have cloned a cDNA for human SHIP and examined mRNA and protein expression of SHIP and related species in bone marrow and blood cells. Flow cytometry indicates that at least 74% of immature CD34+ cells express SHIP cross-reacting protein species, whereas within the more mature population of CD33+ cells, only 10% of cells have similar expression. The majority of T cells react positively with the anti-SHIP antibodies, but significantly fewer B cells are positive. Immunoblotting detects up to seven different cross-reacting SHIP species, with peripheral blood mononuclear cells exhibiting primarily a 100-kD protein and a CD34+ acute myeloblastic leukemia expressing mainly 130-kD and 145-kD forms of SHIP. Overall, these results indicate that there is an enormous diversity in the size of SHIP or SHIP-related mRNA and protein species. Furthermore, the expression of these protein species changes according to both the developmental stage and differentiated lineage of the mature blood cell.
SIGNALING BY THE macrophage colony-stimulating factor (M-CSF) receptor (FMS)1 is a paradigm for developmental control occurring at several stages of hematopoiesis. Not only are the related receptors, KIT and FLT3, present in various early and late lineages, but the signal transduction proteins activated by these and other hematopoietic growth factor receptors are shared. How these various related receptors, operating in different stages and lineages of hematopoietic development, achieve their specificity is a primary concern of our laboratory. We have sought to address some of these issues by characterizing the additional signal transduction proteins in the M-CSF–dependent signaling pathway.
Recently, we characterized and cloned the cDNA for a new signal transduction protein that was initially identified as a 150-kD protein that was tyrosine phosphorylated after M-CSF stimulation of murine myeloid cells expressing FMS.2,3 The same protein was not detectable in fibroblast cells ectopically expressing FMS and stimulated with M-CSF. The protein sequence indicated a unique structure and potential function, with the amino-terminal end containing an SH2 domain and the central portion having structural features of an enzymatic inositol 5-phosphatase domain. Furthermore, two tyrosine phosphorylation target sites (NPXY) for binding the Shc PTB domain were present near the carboxyl-terminal end among at least three polyproline stretches with potential for binding SH3 domains.3 The protein exhibited phosphatidylinositol and inositol 5-phosphatase enzymatic activity and was named SHIP, designating its structure and enzymatic function. The cDNA for this same protein was simultaneously cloned independently by others.4
In contrast to other signal transduction proteins that transform cells or augment cell growth, SHIP has a negative influence on cell growth. This was first demonstrated in myeloid cells3 and later in B cells.5,6 In B cells, cell growth is arrested when the FcγRII associates with the B-cell receptor by interactions of their extracellular domains with immune complexes. Tyrosine phosphorylation of the FcγRII cytoplasmic domain then allows SHIP to bind through its SH2 domain. This interaction is believed to block extracellular Ca2+ uptake and cell growth via the inositol 5-phosphatase activity of SHIP. It is not clear whether SHIP functions similarly in monocytes-macrophages, even though FcγRII splice variants are present in monocytes-macrophages7 and cell growth does slow in M-CSF–treated cells.8 It is puzzling why FMS signaling would activate such a potent growth inhibitory signal. However, tyrosine phosphorylation of SHIP may alleviate its inhibitory signal and therefore permit cell growth.
Several growth factors and cytokines will induce the tyrosine phosphorylation of SHIP in murine hematopoietic cell lines in culture. In appropriate cells, tyrosine phosphorylation of the 140- to 150-kD SHIP protein has been detected after treatment with erythropoietin (EPO), interleukin-2 (IL-2), IL-3, granulocyte-macrophage colony-stimulating factor (GM-CSF), Kit ligand, M-CSF, thrombopoietin (TPO), B-cell receptor cross-linking, T-cell activation, or FLT3 ligand.2 9-14 The importance of SHIP in blood cell development and function is clear from its expression in diverse hematopoietic cells. In addition, the potential loss or modification of the SHIP-negative function could contribute to abnormal growth and/or development. Therefore, to approach the potential involvement of SHIP in human disease, we have cloned the human SHIP cDNA and examined its mRNA and protein expression in blood cells and bone marrow.
MATERIALS AND METHODS
Cells.Samples of human bone marrow were obtained from normal healthy volunteers after informed consent was received under an Institutional Review Board approved protocol. Human leukemia cells were obtained from bone marrow through the Children's Cancer Group reference laboratory, and the peripheral blood leukocytes were obtained from healthy volunteers. The human ML-1 cells and murine FDC-P1 cells have been previously described,8 15 and murine bone marrow cells were freshly prepared from femurs.
DNA hybridization.Phage plaques (2 × 106 pfu) were lifted onto maximum strength Nytran Plus membranes (Schleicher & Schuell, Keene, NH). The phage lifts were autoclaved for 1 minute to release the DNA followed with immobilization by UV cross-linking (1,200 μJ; Stratalinker; Stratagene, LaJolla, CA). Filters were prehybridized, hybridized, and washed as described.16 Probe DNA was labeled with [α32-P]dCTP (NEN, Boston, MA; 3,000 Ci/mmol) by random oligonucleotide primer extension (Ready-to-Go; Pharmacia, Uppsala, Sweden). Filters were washed at a final stringency of 1× SSC, 0.1% sodium dodecyl sulfate (SDS) at 55°C (h9.1 clone) or at 1× SSC, 0.1% SDS at room temperature (h4.2-A2 clone). Probed filters were exposed to film at −80°C with intensifying screens (NEN, DuPont, Boston, MA).
Cloning of a human SHIP cDNA.Human p145Ship was cloned by screening an HL60 cDNA library17 with the murine EML-11 cDNA clone.3 λ Phage growth, plaque purification, DNA extraction, restriction enzyme analysis, and subcloning were performed according to standard procedures.18 The cDNA clones obtained were subjected to automated dye termination sequencing in both directions (Applied Biosystems, Inc, Foster City, CA), and DNA sequences were analyzed using Sequencher software (Gene Codes Corp, Ann Arbor, MI). Twelve positive phage clones were biologically purified. The longest clone obtained (h9.1) was approximately 4.2 kb. Comparison of the murine sequence with the 5′ end of the clone showed that it was not a full-length clone. The HL60 cDNA library (2 × 106 pfu) was plated and screened a second time using a 1.1-kb segment containing the 5′ end of the human cDNA clone (h9.1) as probe. The longest clone (h4.2-A2) from this screen of 14 positive phage was approximately 4.7 kb. This clone still lacked the starting methionine predicted from the murine SHIP sequence. Subsequently, the 5′ end of human p145Ship was obtained by polymerase chain reaction (PCR) amplification of the HL60 library using a sense oligonucleotide specific to the vector (5′-CGAAATTAACCCTCACTAAAGGG) and an antisense oligonucleotide specific to the 5′ end of clone h4.2-A2 (5′-GATGAGCTGGTCCAGCTTG). Amplified products were resolved by agarose gel electrophoresis, and those greater than 400 bp were gel-purified and ligated into the pT7Blue(R) vector (Novagen, Madison, WI). The DNA inserts from recombinant transformants were sequenced in both directions and found to contain the SHIP 5′ coding region.
RNA preparation and Northern analysis.Total RNA from murine bone marrow cells was isolated by lysis in guanidium isothiocyanate and centrifugation through 5.7 mol/L cesium chloride.19 mRNA (poly A+) was purified from total cellular RNA using oligo(dT)-cellulose spin columns (Clontech Labs, Palo Alto, CA). Human bone marrow polyA+ RNA was purchased from Clontech Labs. For Northern analysis, 2 μg of each poly A+ RNA was denatured with glyoxal and dimethyl sulfoxide and resolved through 1% agarose gels,18 followed by vacuum transfer (Vacugene; Pharmacia LKB, Uppsala, Sweden) onto Nytran Plus membranes and then immobilized by UV cross-linking (1,200 μJ; Stratalinker). Filters were prehybridized, hybridized, and washed as described.16 Hybridization was performed as described for DNA with 106 cpm/mL probe corresponding to a 4.7-kb fragment (h4.2-A2) of the human full-length cDNA for p145Ship. Blots were washed at a final stringency of 1× SSC, 0.1% SDS at 60°C for 30 minutes or 0.1× SSC, 0.1% SDS at 65°C for 30 minutes.
Immunoblotting.Human or murine hematopoietic cells were lysed in NP40 buffer containing inhibitors,20 and lysates were clarified by centrifugation at 15,000 rpm for 15 minutes. The protein contents of the lysates were measured using a BioRad (Richmond, CA) protein assay and aliquots of lysates containing equal amounts of protein were resolved on a 7.5% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) gel. The proteins were electroblotted to nitrocellulose and the blot was incubated for 1 hour with a rabbit antiserum to SHIP (#5340; prepared to amino acids 670-868 of the murine protein3 ) diluted 1:5,000 in Block.20 After washing four times for 15 minutes each in Rinse buffer, the blot was incubated for 1 hour with horseradish peroxidase (HRP)-conjugated goat antirabbit secondary antibody (1:10,000 in Block). The blot was washed again as described above and SHIP proteins were detected using Renaissance detection (Dupont, Boston, MA) according to the manufacturer's instructions.
Cell staining.Bone marrow mononuclear cells were isolated by standard Ficoll-Hypaque density centrifugation. Coexpression of intracellular p145Ship and CD2, CD4, CD8, CD19, CD20, CD33, or CD34 was examined using two-color immunofluorescence. Cells were first stained for surface antigens using antibodies directly conjugated to phycoerythrin (Becton Dickinson, Inc, San Jose, CA). After surface staining, cells were rinsed twice in phosphate-buffered saline (PBS) with 2% human-type AB serum (2% HABS) and resuspended at room temperature in 200 μL of Permeafix (Ortho Diagnostic Systems, Raritan, NJ) for fixation and permeabilization of cells. Cells were fixed for 20 minutes, rinsed twice in 2% HABS, and incubated with unlabeled affinity-purified rabbit antibody to the murine SHIP protein or with affinity-purified rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA). The anti-SHIP antibody (#5369) was prepared to a GST fusion protein containing amino acids 889 to 1046 of the murine protein. This GST-SHIP fusion protein was attached to agarose beads and the antibodies were affinity-purified. Preliminary experiments showed that the GST-SHIP fusion protein competed successfully for blood cell labeling, whereas the GST protein did not (not shown). The secondary donkey antibody to rabbit Ig was also affinity-purified and coupled to fluorescein isothiocyanate (FITC; Jackson ImmunoResearch Laboratories).
Flow cytometric analysis.Samples were analyzed on a Becton Dickinson Vantage flow cytometer. Parameters for forward (cell size) and orthoganol (cell granularity) light scatter as well as FITC and phycoerythrin fluorescence were collected for 50,000 ungated events. Data for each sample were then analyzed by setting a large region for the size and granularity that would include all mononuclear cells and exclude only debris and clumps. Another region was set for cells positive for the expression of the surface marker of interest. Cells falling in both regions were then analyzed for expression of SHIP relative to the rabbit Ig control.
Cloning of human SHIP genomic DNA.A human fibroblast genomic library in the Lambda Fix II vector (Stratagene) was screened as described for the cDNA library using the cDNA clone h4.2-A2. A positive genomic clone was selected for fluorescence in situ hybridization (FISH) analysis that contained a recombinant insert of approximately 18 kb. Southern analysis with the h4.2-A2 cDNA probe identified a positive 1.2-kb restriction fragment of the 18-kb insert that was sequenced in both directions. This fragment contained 2 SHIP exons and 3 intron sequences. The exons were located at the beginning of the polyphosphate-5-phosphatase domain. This 18-kb genomic SHIP fragment Hg 4.1-A was used in chromosomal localization studies.
FISH.FISH analysis was performed with two independent human SHIP clones (h4.2-A2 cDNA and Hg4.1-A2 genomic DNA). DNA from each clone was fragmented to 200 to 400 bp and labeled with biotin by nick translation using a Bionick kit (GIBCO-BRL, Gaithersburg, MD). Labeled probe (∼500 ng) was hybridized to metaphase spreads prepared from a phytohemagglutinin-stimulated culture of peripheral blood lymphocytes from a normal male following published procedures.21 The cells were blocked in early S-phase with methotrexate and released in the presence of BrdU before harvest. The hybridization sites were detected with avidin-FITC and the chromosomes were stained with 4,6-Diamidino-2-phenylindole (DAPI) to produce a quinacrine-like banding pattern. Hybridization signals were mapped relative to bands both by visible inspection of the spreads through the microscope (a Zeiss Axiophot; 100× 1.3 na objective; Carl Zeiss, Germany) and of digitized images displayed on a computer screen. Ten metaphase spreads were analyzed for each probe.
RESULTS
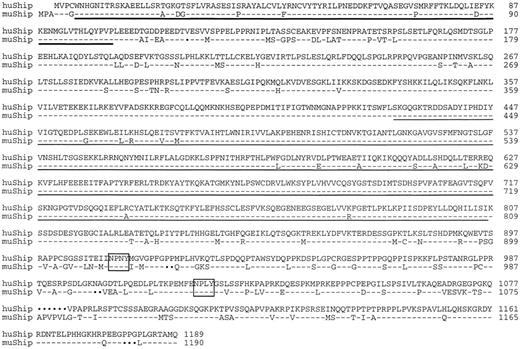
The murine EML-11 cDNA insert3 was used to screen an HL60 cDNA library17 for the human equivalent of the murine p145Ship protein. The 5′ end of the largest clone (h9.1; approximately 4.2 kb) was sequenced and found not to be full-length. The h9.1 clone was missing the starting methionine when compared with the murine p145Ship cDNA sequence (Fig 1). A new probe was made from the 5′ end of clone h9.1 and the HL60 library was screened again. The largest clone from this screen, h4.2-A2, contained an insert of approximately 4.7 kb. This clone was subjected to DNA sequencing in both directions. Clone h4.2-A2 was found to be short of the starting methionine by 81 nucleotides, compared with the murine cDNA sequence. The remaining 5′ end of the human SHIP cDNA was isolated by direct PCR amplification of the HL60 cDNA library and sequenced in both directions. The PCR-amplified product overlapped with clone h4.2-A2 to provide the full-length sequence. This resulted in a gene of 4870 nucleotides with an open reading frame of 3567 bp, including the stop codon and encoding an 1189 amino acid protein.
Amino acid sequence comparison of human SHIP with the murine p145Ship protein. The SH2 domain is designated with a bold underline; the region homologous to the catalytic domain of inositol polyphosphate-5-phosphatase is a single underline; the open boxes show NPXY motifs; and the solid dots show amino acid insertions and/or deletions. The GenBank accession number is U84400.
Amino acid sequence comparison of human SHIP with the murine p145Ship protein. The SH2 domain is designated with a bold underline; the region homologous to the catalytic domain of inositol polyphosphate-5-phosphatase is a single underline; the open boxes show NPXY motifs; and the solid dots show amino acid insertions and/or deletions. The GenBank accession number is U84400.
Human SHIP is very similar to the previously described murine p145Ship 3 and contains an SH2 domain at the amino-terminal end, a central inositol polyphosphate 5-phosphatase catalytic domain, and a carboxyl-terminal region with three polyproline regions with potential for binding SH3 domains. Human SHIP also retains the carboxyl-terminal region NPNY and NPLY motifs found in murine SHIP. One or both of these motifs may participate in binding to the PTB domain of SHC, as we have previously shown.3 Overall, there are 158 amino acid differences between murine and human SHIP. These differences include 8 amino acid additions throughout the human sequence and a contiguous 6 amino acid deletion in the C-terminal end of human SHIP relative to the murine SHIP. The inositol polyphosphate-5-phosphatase region is also conserved with 96% identity. In the human protein, the initiation methionine corresponding to the murine sequence is not present, but initiation occurs 3 amino acids downstream at a second methionine also present in the murine protein. At the nucleotide level, the untranslated regions are poorly conserved.
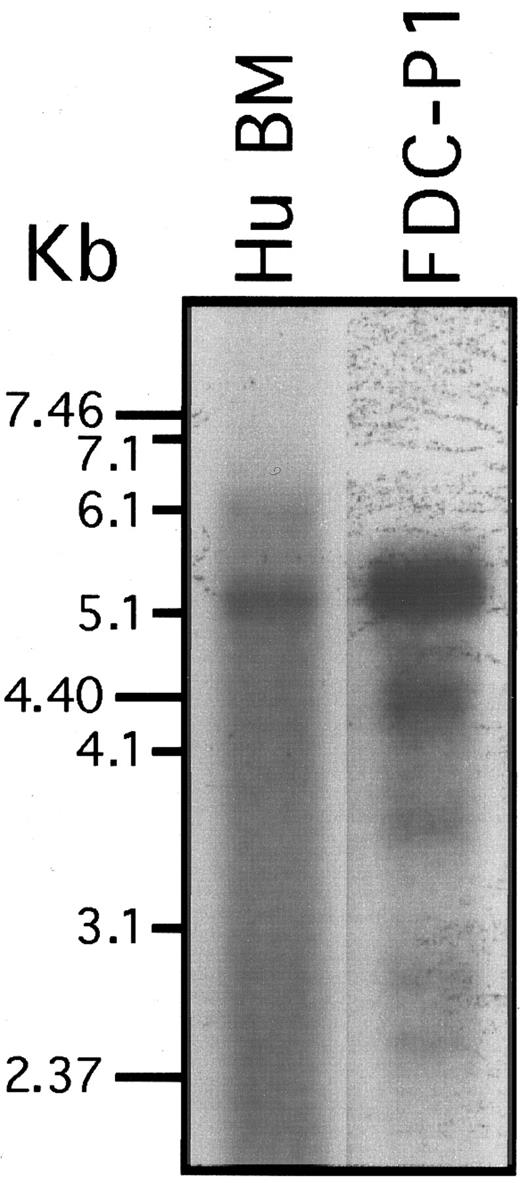
The murine SHIP gene is expressed in several cell lines of hematopoietic origin but is not detectable in fibroblast cell lines.3,4 We therefore examined human bone marrow cells for SHIP RNA expression by Northern analysis (Fig 2). A 4.7-kb fragment of the human SHIP cDNA was used to probe polyA+ RNA from human bone marrow and was compared with polyA+ RNA from murine FDC-P1 cells. As we have previously shown, the murine FDC-P1 cells express a major transcript of about 5 kb encoding the entire reading frame of the p145Ship.3 This 5-kb transcript was also present in the human bone marrow polyA+ RNA. In addition, we also found that bone marrow contained a larger 6-kb transcript not detectable in the FDC-P1 cells even after longer exposure of the blot. Both the human bone marrow 6-kb and 5-kb mRNA species remained at the same relative abundance after successively more stringent washing and are likely to represent SHIP transcripts or products from highly related genes. The nature of the 6-kb transcript is being explored. In the FDC-P1 cells several smaller hybridizing transcripts of approximately 4.0, 3.9, 2.9, and 2.4 kb were observed, and these may represent spliced variants of lower abundance or related genes. These smaller transcripts were not detectable in the human polyA+ RNA under the conditions of the experiments.
Northern analysis of SHIP RNA expression. PolyA+ RNA from human bone marrow cells and from the previously characterized murine FDC-P1 cells was subjected to Northern blot analysis with a human SHIP cDNA probe. The blots were washed at a stringency of 1× SSC, 0.1% SDS at 55°C. Under more stringent washing conditions (0.1× SSC, 0.1% SDS at 65°C), the murine signal was lost but both human mRNA species remained (not shown). The FDC-P1 lane was exposed much longer than the HuBM lane.
Northern analysis of SHIP RNA expression. PolyA+ RNA from human bone marrow cells and from the previously characterized murine FDC-P1 cells was subjected to Northern blot analysis with a human SHIP cDNA probe. The blots were washed at a stringency of 1× SSC, 0.1% SDS at 55°C. Under more stringent washing conditions (0.1× SSC, 0.1% SDS at 65°C), the murine signal was lost but both human mRNA species remained (not shown). The FDC-P1 lane was exposed much longer than the HuBM lane.
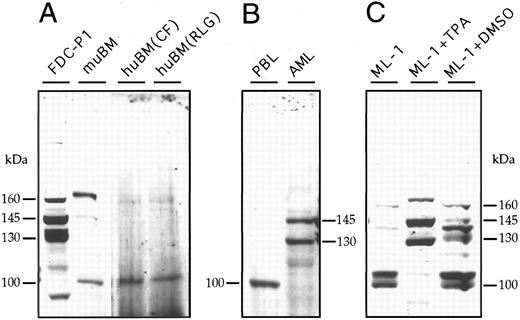
The expression of SHIP protein was examined in blood and bone marrow cells by Western blotting using antibody prepared to an amino acid region of the murine p145Ship that was 96% identical with the human SHIP sequence. These antibodies do not detect p145Ship in mammalian fibroblast cells, but after ectopic expression of the murine SHIP cDNA, several SHIP protein species are detectable.3 The results in Fig 3A show that the murine myeloid cell line FDC-P1 previously examined2 expresses multiple molecular weight proteins that cross-react with the SHIP antibody. The main species was 145 kD, corresponding to the full-length protein obtained from ectopic expression of the murine cDNA in rodent fibroblast cells.3 These cells also contained a single larger cross-reactive protein at approximately 165 kD and two prominent species of 133 and 130 kD. Several additional discrete protein species of less than 65 kD also were detected (not shown).
Western blot analysis of SHIP protein expression in cells derived from bone marrow or blood. Western blotting with antibodies to murine SHIP was performed on cell lysates derived from different sources. (A) Extracts from murine FDC-P1 cells and murine bone marrow cells (muBM) were compared with extracts from two different human bone marrow (huBM) samples. (B) Peripheral blood leukocytes (PBL) and a CD34+ AML sample taken from peripheral blood. (C) Myelomonocytic leukemia cell line, ML-1, either untreated or differentiated to monocytes-macrophages by 2 days of growth in 5 × 10−10 mol/L TPA or to granulocytes by treatment with 1.25% DMSO.
Western blot analysis of SHIP protein expression in cells derived from bone marrow or blood. Western blotting with antibodies to murine SHIP was performed on cell lysates derived from different sources. (A) Extracts from murine FDC-P1 cells and murine bone marrow cells (muBM) were compared with extracts from two different human bone marrow (huBM) samples. (B) Peripheral blood leukocytes (PBL) and a CD34+ AML sample taken from peripheral blood. (C) Myelomonocytic leukemia cell line, ML-1, either untreated or differentiated to monocytes-macrophages by 2 days of growth in 5 × 10−10 mol/L TPA or to granulocytes by treatment with 1.25% DMSO.
Murine bone marrow, examined immediately after recovery from femurs, also contained a higher 175-kD SHIP cross-reactive species, the expected 145-kD protein, and a smaller protein of about 100 kD (Fig 3A). Several human bone marrow samples were examined for SHIP protein expression but none gave satisfactory results because of low detectability of SHIP-related proteins and high background. Results from two of the human bone marrow samples are shown in Fig 3A. A 100-kD protein was detectable as the most prominent species, and a weak but detectable band at 165 kD was seen.
The mononuclear cell fraction was isolated from human blood and SHIP protein expression detected by Western blotting (Fig 3B). As in the human bone marrow samples, the most prominent cross-reactive species was a 100-kD protein band with two very faint bands representing larger size proteins. This suggested that the bone marrow samples may have been composed primarily of mature blood cells from intermixed blood obtained during aspiration of the bone marrow. This could explain why SHIP expression in the human blood and bone marrow samples looked the same, whereas the murine bone marrow sample was distinctly different in SHIP expression.
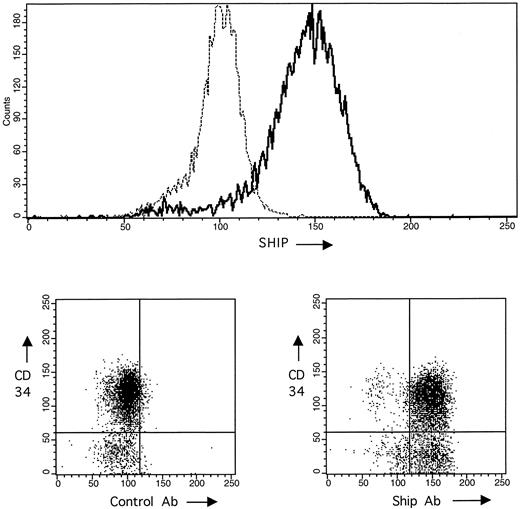
It was not possible to determine the SHIP protein expression in hematopoietic progenitor cells by Western blotting because it was not possible to obtain sufficient quantities of cells. Therefore, to address the question of the SHIP expression in immature hematopoietic cells, we looked at acute myeloblastic leukemia (AML) cells expressing the early marker, CD34. Several leukemias were analyzed by flow cytometry using both antibodies to the CD34 antigen and to the SHIP protein. All were positive (to various degrees) for CD34 and SHIP-related proteins, and the results in Fig 4 show one of these leukemias highly positive for both SHIP cross-reactive proteins and CD34 antigen. These AML cells were very homogenous and Western blotting for the SHIP-related proteins (shown in Fig 3B) demonstrated that two prominent SHIP proteins of 145 and 130 kD were present. The larger 165- to 175-kD proteins were not present in cells of this leukemia.
Analysis of SHIP and CD34 expression on AML by flow cytometry. Several AML samples were analyzed for coexpression of SHIP and CD34. The AML shown expressed high levels of both proteins. The upper panel shows the single-parameter analysis of SHIP relative to a control rabbit Ig fraction; the lower two panels show two-parameter analysis of CD34 versus control Ig (left) and CD34 versus SHIP expression (right) in the same AML cells.
Analysis of SHIP and CD34 expression on AML by flow cytometry. Several AML samples were analyzed for coexpression of SHIP and CD34. The AML shown expressed high levels of both proteins. The upper panel shows the single-parameter analysis of SHIP relative to a control rabbit Ig fraction; the lower two panels show two-parameter analysis of CD34 versus control Ig (left) and CD34 versus SHIP expression (right) in the same AML cells.
More mature myeloid cells were examined for SHIP expression using the ML-1 myeloblastic leukemia that differentiates to monocytes in the presence of 12-O-tetradecanoylphorbol-13-acetate (TPA) and to granulocytes when treated with dimethylsulfoxide (DMSO).22 We have used these cells previously for identification of the human FMS (the M-CSF receptor) by its restricted expression in the monocyte differentiation lineage.15 Untreated ML-1 cells expressed the same size (100 kD) SHIP cross-reactive protein detected in the peripheral blood leukocyte sample plus a 105-kD protein. Induction of granulocyte formation by DMSO resulted in the continued expression of both 100-kD and 105-kD species along with an array of higher molecular weight proteins. A 140-kD protein was present as one of the more prominent higher molecular weight species in the cells differentiated to granulocytes, and a 165-kD cross-reactive species was observed. ML-1 cells that had become monocytes in the presence of TPA expressed a 175-kD, a 145-kD, and a 130-kD species with little or none of the proteins around 100 kD. The proteins in the TPA-treated ML-1 cells resembled those seen in the AML cells, with the exception that the AML cells lacked the highest molecular weight forms. Antibodies prepared to two different regions of the murine p145Ship detected similar but not identical protein species, suggesting that the majority represent the human SHIP protein or a very close relative (data not shown). These results indicate that SHIP expression changes dramatically with the differentiated state of the cells.
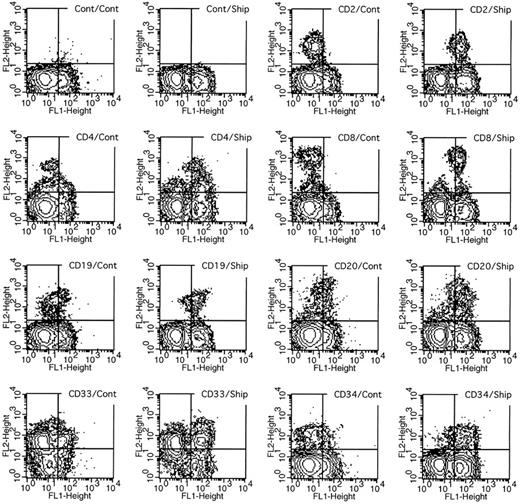
The human bone marrow cells expressed a low level of SHIP cross-reactive proteins detectable by Western blotting, but more sensitive detection was possible with flow cytometry. Bone marrow cells were simultaneously labeled with antibodies to individual lineage-specific cell surface markers and with antibodies to SHIP in permeabilized cells. Gates for forward scatter versus side scatter were set to focus on individual cell populations, and coexpression of the lineage marker and SHIP was measured by two parameter sorting. The results in Fig 5 show the expression levels of SHIP in individual lineage-marker positive cells, and the results are quantitated in Table 1. SHIP expression in total bone marrow cells is higher than control (Cont/Cont v Cont/Ship panels). However, when a single lineage marker, such as the T-cell marker, CD2, is used, the double-positive cells (shown in the CD2/Cont v CD2/Ship panels, Fig 5) indicate that at least 75% of the CD2+ cells also express SHIP (Table 1). Likewise, CD4+ and CD8+ T cells also express high SHIP levels. The CD19 and CD20 antigens are present on B cells, whereas CD19 is also found on B-cell precursors. Again, SHIP is detected on both of these cell types, but the expression level was perhaps one third of that detected in the T cells. Finally, SHIP expression was examined in CD33+ versus CD34+ bone marrow cells. The results indicated that the number of CD34+ progenitor cells that contained SHIP protein was similar to that found in mature T cells. In contrast, only 10% of CD33+ cells, representing mature granulocytes and monocytes/macrophages, contained SHIP cross-reactive protein. The CD33+ population was the only group of those examined that contained a clear SHIP-negative fraction. We do not know which cell types within the CD33+ population were negative.
Two-parameter analysis of SHIP expression in lineage-tagged bone marrow cells. Human bone marrow cells were labeled with rat monoclonal antibodies to the various CD markers shown (or a control rat Ig) and affinity-purified antibodies to the murine SHIP protein (or control rabbit Ig). The upper right-hand corner of each graph indicates the combination of antibodies used for cell labeling, with the first representing the FL2 axis and the second the FL1 axis. Thus, SHIP-positive cells appear to the right and CD-positive cells toward the top of each graph. The doubly labeled cells are detectable in the upper right-hand quadrant.
Two-parameter analysis of SHIP expression in lineage-tagged bone marrow cells. Human bone marrow cells were labeled with rat monoclonal antibodies to the various CD markers shown (or a control rat Ig) and affinity-purified antibodies to the murine SHIP protein (or control rabbit Ig). The upper right-hand corner of each graph indicates the combination of antibodies used for cell labeling, with the first representing the FL2 axis and the second the FL1 axis. Thus, SHIP-positive cells appear to the right and CD-positive cells toward the top of each graph. The doubly labeled cells are detectable in the upper right-hand quadrant.
Quantification of SHIP Expression in Surface Marked Cells
| Normal Bone Marrow Mononuclear Cell Phenotype . | Percentage Positive for SHIP . |
|---|---|
| CD2+ | 75.2 |
| CD4+ | 82.8 |
| CD8+ | 80.3 |
| CD19+ | 27.7 |
| CD20+ | 23.9 |
| CD33+ | 10.0 |
| CD34+ | 74.0 |
| Normal Bone Marrow Mononuclear Cell Phenotype . | Percentage Positive for SHIP . |
|---|---|
| CD2+ | 75.2 |
| CD4+ | 82.8 |
| CD8+ | 80.3 |
| CD19+ | 27.7 |
| CD20+ | 23.9 |
| CD33+ | 10.0 |
| CD34+ | 74.0 |
Quantification of SHIP expression in bone marrow cells from the results in Fig 5. The percentage of SHIP-positive cells in each CD-labeled cell population was determined as the percentage of SHIP-positive cells greater than 95% above the fluorescence obtained with the control antibody.
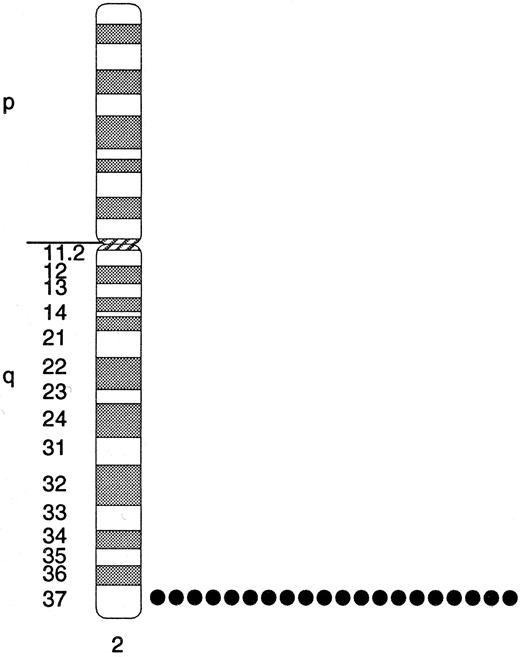
FISH was used to map the SHIP gene to human chromosome 2q37. This location was determined using a 4.7-kb cDNA clone (h4.2-A2) and confirmed using an 18-kb genomic clone (Hg4.1-A). Figure 6 summarizes the FISH results for the cDNA clone. Hybridization signals were observed at 2q37 in all 10 metaphases analyzed. No other sites showed specific hybridization. The genomic SHIP clone, containing at least two exons derived from the beginning of the inositol 5-phosphatase domain, was used in chromosomal localization studies and also mapped to 2q37 (95% detection efficiency). A high resolution color figure of the FISH results can be viewed and downloaded from our world wide web site (http://www.fhcrc.org/∼lrr).
Ideogram of the SHIP localization on human chromosome 2. The human SHIP gene was mapped to human chromosomes using FISH. Ten metaphase cells were analyzed for FISH signals using a 4.7-kb human SHIP cDNA clone. Each dot represents the occurrence of an FISH signal on one chromosome. Twenty of a possible 20 signals were observed, representing a detection efficiency of 100%. Specific signals were not seen on other chromosomes. The same location, 2q37, was found using an 18-kb genomic clone for SHIP.
Ideogram of the SHIP localization on human chromosome 2. The human SHIP gene was mapped to human chromosomes using FISH. Ten metaphase cells were analyzed for FISH signals using a 4.7-kb human SHIP cDNA clone. Each dot represents the occurrence of an FISH signal on one chromosome. Twenty of a possible 20 signals were observed, representing a detection efficiency of 100%. Specific signals were not seen on other chromosomes. The same location, 2q37, was found using an 18-kb genomic clone for SHIP.
DISCUSSION
Analysis of the expression and potential function(s) of SHIP in human blood and bone marrow cells required that we first clone a cDNA for the human SHIP gene. The complete open reading frame was obtained from an HL60 cDNA library using a 4.7-kb murine cDNA probe (Fig 1). The human SHIP open reading frame contained 3567 nt and encoded an amino acid sequence of 1189 residues, with only 158 amino acids different than the murine SHIP protein. The major sequence motifs and domain structures are conserved between the murine and human proteins (see Results). A cDNA clone for human SHIP from a human megakaryocytic cell line was also reported recently.23 Others have obtained several human cDNA sequences for inositol 5-phosphatases from human brain using degenerate PCR, and one of the PCR clones encoded the SHIP protein.24 In addition, partial cDNAs for human SHIP proteins have been cloned from human placenta and lung.25 Comparison of the sequences from all four publications indicates that the variability among the SHIP proteins involves only five amino acid differences. These differences include amino acid residues 25-26, 116, 1029, and 1169 of the SHIP sequence reported in this study. Of four independent SHIP clones we obtained, two had aspartate-glycine (DG) at positions 25 and 26 and two had glycine-threonine (GT) at these sites. All other reported sequences contained only DG residues. Two of the reported sequences have a deletion of the valine at amino acid 116. Single amino acid substitutions were also evident at positions 1029 (P to H; this study) and 1169 (H to Y24). Such variations, especially from the same library, suggest that these small differences may represent allelic variations within the population.
The presence of spliced SHIP mRNAs has been reported based on the 5′ sequences of two partial SHIP cDNAs.25 Two laboratories have reported Northern analysis of human SHIP mRNA and detected the 5-kb transcript in every tissue examined.23 24 However, even though the same commercially available polyA+ RNA blots were used in each study, each laboratory reported completely disparate results for the expression of a larger SHIP transcript.
The Northern analysis of SHIP mRNA expression in human bone marrow (Fig 2) detected at least two species of 5 kb and ∼6 kb. The 5-kb mRNA corresponded to the approximate size of the full-length murine cDNA clone3 that encoded an SHIP protein calculated to have a molecular mass of ∼133,000 but whose size on gel electrophoresis was 145 kD (we previously referred to this protein as 150 kD but will now adopt the 145-kD designation to maintain a nomenclature consistent with other laboratories). Thus, the human SHIP 5-kb mRNA in bone marrow probably encodes the full-length p145Ship, as we have shown for the murine protein.3 The 6-kb mRNA is assumed to encode a larger size protein and, although this need not be the case, larger size forms of SHIP-related proteins are present in bone marrow and blood cells. Further characterization of both genomic and cDNA sequences will be needed to understand the origin and function of this larger transcript. Also, although the smaller SHIP mRNA species of less than 5 kb were detected in the murine FDC-P1 cells, they were not clearly present in the human bone marrow polyA+ RNA. However, PCR analysis of the polyA+ RNA from the human bone marrow and from ML-1 cells (data not shown) showed the existence of the spliced smaller SHIP mRNA in addition to the larger SHIP mRNA, reported previously as encoding SIP110 and SIP145, respectively.25 Therefore, at least one of these smaller spliced SHIP mRNA species (lacking the SH2 domain) is present in the bone marrow cells although not detectable by Northern analysis. Thus, at least three differentially spliced transcripts may encode the SHIP proteins. The 6-kb mRNA could encode one of the larger SHIP proteins (165 kD or 175 kD) with perhaps another yet undetected splice variant accounting for the other large protein. The 5-kb transcript encodes the 145-kD protein,3 and smaller transcripts could encode a SHIP protein of about 130 kD or smaller, with a short spliced N-terminus replacing the entire SH2 domain as in SIP110.25
The immunoblotting analysis of protein expression in bone marrow and blood cells showed a complex pattern of SHIP cross-reactive protein expression. As many as seven different size proteins were detected by the antibodies prepared to murine p145Ship. Two different antibodies, prepared to separate regions of the murine SHIP sequence, were used in these studies and each had specific advantages. For the immunoblotting, an antibody prepared to a highly conserved region between murine and human sequences was used (96% identity in amino acid sequence). This increased the probability of detecting human SHIP but also gave some chance of detecting related proteins. For example, the most closely related protein in the database, 51C,3,26 contains 52% amino acid identity in this same region and encodes an ∼145-kD protein. Other inositol 5-phosphatases may not cross-react because the most closely related member, IP5P,27 28 contains only 14% identity in that region. Therefore, the antibody used for immunoblotting could detect other related proteins but had the highest potential cross-reactivity with human SHIP. A second affinity-purified antibody was prepared to the more unique amino acid region near the carboxyl tail of the murine p145Ship. This amino acid region was still 76% identical to the human sequence and clearly cross-reacted with human SHIP proteins by immunoblotting (not shown). This antibody had the added advantage that it was less likely to recognize the 51C and IP5P proteins (20% and 14% identity in the corresponding amino acid region, respectively) and was used in the flow cytometry analysis because of its selectivity for human SHIP with less potential for recognizing the related proteins.
The immunoblotting experiments showed that the murine FDC-P1 cells and the bone marrow and blood cell samples contained SHIP cross-reactive proteins of similar sizes, suggesting that the same SHIP proteins may be expressed in these cells. A 175-kD SHIP protein was seen in murine bone marrow and a 165-kD SHIP protein was present in FDC-P1 cells. A SHIP protein of similar size has been detected in B cells,6 and a protein of about this size is also visible in human bone marrow cells (Fig 3A). As mentioned above, the 145-kD SHIP protein is encoded by the ∼5-kb mRNA and detectable in both FDC-P1 cells and in the murine bone marrow. This protein may be in the human bone marrow as well but not detectable due to its low abundance and the presence of mature blood cells contaminating the two human bone marrow samples (see Results).
Both human and murine bone marrow samples exhibited a 100-kD SHIP cross-reactive protein. The presence of the 100-kD SHIP-related protein in the human peripheral blood leukocyte sample resembled the results with the human bone marrow, except that there was little evidence of the larger forms of SHIP. It is not known which cells of the blood express the 100-kD SHIP-related protein, but flow cytometry of blood cells indicated that almost all peripheral blood mononuclear cells express SHIP proteins (with the exception of some CD14+ cells; ie, monocytes and perhaps some granulocytes7; data not shown). Therefore, the 100-kD SHIP-related protein is probably expressed in most of the blood cells. The quiescent state of blood cells in terms of growth and development may be related to the expression of this protein.
In contrast, a human CD34+ AML was analyzed as a substitute for hematopoietic progenitor cells and found to express primarily 145-kD and 130-kD SHIP proteins. These results show that cells at different stages of hematopoietic cell development express entirely different forms of the SHIP (or related) proteins and suggest that each species may be performing a distinct (but related) function.
The clearest example of the diversity in SHIP expression pattern accompanying differentiation was obtained with the myelomonocytic leukemia ML-1 cells. Under normal growth conditions, the expression pattern of proteins detectable with the anti-SHIP antibody resembled that seen in the PBL cells (except that two proteins around 100 kD were detectable). Upon differentiation to macrophages, the higher molecular mass forms of SHIP were expressed and the pattern resembled that seen in the FDC-P1 cells. In contrast, after differentiation to granulocytes, the pattern of SHIP cross-reactive proteins was entirely different. This suggests that the size and number of SHIP and SHIP-related proteins is lineage-dependent in the hematopoietic developmental scheme. It does not seem to be growth related but clearly changes with the developmental stage.
The chromosomal location of the human SHIP gene was mapped to 2q37. Both 4.7-kb cDNA and a genomic 18-kb DNA gave identical results. Although abnormalities at this site do not represent a hallmark of any one leukemia, aberrant translocations at this chromosomal location have been detected in some chronic myeloid leukemia29 and sporadic abnormalities in this region have been reported in several leukemias. Certainly, our current understanding of the activity of p145Ship suggests a role in both growth and development signaling as well as in B- and T-cell immunity. Therefore, abnormalities may be anticipated in pathological conditions such as leukemia and autoimmune diseases. The complexity of protein expression from this site, along with the potential functional differences in expression of individual SHIP proteins, will make the task of characterizing the role in normal and abnormal hematopoiesis difficult yet intriguing. Our current effort, therefore, is directed toward further defining the genomic locus and characterizing the various SHIP transcripts within hematopoietic cells.
ACKNOWLEDGMENT
We gratefully acknowledge the assistance of Mario Lioubin during the initial stages of this work, Irv Bernstein for help in obtaining human leukemia samples, members of the Rohrschneider laboratory for discussions and suggestions, and Ruth White for help in preparing the manuscript.
Supported in part by grants from the National Institutes of Health (CA 20551 [LRR]; CA 13539 [Children's Cancer Group]) and the US Department of Energy (DE-FG06-93ER61553 [B.T.]). P.A.A. was supported by a National Cancer Institute National Research Service Award (CA 66261).
Address reprint requests to Larry R. Rohrschneider, PhD, Division of Basic Science, Fred Hutchinson Cancer Research Center, 1124 Columbia St, B2-152, Seattle, WA 98104-2092.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal