Abstract
Vascular endothelial growth factor (VEGF ) is a multifunctional cytokine involved in angiogenesis, inflammation, and wound healing. It is secreted by a variety of tumor cell lines, including hematopoietic lines. Therefore, we investigated expression of VEGF and its receptors on fresh leukemic blasts. VEGF-specific transcripts were detected by polymerase chain reaction (PCR) in 20 of 28 patients with de novo acute myeloid leukemia (AML) and in 3 of 5 patients with secondary AML. Using immunocytochemistry, we found VEGF protein in 2 leukemic cell lines and in 8 AML patients, in concordance with PCR results. Supernatants of fresh leukemic cells from 24 AML patients contained significantly more VEGF than supernatants from bone marrow cells of 9 normal donors or of CD34-enriched cells from 3 normal volunteer donors as determined by an enzyme-linked immunosorbent assay. VEGF possesses two high-affinity receptors, KDR and FLT1. Using a sensitive nested PCR assay, we detected expression of FLT1 in 10 of 20 patients with de novo AML and 3 of 5 patients with secondary AML. KDR was expressed in 4 of 22 patients with de novo AML and 1 of 4 with secondary AML. To study possible paracrine growth stimulation of AML blasts, endothelial cells from human umbilical cords were incubated with increasing concentrations of VEGF. A dose-dependent increase of granulocyte-macrophage colony-stimulating factor secretion from endothelial cells was identified.
VASCULAR ENDOTHELIAL growth factor (VEGF ) is a multifunctional, secreted cytokine that stimulates endothelial cells to proliferate, to migrate, and to increase their permeability to plasma proteins, as has recently been reviewed.1 VEGF has been purified from conditioned media and has been molecularely cloned.2 It shows sequence homology to the A and B chain of PDGF and occurs naturally as a homodimer.3 Because of differential splicing, various isoforms exist. The most abundant have 121, 165, or 189 amino acids, respectively.4 VEGF expression is physiologically induced by hypoxia5 and plays a role in inflammatory diseases such as rheumatoid arthritis, wound healing, and diabetic retinopathy.3,6-8 Furthermore, VEGF is secreted by a variety of malignant tumors, including Kaposi's sarcoma, melanoma, glioma, and renal cell cancer, contributing to neoangiogenesis.9-11 Activation of oncogenes such as H-Ras, K-Ras, and Raf or overexpression of the Src oncogene may result in upregulation of VEGF secretion.12-14 On the other hand, wild-type p53 may suppress VEGF expression and angiogenesis.14
Two receptors for VEGF have been identified, FMS-like tyrosine kinase-1 (FLT1) and fetal liver kinase-1 (FLK-1/KDR).15-17 Both belong to the class III of receptor tyrosine kinase (RTK). They show sequence homology to each other and to other members of this class of RTK such as FMS and KIT. During hypoxia, at least one of the receptors, KDR, is upregulated.18
Because VEGF was originally cloned from a human leukemic cell line HL60,2 we investigated spontaneous expression of VEGF and its receptor by leukemic blasts from patients with acute myeloid leukemia (AML). Furthermore, we studied paracrine provision of granulocyte-macrophage colony-stimulating factor (GM-CSF ) by endothelial cells after stimulation with VEGF.
MATERIALS AND METHODS
Isolation of peripheral blood or bone marrow mononuclear cells.Peripheral blood or bone marrow samples from 28 consecutive patients with newly diagnosed or relapsed AML were obtained before chemotherapy. Mononuclear cells were separated by density gradient centrifugation on Ficoll-Hypaque (Pharmacia, Uppsala, Sweden). These preparations contained at least 90% myeloblasts as judged by morphologic criteria on Papenheim smears. A control group of 9 healthy volunteer bone marrow donors was included.
Preparation of CD34+ cells.Mononuclear cells from about 30 mL of bone marrow from normal human volunteers were recovered from a Ficoll-Hypaque gradient, washed twice, and counted. To select for CD34+ cells, about 108 mononuclear cells were applied to the Mini Macs column (Miltenyi Biotech, Bergisch Gladbach, Germany) according to the directions of the supplier. Recovered cells were evaluated for purity by fluorescence-activated cell sorter (FACS) analysis using a CD34 monoclonal antibody that recognizes a epitope different from the one used for the Mini Macs column (Anti HPCA-2; Becton Dickinson, San Jose, CA). The purity of obtained cells was at least 93% (not shown).
Preparation of c-DNA and reverse transcription-polymerase chain reaction (RT-PCR).Mononuclear cells were washed twice in phosphate-buffered saline (PBS) and collected by centrifugation. Total cellular RNA was prepared using Qiagen minicolumns (Qiagen, Hilden, Germany) as described by the manufacturer. One microgram of RNA was used for c-DNA synthesis, employing avian myeloblastosis virus (AMV) reverse transcriptase and oligo dT as primer.
Different aliquots of c-DNA were amplified with specific primers for VEGF, KDR, FLT1, and actin as control for successful c-DNA synthesis. For KDR and FLT1, two rounds were performed and for VEGF and actin one round was performed of 35 cycles of PCR in a programmable heat block at 94°C for 1.5 minutes, at 60°C for 3 minutes, and at 72°C for 4 minutes. PCR products were separated on 1% agarose gels, stained with ethidium bromide, and visualized under UV light. Primer sequences were as follows: VEGF sense primer, 5′-TCGGGCCTCCGAAACCATGA-3′; VEGF antisense primer, 5′-CCTGGTGAGAGATCTGGTTC-3′; KDR outer sense primer, 5′-GTCAAGGGAAAGACTACGTTGG-3′; KDR outer antisense primer, 5′-AGCAGTCCAGCATGGTCTG-3′; KDR inner sense primer, 5′-CAGCTTCCAAGTGGCTAAGG-3′; KDR inner antisense primer, 5′-TCAAAAATTGTTTCTGGGGC-3′; FLT1 outer sense primer, 5′-ATTTGTGATTTTGGCCTTGC-3′; FLT1 outer antisense primer, 5′-CAGGCTCATGAACTTGAAAGC-3′; FLT1 inner sense primer, 5′-CACCAAGAGCGACGTGTG-3′; FLT1 inner antisense primer, 5′-TTTTGGGTCTCTGTGCCAG-3′; actin sense primer, 5′-CGCTGCGCTGGTCGTCGACA-3′; and actin antisense primer, 5′-GTCACGCACGATTTCCCGCT-3′.
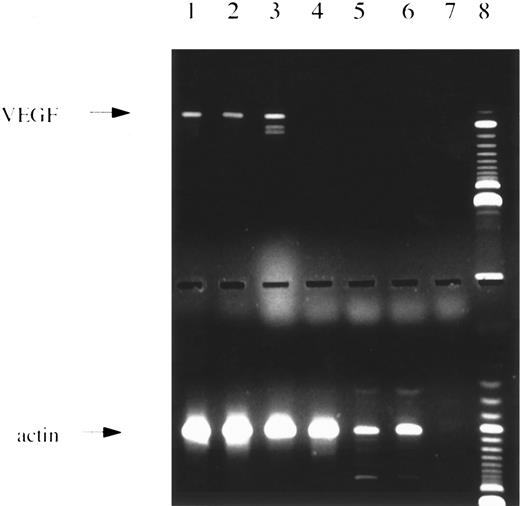
PCR analysis. (Upper panel) Amplification with VEGF-specific primers showing the 3 major splice variants. Lanes 1 through 4 and 6, patients with AML; lane 5, normal bone marrow; lane 7, water control; lane 8, 100-bp ladder size marker. (Lower panel) Same c-DNAs as in lanes given above amplified with actin-specific primers.
PCR analysis. (Upper panel) Amplification with VEGF-specific primers showing the 3 major splice variants. Lanes 1 through 4 and 6, patients with AML; lane 5, normal bone marrow; lane 7, water control; lane 8, 100-bp ladder size marker. (Lower panel) Same c-DNAs as in lanes given above amplified with actin-specific primers.
PCR Analysis: Number of Patients and Normal Controls PCR Positive for VEGF, KDR, or FLT-1 Per Number of Studied Subjects According to FAB Type
| . | VEGF . | KDR . | FLT-1 . |
|---|---|---|---|
| M0 | 1/1 (100) | 0/1 (0) | 0/1 (0) |
| M1 | 4/5 (80) | 0/2 (0) | 3/3 (100) |
| M2 | 2/3 (67) | 2/2 (100) | 1/2 (50) |
| M3 | 5/6 (83) | 0/4 (0) | 0/2 (0) |
| M4 | 6/11 (54) | 2/11 (18) | 5/9 (55) |
| M5 | 2/2 (100) | 0/2 (0) | 1/2 (50) |
| M6 | ND | ND | 0/1 (0) |
| Total | 20/28 (71) | 4/22 (18) | 10/20 (50) |
| Sek. AML | 3/5 (60) | 1/4 (25) | 3/5 (60) |
| Total | 23/32 (72) | 5/26 (19) | 13/25 (52) |
| Normal | 2/7 (28) | 0/8 (0) | 2/6 (33) |
| CD34+ | 1/3 (33) | 0/3 (0) | 1/3 (33) |
| . | VEGF . | KDR . | FLT-1 . |
|---|---|---|---|
| M0 | 1/1 (100) | 0/1 (0) | 0/1 (0) |
| M1 | 4/5 (80) | 0/2 (0) | 3/3 (100) |
| M2 | 2/3 (67) | 2/2 (100) | 1/2 (50) |
| M3 | 5/6 (83) | 0/4 (0) | 0/2 (0) |
| M4 | 6/11 (54) | 2/11 (18) | 5/9 (55) |
| M5 | 2/2 (100) | 0/2 (0) | 1/2 (50) |
| M6 | ND | ND | 0/1 (0) |
| Total | 20/28 (71) | 4/22 (18) | 10/20 (50) |
| Sek. AML | 3/5 (60) | 1/4 (25) | 3/5 (60) |
| Total | 23/32 (72) | 5/26 (19) | 13/25 (52) |
| Normal | 2/7 (28) | 0/8 (0) | 2/6 (33) |
| CD34+ | 1/3 (33) | 0/3 (0) | 1/3 (33) |
Values are the number positive/total number, with percentages in parentheses.
Abbreviation: ND, not done.
The VEGF primers correspond to sequences in the untranslated 5′ and 3′ region resulting in amplification of four different splice variants of a size of 516, 648, 720, and 771 bp. Primers specific for KDR, FLT1, and actin recognize coding sequences. PCR product sizes are, for KDR outer primer pair, 591 bp; for KDR inner primer pair, 213 bp; for FLT1 outer primer pair, 555 bp; for FLT1 inner primer pair, 196 bp; and for actin, 619 bp, respectively. To avoid cross-contamination, the set up of PCR reactions and gel electrophoresis was performed in different rooms using different sets of pipettes. Appropriate control reactions remained always negative. For each primer pair, PCR products were subcloned using the TA cloning kit (Invitrogen, San Diego, CA). Single bacterial colonies were picked and cultured overnight. Bacteria were diluted in destilled water and boiled for 10 minutes. PCR reactions were performed with primers recognizing sites flanking the PCR product (Dynal A and B; Dynal, Hamburg, Germany). Because one of the primers is biotinylated, strandseparation was performed using streptavidin-coated Dynabeads (Dynal). DNA sequencing was performed with the Taq cycle sequencing kit (Applied Biosystems, Foster City, CA) and the automated DNA sequencer 373A (Applied Biosystems). The identified DNA sequences corresponded to the published ones for all primer pairs.
Immunocytochemistry.Cells were transferred from culture into the chamber slides, where they were washed twice with PBS for 10 minutes. They were then fixed for 15 minutes with fresh paraformaldehyd (4%) at room temperature. Afterwards, they were washed in PBS and further processed for the visualization of VEGF. Polyclonal antibodies against VEGF were purchased from Santa Cruz (Santa Cruz, CA) and used in a 1:1,000 dilution. A detailed description of the methods used was reported elsewhere.19,20 In brief, we used an amplification combination of the peroxidase antiperoxidase (PAP) and the avidin-biotin-peroxidase complex (ABC) techniques. The peroxidase activity was visualized by means of the nickel-glucose oxidase technique.21 22 Controls included preabsorption with VEGF peptide, replacement of primary and secondary antibody with PBS, visualization of peroxidase only, and incubation of cells with normal rabbit serum (Sigma, Deisenhofen, Germany) in concentrations ranging from 0.1% to 0.01% instead of primary antiserum.
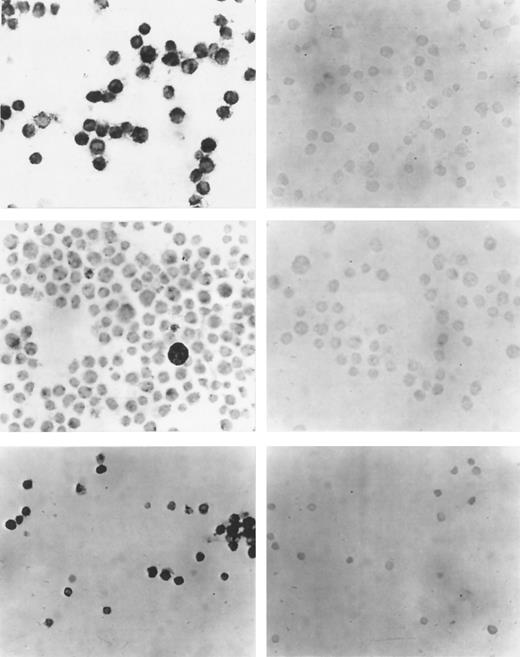
(Left side) Immunocytochemistry with a polyclonal antibody against VEGF. (Right side) Control reactions with the VEGF antibody after preabsorption with specific peptide. (Upper panel) Cell line U 937. (Middle panel) Cell line TF-1. (Lower panel) Cells from a representative patient with AML. VEGF expression in all three cases. (Original magnification × 100.)
(Left side) Immunocytochemistry with a polyclonal antibody against VEGF. (Right side) Control reactions with the VEGF antibody after preabsorption with specific peptide. (Upper panel) Cell line U 937. (Middle panel) Cell line TF-1. (Lower panel) Cells from a representative patient with AML. VEGF expression in all three cases. (Original magnification × 100.)
Preparation of supernatants from leukemic cells, normal bone marrow, and CD34+ cells.Mononuclear cells from 24 AML patients and from 9 normal bone marrow donors and CD34+ cells from 3 normal bone marrow donors were incubated at a density of 1 million cells per milliliter in RPMI 1640 medium with 10% fetal calf serum (FCS). After 3 days, supernatants were removed and stored at −70°C until further use.
Preparation and stimulation of human umbilical vein endothelial cells (HUVEC) with VEGF.HUVEC were isolated from umbilical cords as described.23 Cells were grown to confluency on fibronectin (GIBCO, Eggenstein, Germany) -coated plasticware in medium 199 (GIBCO) supplemented with 20% FCS and endothelial cell growth supplement (ECGS; Sigma) as indicated by the supplier, 50 μg/mL heparin, and glutamine. Cells were starved for 24 hours in medium 199 supplemented with 5% FCS. HUVEC were stimulated with VEGF (R&D Systems, Abingdon, UK) at a concentration of 0, 2, 10, or 50 ng/mL in starvation medium and incubated for 24, 48, or 72 hours. Supernatants were removed and kept at −70°C until further use. HUVEC were trypsinized and c-DNA was prepared as indicated above.
VEGF and GM-CSF enzyme-linked immunosorbent assay (ELISA).VEGF and GM-CSF ELISA kits were purchased from R&D Systems. VEGF and GM-CSF determinations were performed as indicated by the supplier. All samples were run in duplicate.
Statistical analysis.The nonparametric, signed rank Wilcoxon test was used for statistical analysis. The level of significance was set to 5%.
RESULTS
VEGF expression was studied by PCR analysis in leukemic blasts of 28 patients with de novo AML at diagnosis or relapse. Five additional patients with secondary AML were analyzed. Seventy-one percent of patients with de novo AML and 60% of patients with secondary AML were found to express VEGF transcripts. A representative example of a RT-RCR analysis is shown in Fig 1. Table 1 gives the rates of VEGF expression according to French-American-British (FAB) type. In normal bone marrow, VEGF m-RNA was detected in 2 of 7 volunteer donors, possibly due to expression in macrophages.
The cell lines U 937 and TF-1 and leukemic blasts from 8 patients were investigated for VEGF protein expression with immunocytochemistry using a modified APAP method. In concordance with the PCR analysis, U937 and TF-1 cells were positive for VEGF protein expression. In Fig 2, photomicrographs of immunocytochemistry of cell lines and representative AML patients are shown. A good correlation existed between PCR and immunocytochemistry results.
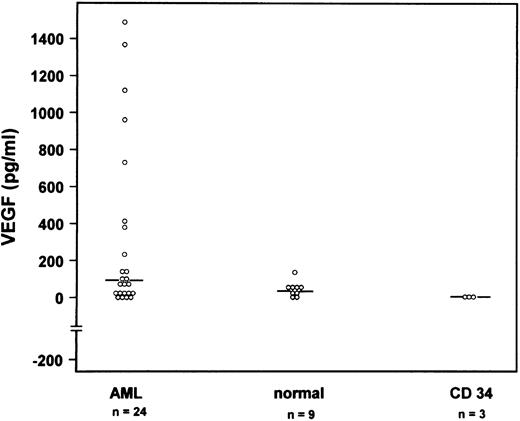
To obtain quantitative data on VEGF protein expression, we performed a VEGF ELISA of cell culture supernatants. One million fresh leukemic cells, normal bone marrow cells, or CD34+ cells per milliliter were cultured for 3 days. Supernatants were harvested and VEGF concentrations were determined with a VEGF ELISA. The mean VEGF concentration for 24 patients with AML was 313 pg/mL (range, 0 to 1,488 pg/mL), for low-density bone marrow cells of 9 normal volunteers was 46 pg/mL (range, 0 to 136 pg/mL), and for CD34+ cells from 3 independent donors was 3.4 pg/mL (range, 0 to 7.3 pg/mL; Fig 3). The difference between the VEGF concentrations of AML supernatants compared with the supernatants of normal bone marrow cells or CD34+ cells was statistically significant using the Wilcoxon test. VEGF receptor expression was studied by a sensitive nested RT-PCR. As shown in Table 1, KDR expression was an infrequent event detected in only 4 of 22 cases with de novo AML and 1 of 4 cases with secondary AML. KDR expression was found neither in normal bone marrow nor in enriched CD34+ cells (Table 1). On the contrary, FLT1 m-RNA was shown in 10 of 20 de novo AML cases and in 3 of 5 patients with secondary AML. Interestingly FLT1 expression was found in 2 of 6 normal bone marrow samples and 1 of 3 samples enriched for CD34+ cells.
VEGF concentrations in supernatants from fresh leukemic cells of 24 individual AML patients (first column), of bone marrow cells from 9 normal donors (second column), and of CD34+ cells from bone marrow from 3 donors (third column) after 3 days of culture determined by ELISA. Median concentrations are indicated by a horizontal line.
VEGF concentrations in supernatants from fresh leukemic cells of 24 individual AML patients (first column), of bone marrow cells from 9 normal donors (second column), and of CD34+ cells from bone marrow from 3 donors (third column) after 3 days of culture determined by ELISA. Median concentrations are indicated by a horizontal line.
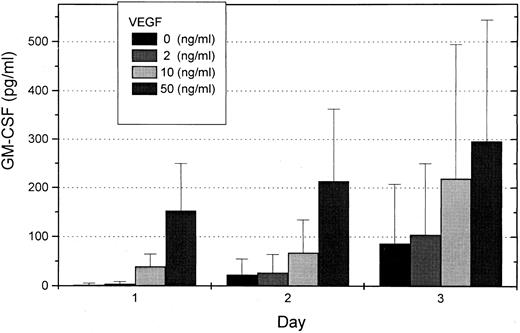
To investigate whether VEGF can induce secretion of hematopoietic growth factor in endothelial cells, we studied GM-CSF concentration in supernatants of HUVEC. Endothelial cells were prepared from human umbilical cords and grown to confluency. After starvation for 24 hours, HUVEC were stimulated in medium containing 0, 2, 10, or 50 ng/mL VEGF for 1 to 3 days. Supernatants were investigated for GM-CSF concentrations with a GM-CSF ELISA. A dose- and time-dependent concentration curve for GM-CSF induction by VEGF was found (Fig 4). RT-PCR analyses from endothelial cells with and without VEGF stimulation were also performed. Induction of GM-CSF m-RNA was found, indicating that GM-CSF induction occurred mainly on the transcriptional level.
Mean GM-CSF concentrations in supernatants of HUVEC after stimulation with 0, 2, 10, or 50 ng/mL VEGF for 1 to 3 days determined with a GM-CSF ELISA. Each column represents three independent experiments.
Mean GM-CSF concentrations in supernatants of HUVEC after stimulation with 0, 2, 10, or 50 ng/mL VEGF for 1 to 3 days determined with a GM-CSF ELISA. Each column represents three independent experiments.
DISCUSSION
VEGF expression of fresh leukemic blasts from patients with AML was studied on the transcriptional and protein level using PCR analysis and immunocytochemistry. Twenty-three of 32 patients with de novo or secondary AML expressed VEGF transcripts. Results of immunocytochemistry performed in 8 patients and two cell lines were compatible with the PCR analysis. Using the ELISA technique, VEGF concentrations of supernatants of fresh leukemic blasts of 24 AML patients were significantly higher than VEGF levels of supernatants of low-density bone marrow cells of 9 normal donors or of CD34-enriched cells of 3 additional volunteers.
Because leukemic blasts of about 50% of the AML patients express FLT1 receptors and 20% express KDR receptors, autocrine effects of VEGF on leukemic blasts are possible. We demonstrated binding of biotinylated VEGF to CD34+ TF-1 cells using a double-labeling FACS technique (data not shown). This indicates that VEGF receptors on leukemic cells are functional. VEGF has been evaluated for its capacity to induce colony formation on normal hematopoietic progenitor cells. It has been shown that VEGF enhances colony formation of more mature progenitor cells in concert with colony-stimulating factors, but suppresses growth of immature progenitors that depend on Steel factor or FLT3 ligand.24 We did not find colony formation of leukemic blasts of 3 AML patients with VEGF alone. The addition of VEGF to submaximal concentrations of granulocyte colony-stimulating factor and GM-CSF reduced leukemic colony formation slightly (own unpublished observation). But VEGF may exert effects on leukemic cells that do not result in proliferation. It has been shown that VEGF reduces apoptotic cell death of normal hematopoietic stem cells and CMK86 cells after ionizing radiation.25
Endothelial cells are found in the stroma layer of long-term bone marrow cultures in close contact to hematopoietic cells. Porcine brain microvascular endothelial cells have been shown to support expansion of human progenitor cells in concert with hematopoietic growth factors.26 In vivo, increased amounts of von Willebrand factor-positive endothelial cells have been detected in histologic sections of bone marrow biopsies from patients with AML.27 In multiple myeloma, increased microvessel density in bone marrow biopsies was significantly correlated with labeling index and prognosis.28 Therefore, paracrine exchange of growth factors may contribute to growth stimulation of AML cells. Because we could establish a dose-response curve between VEGF concentration in the culture medium and secretion of GM-CSF by HUVEC, such a paracrine loop may exist between AML cells and bone marrow endothelial cells. Similar paracrine mechanisms have been described for interleukin-1 and tumor necrosis factor-α secreted by leukemic blasts.29 30 Work in our laboratory is in progress to isolate human bone marrow endothelial cells and to study paracrine growth mechanisms between these cells and normal and leukemic hematopoietic cells.
Paracrine growth stimulation may not only be restricted to the bone marrow microenvironment, but may also take place at extramedullary sites. Circulating AML blasts may profit from paracrine provision of growth factors in various capillary beds. This may result in their expansion in peripheral blood. Under favorable conditions, extramedullary manifestations of AML, such as chloromas, gingiva hyperplasia, or organ infiltration, may be initiated by this mechanism.
Supported in part by grants from Deutsche Forschungsgemeinschaft Fi389/2-3, Gr 984/3-1, and Roggenbuck Stiftung.
Address reprint requests to Walter Fiedler, MD, Department of Oncology/Hematology, University Hospital Eppendorf, Martinistraβe 52, 20246 Hamburg, Germany.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal