Abstract
Previously, we found that, in the myeloproliferative disorder polycythemia vera (PV), circulating erythroid progenitor cells were hypersensitive to insulin-like growth factor I (IGF-I), an effect shown to occur through the IGF-I receptor. Also, in cells of PV patients, the IGF-I receptor was hyperphosphorylated on tyrosine residues under basal conditions, and its tyrosine phosphorylation in response to exogenous IGF-I was strongly augmented. Thus, because IGF-I appeared to play a role in the pathogenesis of PV, we wished to assess its level in the circulation of these patients. Normally, most of the circulating IGF-I is bound to specific high-affinity IGF binding proteins that can regulate its activity. We determined the circulating levels of IGF-I and two of its key binding proteins, IGFBP-1 and IGFBP-3. In two separate experiments, plasma samples from a total of 23 PV patients age- and sex-matched with 41 normal individuals were compared by radioimmunoassay. The levels of IGFBP-1 in patients with PV (37.80 ± 4.33 μg/L) were more than fourfold higher than in normals (9.34 ± 1.34 μg/L) or patients with secondary erythrocytosis (9.47 ± 1.96 μg/L), whereas the plasma concentrations of IGFBP-3 and IGF-I in these patients were similar to those of normal subjects. Because circulating IGFBP-1 levels may be influenced by insulin, we measured the concentrations of insulin in the same samples. Our data showed that the elevation of circulating IGFBP-1 in PV could not be attributed to low levels of insulin in these patients. The substantial increase in concentration of IGFBP-1 was confirmed on ligand blots performed with 125I–IGF-I. IGFBP-1 can be either inhibitory or stimulatory to the action of IGF-I under different conditions. We reasoned that if IGFBP-1 were stimulatory for erythropoiesis, an elevated IGFBP-1 level could help to explain the increased sensitivity to IGF-I observed in PV. If IGFBP-1 were inhibitory, it might suggest a compensatory mechanism in which a hyperphosphorylated IGF-I receptor in PV might induce a negative modulator of IGF-I action, in this case IGFBP-1. To distinguish between these two hypotheses, we titrated the effect of IGFBP-1 in the presence of IGF-I with respect to erythroid burst formation and found that IGFBP-1 was strikingly stimulatory. The elevated level of IGFBP-1 coupled with its ability to stimulate erythroid burst formation provide an attractive mechanism to account for the increased sensitivity of erythroid progenitor cells to IGF-I and the consequent overproduction of red blood cells characteristic of PV.
POLYCYTHEMIA VERA (PV) is a chronic myeloproliferative disorder characterized by hyperplasia of the three major myeloid lineages, but with particular emphasis on the erythroid lineage.1 A relentless overproduction of red blood cells occurs in the presence of normal oxygen saturation and with levels of serum erythropoietin often depressed.2 Like erythropoietin, insulin-like growth factor I (IGF-I) can stimulate erythroid progenitor cell proliferation and differentiation, but it normally requires a much higher concentration to bring about this effect.3 We have previously shown that, in PV, circulating erythroid progenitor cells are hypersensitive to IGF-I with respect to burst formation in serum-free culture and that this effect occurred through the IGF-I receptor.4 Recently, we have found that tyrosine phosphorylation of the IGF-I receptor β subunit was increased in the cells of PV patients in the absence of exogenous ligand. In the presence of ligand, tyrosine phosphorylation in PV cells occurred more rapidly, at lower concentrations of IGF-I, and attained a higher level of phosphorylation than in normal subjects.5 These findings strongly suggest that IGF-I and/or its receptor play a role in the pathogenesis of PV.
IGF-I is a highly conserved 70 amino acid 7.5-kD protein that is produced ubiquitously.6 It exerts acute anabolic effects on protein and carbohydrate metabolism in a wide variety of tissues and more long-term effects on cell proliferation and differentiation.7
There is very little free IGF-I in the circulation.8 Most of the IGF-I circulates bound to specific high-affinity binding proteins. These homologous but distinct IGF binding proteins (IGFBP-1 to -6) regulate both the bioavailability and the bioactivity of IGF-I in extracellular fluids.9 The binding proteins exhibit tissue and developmental specificity with respect to their expression and thus are thought to play an important physiologic role in IGF transport, localization, and action, but the exact mechanisms remain to be elucidated. Two key IGF binding proteins are IGFBP-3 and IGFBP-1. Most of the IGF-I in circulation is bound by IGFBP-3, whose circulating levels are more than 10-fold higher than any of the other binding proteins.9 IGFBP-3, regulated by growth hormone, is known to inhibit the biological activity of IGF-I.10-12 IGFBP-1, regulated by a variety of factors,13 is believed to be an acute modulator of IGF-I action, under different conditions either enhancing14 or attenuating its activity.15 16
The potential importance of IGF-I in PV prompted us to investigate the levels of IGF-I and its binding proteins IGFBP-1 and IGFBP-3 in the circulation of patients with this disorder. In the present study, we found that the level of circulating IGFBP-1 was substantially and specifically increased in PV, an observation that led us to ask whether this binding protein was inhibitory or stimulatory for erythroid progenitor cell proliferation.
MATERIALS AND METHODS
Patients and normal controls.Patients clinically diagnosed as having PV with the help of the PV Study Group guidelines17 were used in this study. All patients were being managed by phlebotomy at the time of study; one PV patient had previously been treated with hydroxyurea. Patients categorized as having secondary erythrocytosis in this study had an increase in the number of circulating red blood cells, but were clinically ruled out as having PV according to the PV Study Group guidelines.17 Normal volunteers were healthy individuals who were age- and sex-matched to PV patients.
Radioimmunoassay (RIA).The levels of IGFBP-1, IGFBP-3, IGF-I, and insulin were determined by radioimmunoassay as previously described.18 The assays for IGFBP-1 and IGFBP-3 were performed with commercially available kits (Diagnostic Systems Laboratories, Webster, TX). The kits had an intraassay variability of 5.2% and 6.7%, respectively. Their interassay variability was 4.3% for IGFBP-1 and 3.5% for IGFBP-3. Serum IGF-I levels were determined with a kit from Nichols Institute Diagnostics (San Juan Capistrano, CA), which had an intraassay variability of 3.0% and an interassay variability of 5.2%. Serum insulin levels were determined with a commercially available kit (ICN Biomedicals, Costa Mesa, CA), with a sensitivity of 1.4 mU/L and intraassay variations of 13.1% and 2.5% for 5.0 and 26.0 mU/L, respectively. All assays were performed in duplicate, and the same plasma sample was used for IGFBP-1, IGFBP-3, IGF-I, and insulin determinations.
Immunoprecipitation of IGFBP-1 and ligand blotting.In brief, after informed consent was obtained, patient and normal blood samples were collected in EDTA vacutainers (Becton Dickinson, Rutherford, NJ) and centrifuged at 450g for 10 minutes, the plasma was removed and was either stored at −20°C or used immediately. Samples of plasma were centrifuged at 3,000g and 200 μL of the resulting supernatant was precleared overnight at 4°C with 100 μL of a prepared 1:1 slurry of protein G-Sepharose (Sigma, St Louis, MO) plus dilution buffer (0.1% Triton X-100, 0.1% bovine hemoglobin in TSA [10mmol/L Tris-HCl, pH 8.0, 140 mmol/L NaCl, 0.025% NaN3]) and the supernatant was removed. Supernatants in a 200 μL final volume were placed in 1.5-mL microfuge tubes precoated with dilution buffer, a 1:500 dilution of rabbit antihuman IGFBP-1 polyclonal antibody (UBI, Lake Placid, NY) was added, and the contents of the tube were incubated at 4°C on an orbital shaker for 1 hour. Then, 25 μL of a prepared slurry of protein G-Sepharose was added to the tubes and the samples were incubated at 4°C overnight on an orbital shaker. The complexed Sepharose was then washed four times: twice in dilution buffer, once in TSA, and finally in 50 mmol/L Tris-Cl, pH 6.8. After the final wash, the immunoprecipitated samples were prepared with nondenaturing loading buffer and boiled for 5 minutes. The ligand blots were otherwise performed as previously described.19 The molecular weight markers (Bio-Rad, Hercules, CA) and prepared samples were subjected to electrophoresis on a 12% sodium dodecyl sulfate (SDS) gel under nondenaturing conditions. Proteins were electrophoretically transferred to supported nitrocellulose membrane (Bio-Rad). The membrane was blocked with nonspecific protein (1% fatty acid-free and globulin-free bovine serum albumin, 0.1% Tween 20 [T], in Tris-buffered saline [TBS]) for 1 hour. After primary blocking, the membrane was incubated overnight with fresh blocking solution plus 2 μCi 125I–IGF-I (ICN, Montreal, Quebec, Canada). The nitrocellulose membrane was subsequently washed with TTBS three times for 15 minutes each and the membrane was used to expose x-ray film at −70°C. The x-ray film was analyzed with an LKB Ultrascan XL Laser Densitometer (LKB Biochrom, Cambridge, UK) to quantitate the IGFBP-1 bands found in PV and normal individuals as well as the recombinant IGFBP-1 (UBI) used later in this study.
Cell preparations and colony assay in vitro.After informed consent, peripheral blood was obtained by venipuncture from healthy volunteer donors and patients and was immediately placed in a polypropylene tube containing 10 U/mL preservative-free sodium heparin (#820 5077MF; GIBCO, Grand Island, NY). Within 2 to 4 hours, the heparinized blood was layered onto 15 mL of Ficoll-Hypaque (Pharmacia, Montreal, Quebec, Canada) and the light-density peripheral blood mononuclear cells were collected after centrifugation at 450g for 30 minutes at room temperature. Cell suspensions were washed three times (450g for 10 minutes at room temperature) in α-minimal essential medium (αMEM) containing 0.1% fatty acid-free and globulin-free bovine serum albumin (FAF-BSA), and the cells were counted.
The culture assays for erythroid bursts were performed in serum-free medium (SeroZero/Stem Cell Medium [SCM]; US Patent No. 5,397,706; March 14, 1995) with methylcellulose as previously described,3 with the following changes. Recombinant human IGFBP-1 (UBI) was titrated (3 × 10−14 mol/L to 3 × 10−11 mol/L) in the presence of 3 × 10−11 mol/L recombinant human IGF-I (R&D Systems, Minneapolis, MN) in αMEM plus 0.1% FAF-BSA and preincubated for 1 hour at room temperature to allow complex formation to occur. The IGF-I/IGFBP-1 putative complex was added to Ficoll-separated peripheral blood mononuclear cells and the cells were plated as described.3 Hemoglobinized burst component colonies of ≥50 cells were scored at 14 days.
Statistical analyses.Data on concentrations of circulating IGF-I, IGFBP-1, IGFBP-3, and insulin obtained for each experiment were tested with the Wilcoxon rank sum test for nonparametric data. The mean ± standard error of the mean was calculated for the pooled data from the two experiments and the results were analyzed with the Student's unpaired t-test.
RESULTS
Levels of circulating IGF-I and its binding proteins IGFBP-3 and IGFBP-1 in patients with PV.Levels of plasma IGF-I, IGFBP-3, and IGFBP-1 were determined in two separate experiments for a total of 23 PV patients age- and sex-matched with those of 41 normal individuals. The results are presented in Figs 1 and 2. We found that the circulating level of IGF-I in patients with PV (151.30 ± 13.73 μg/L) was not significantly different (.7 > P > .6) from that of normal controls (143.85 ± 11.35 μg/L). Similarly, when we compared the circulating levels of IGFBP-3, we found that the level in PV (2.89 ± 0.24 mg/L) was not different (.3 > P > .2) from that of the normal individuals (2.47 ± 0.14 mg/L). However, the level of circulating IGFBP-1 in PV (37.80 ± 4.33 μg/L) was significantly greater (P < .001) than that of normal individuals (9.34 ± 1.34 μg/L). Thus, in PV, the circulating levels of IGFBP-1 were more than fourfold higher than in the normal controls, whereas the plasma concentrations of IGFBP-3 and IGF-I in these same patients were not significantly different from those of normal individuals. Because high levels of IGFBP-1 can be associated with low levels of insulin,20 we determined the concentrations of insulin in these same plasma samples in PV (144.47 ± 37.22 pmol/L) and found them not to be significantly different (.10 > P > .05) from those of normal individuals (222.73 ± 25.93 pmol/L). As an independent test, we compared IGFBP-1 concentrations in a group of 5 PV patients and 5 normal individuals with comparable (.9 > P > .8) levels of insulin (171.60 ± 18.26 and 176.80 ± 19.03 pmol/L, respectively). We determined that the level of IGFBP-1 in these PV patients (23.04 ± 4.23 μg/L) was significantly greater than in the normal subjects (3.86 ± 1.32 μg/L; .02 < P < .05) for comparable levels of insulin. Thus, the elevation of IGFBP-1 in PV was independent of insulin and therefore could not be attributed to low levels of insulin in these patients.
Circulating levels of IGF-I, two key binding proteins, and insulin as determined by RIA for patients with PV and normal individuals. Data from two separate experiments (experiment no. 1 [○, •] and experiment no. 2 [□, ▪]) compare plasma levels of IGF-I in micrograms per liter (A), IGFBP-3 in milligrams per liter (B), IGFBP-1 in micrograms per liter (C), and insulin in picomoles per liter (D) for age- and sex-matched normal individuals (N) and patients with PV. Each point represents one individual, whereas the horizontal bars indicate the median value for each group. Levels of IGF-I, IGFBP-3, and insulin are normal in patients with PV, but their IGFBP-1 levels are significantly elevated.
Circulating levels of IGF-I, two key binding proteins, and insulin as determined by RIA for patients with PV and normal individuals. Data from two separate experiments (experiment no. 1 [○, •] and experiment no. 2 [□, ▪]) compare plasma levels of IGF-I in micrograms per liter (A), IGFBP-3 in milligrams per liter (B), IGFBP-1 in micrograms per liter (C), and insulin in picomoles per liter (D) for age- and sex-matched normal individuals (N) and patients with PV. Each point represents one individual, whereas the horizontal bars indicate the median value for each group. Levels of IGF-I, IGFBP-3, and insulin are normal in patients with PV, but their IGFBP-1 levels are significantly elevated.
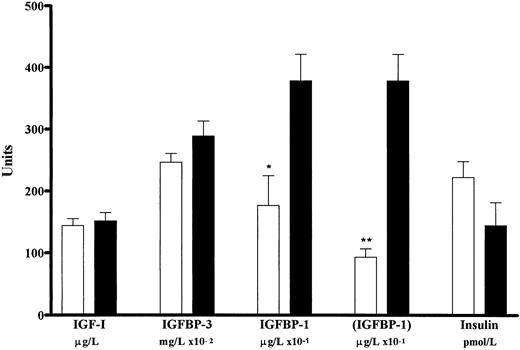
Plasma levels of IGF-I, IGFBP-3, IGFBP-1, and insulin in normal individuals and patients with PV (pooled data from two experiments). Values for IGF-I are given in micrograms per liter (n = 41 for normal and n = 23 for PV), IGFBP-3 in milligrams per liter × 10−2 (n = 41 for normal and n = 23 for PV), IGFBP-I in micrograms per liter × 10−1 (n = 41 for normal and n = 23 for PV), (IGFBP-1) in micrograms per liter × 10−1 (n = 38 for normal and n = 23 for PV), and insulin in picomoles per liter (n = 40 for normal and n = 23 for PV) for normal individuals (□) and patients with PV (▪). The level of plasma IGFBP-1 in PV is significantly higher than that in normal individuals (*P < .01; **P < .001). However, the circulating levels of IGF-I, IGFBP-3, and insulin are not significantly different in PV versus normal. Three values for IGFBP-1 greater than 104 μg/L in the normal (N) of experiment 1 (Fig 1) were statistically shown to lie outside the confidence limits of both the normal (P < .001) and the PV (P < .01) populations sampled. The mean ± standard error of the mean for the level of IGFBP-1 in normals, if these values were to be excluded, is given in brackets.
Plasma levels of IGF-I, IGFBP-3, IGFBP-1, and insulin in normal individuals and patients with PV (pooled data from two experiments). Values for IGF-I are given in micrograms per liter (n = 41 for normal and n = 23 for PV), IGFBP-3 in milligrams per liter × 10−2 (n = 41 for normal and n = 23 for PV), IGFBP-I in micrograms per liter × 10−1 (n = 41 for normal and n = 23 for PV), (IGFBP-1) in micrograms per liter × 10−1 (n = 38 for normal and n = 23 for PV), and insulin in picomoles per liter (n = 40 for normal and n = 23 for PV) for normal individuals (□) and patients with PV (▪). The level of plasma IGFBP-1 in PV is significantly higher than that in normal individuals (*P < .01; **P < .001). However, the circulating levels of IGF-I, IGFBP-3, and insulin are not significantly different in PV versus normal. Three values for IGFBP-1 greater than 104 μg/L in the normal (N) of experiment 1 (Fig 1) were statistically shown to lie outside the confidence limits of both the normal (P < .001) and the PV (P < .01) populations sampled. The mean ± standard error of the mean for the level of IGFBP-1 in normals, if these values were to be excluded, is given in brackets.
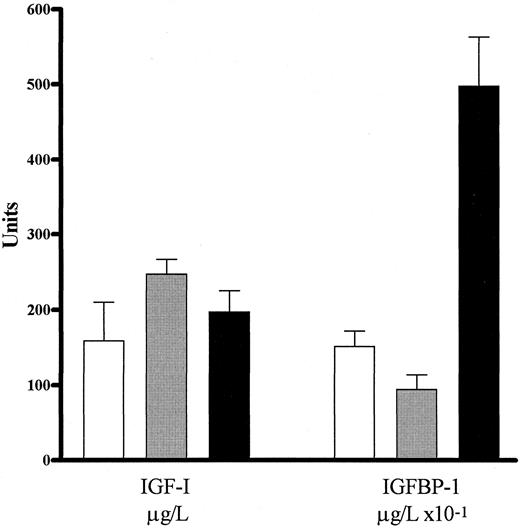
We wished to determine whether IGFBP-1 was specifically elevated in patients with PV or was simply either the cause or the result of a nonspecific increase in the number of red blood cells. In a separate experiment, we compared the levels of circulating IGF-I and IGFBP-1 in 7 patients with secondary erythrocytosis, 3 normal individuals, and 4 patients with PV. The results are shown in Fig 3. We found that the plasma IGF-I levels in patients with secondary erythrocytosis (237.67 ± 24.62 μg/L) were not different (.2 > P > .1) from those of patients with PV (176.25 ± 34.86 μg/L). Also, the plasma levels in PV were not different (.5 > P > .4) from normal (145.00 ± 25.51 μg/L). In contrast, IGF-I levels in patients with secondary erythrocytosis appeared to be slightly elevated in comparison to normal (.05 > P > .02). The plasma IGFBP-1 levels in patients with secondary erythrocytosis (9.47 ± 1.96 μg/L) were not significantly different (.1 < P < .05) from those of normal individuals (15.17 ± 2.07 μg/L). However, the IGFBP-1 levels in patients with PV (49.7 ± 6.56 μg/L) were significantly greater (P < .001) than those observed for either patients with secondary erythrocytosis, or normal individuals. Thus, it appeared that IGFBP-1 is specifically elevated in PV.
RIA data for the levels of circulating IGF-I and IGFBP-1 for (□) normal individuals, () patients with secondary erythrocytosis (E), and (▪) patients with PV are shown as the mean ± standard error of the mean. Values are given in micrograms per liter for IGF-I (n = 3 for normal, n = 6 for E, and n = 4 for PV) and in micrograms per liter × 10−1 for IGFBP-1 (n = 3 for normal, n = 7 for E, and n = 4 for PV). The level of IGF-I in PV was not different from those in patients with secondary erythrocytosis or normals (.2 < P < .1 and .5 < P < .4, respectively). In contrast, IGFBP-1 levels were significantly higher in PV compared with either patients with secondary erythrocytosis or normal individuals (P < .001). Therefore, IGFBP-1 appeared to be specifically elevated in patients with PV.
RIA data for the levels of circulating IGF-I and IGFBP-1 for (□) normal individuals, () patients with secondary erythrocytosis (E), and (▪) patients with PV are shown as the mean ± standard error of the mean. Values are given in micrograms per liter for IGF-I (n = 3 for normal, n = 6 for E, and n = 4 for PV) and in micrograms per liter × 10−1 for IGFBP-1 (n = 3 for normal, n = 7 for E, and n = 4 for PV). The level of IGF-I in PV was not different from those in patients with secondary erythrocytosis or normals (.2 < P < .1 and .5 < P < .4, respectively). In contrast, IGFBP-1 levels were significantly higher in PV compared with either patients with secondary erythrocytosis or normal individuals (P < .001). Therefore, IGFBP-1 appeared to be specifically elevated in patients with PV.
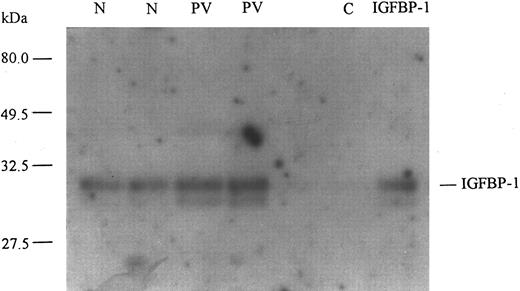
Plasma samples obtained from normal individuals and PV patients were used to immunoprecipitate IGFBP-1. These immunoprecipitates were then subjected to ligand blotting with 125I–IGF-I (Fig 4). A representative autoradiograph shows the relative amounts of IGFBP-1 in plasma samples from normal individuals and patients with PV. It demonstrates that the amount of circulating IGFBP-1 was increased in PV over that in normals. The increase was determined to be approximately threefold by densitometric analysis. Thus, the ligand blots confirmed the specific increase of IGFBP-1 in PV observed by RIA. They also showed that there are at least two species of IGFBP-1 present in circulation of both PV patients and normals. The approximately 30-kD band represents a putative nonphosphorylated species of IGFBP-1, whereas the approximately 28-kD band probably represents a phosphorylated species.21 The ratio of nonphosphorylated to phosphorylated IGFBP-1 (approximately 4:1 by densitometric analysis) appeared to be similar in PV and normal, both of which appeared to be similar to that of the recombinant IGFBP-1 used later in this study.
Ligand blot of circulating IGFBP-1 in normal individuals and patients with PV. A representative ligand blot shows plasma levels of IGFBP-1 in PV patients and normal individuals (N). Plasma samples from 2 PV patients and 2 age- and sex- matched normals were used to immunoprecipitate IGFBP-1 and these were separated by SDS-polyacrylamide gel electrophoresis under nondenaturing conditions, transferred to nitrocellulose membrane, and ligand blotted with 125I–IGF-I. The membrane was then washed and used to expose x-ray film. Recombinant human IGFBP-1 (UBI) was run as a positive control and C indicates a negative control for the antibody used. Relative molecular weights are shown in kilodaltons and were determined by prestained molecular weight markers (Bio-Rad). The position of the putative nonphosphorylated (30 kD) IGFBP-1 species is indicated. Using densitometric analysis, we found the amounts of nonphosphorylated IGFBP-1 in the 2 normal samples to be 1.021 and 1.167, respectively, in arbitrary units; in the 2 PV patients, the amounts were found to be 2.897 and 3.115, respectively, whereas in the recombinant control, it was 1.067. The amounts of the phosphorylated IGFBP-1 species (28 kD) were 0.188 and 0.256 in the 2 normal subjects, respectively; in the 2 PV patients, they were 0.707 and 0.736, respectively, whereas in the recombinant control it was 0.292. The ligand blots confirm that the level of circulating IGFBP-1 in PV is greater than in normal.
Ligand blot of circulating IGFBP-1 in normal individuals and patients with PV. A representative ligand blot shows plasma levels of IGFBP-1 in PV patients and normal individuals (N). Plasma samples from 2 PV patients and 2 age- and sex- matched normals were used to immunoprecipitate IGFBP-1 and these were separated by SDS-polyacrylamide gel electrophoresis under nondenaturing conditions, transferred to nitrocellulose membrane, and ligand blotted with 125I–IGF-I. The membrane was then washed and used to expose x-ray film. Recombinant human IGFBP-1 (UBI) was run as a positive control and C indicates a negative control for the antibody used. Relative molecular weights are shown in kilodaltons and were determined by prestained molecular weight markers (Bio-Rad). The position of the putative nonphosphorylated (30 kD) IGFBP-1 species is indicated. Using densitometric analysis, we found the amounts of nonphosphorylated IGFBP-1 in the 2 normal samples to be 1.021 and 1.167, respectively, in arbitrary units; in the 2 PV patients, the amounts were found to be 2.897 and 3.115, respectively, whereas in the recombinant control, it was 1.067. The amounts of the phosphorylated IGFBP-1 species (28 kD) were 0.188 and 0.256 in the 2 normal subjects, respectively; in the 2 PV patients, they were 0.707 and 0.736, respectively, whereas in the recombinant control it was 0.292. The ligand blots confirm that the level of circulating IGFBP-1 in PV is greater than in normal.
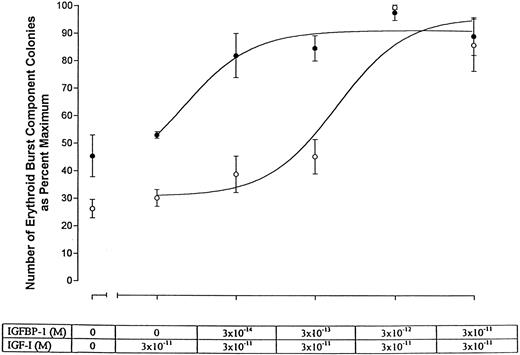
Effect of IGFBP-1 on erythroid burst formation in vitro.Increased levels of IGFBP-1 in PV could have strikingly different consequences depending on whether this binding protein had an inhibitory or stimulatory effect on the action of IGF-I. To determine the biological effect of IGFBP-1 on erythroid burst formation in vitro, we titrated IGFBP-1 in the presence of IGF-I and assayed its effects on erythroid burst formation in semisolid serum-free culture. IGFBP-1 was titrated in the presence of 3 × 10−11 mol/L IGF-I, a concentration previously shown to be stimulatory for erythroid burst formation in PV patients but not in normal subjects.5 The results are presented in Fig 5 and Table 1. We found that in both normal individuals and patients with PV, IGFBP-1 was strikingly stimulatory for erythroid burst formation in the presence of IGF-I. This stimulatory action occurred in the normal at IGFBP-1 concentrations as low as 3 × 10−12 mol/L IGF-I. It should be noted that normal erythroid progenitors usually do not respond to IGF-I levels less than 10−10 mol/L. Therefore, IGFBP-1 appeared to be able to stimulate erythroid burst formation by markedly increasing progenitor cell sensitivity to IGF-I. In PV, the same stimulation of erythroid burst formation occurred at IGFBP-1 concentrations as low as 3 × 10−14 mol/L. This indicates that, although IGFBP-1 stimulated erythroid burst formation in both PV and normal, progenitor cells in PV appeared to require considerably less IGFBP-1. In terms of half-maximum effects, erythroid burst formation occurred in PV at an IGFBP-1 concentration ∼60-fold lower in PV (∼1 × 10−14 mol/L) than in normal (∼6 × 10−13 mol/L).
Plot of the number of erythroid burst component colonies as a percentage of maximum versus the molar concentration of IGFBP-1 in the presence of 3 × 10−11 mol/L IGF-I. Representative data obtained for cells of normal individuals (○) and PV patients (•) are shown. The half-maximum value for PV occurs at ∼1 × 10−14 mol/L IGFBP-1, whereas that for the normal occurs at ∼6 × 10−13 mol/L IGFBP-1. Thus, with respect to burst formation, erythroid progenitor cells in PV are approximately 60-fold more sensitive than normal to IGFBP-1 in the presence of IGF-I.
Plot of the number of erythroid burst component colonies as a percentage of maximum versus the molar concentration of IGFBP-1 in the presence of 3 × 10−11 mol/L IGF-I. Representative data obtained for cells of normal individuals (○) and PV patients (•) are shown. The half-maximum value for PV occurs at ∼1 × 10−14 mol/L IGFBP-1, whereas that for the normal occurs at ∼6 × 10−13 mol/L IGFBP-1. Thus, with respect to burst formation, erythroid progenitor cells in PV are approximately 60-fold more sensitive than normal to IGFBP-1 in the presence of IGF-I.
Burst Component Colonies Produced In Vitro by Normal and PV Erythroid Progenitor Cells With Increasing Amounts of IGFBP-1 in the Presence of IGF-I
| IGF-I (mol/L) | 3 × 10−11 | 3 × 10−11 | 3 × 10−11 | 3 × 10−11 | 3 × 10−11 |
| IGFBP-1 (mol/L) | 0 | 3 × 10−14 | 3 × 10−13 | 3 × 10−12 | 3 × 10−11 |
| Normal | 89 ± 7.2 | 84 ± 7.8 | 101 ± 8.0 | 164 ± 4.7 | 166 ± 12.8 |
| Normal | 72 ± 3.3 | 92 ± 8.8 | 107 ± 5.0 | 244 ± 24.6 | 209 ± 25.4 |
| PV | 134 ± 3.3 | 207 ± 13.4 | 214 ± 4.7 | 247 ± 10.0 | 228 ± 27.0 |
| PV | 29 ± 2.0 | 39 ± 3.4 | 61 ± 7.1 | 74 ± 5.0 | 88 ± 4.8 |
| PV | 202 ± 13.4 | 218 ± 9.0 | 186 ± 11.3 | 318 ± 9.6 | 280 ± 15.0 |
| IGF-I (mol/L) | 3 × 10−11 | 3 × 10−11 | 3 × 10−11 | 3 × 10−11 | 3 × 10−11 |
| IGFBP-1 (mol/L) | 0 | 3 × 10−14 | 3 × 10−13 | 3 × 10−12 | 3 × 10−11 |
| Normal | 89 ± 7.2 | 84 ± 7.8 | 101 ± 8.0 | 164 ± 4.7 | 166 ± 12.8 |
| Normal | 72 ± 3.3 | 92 ± 8.8 | 107 ± 5.0 | 244 ± 24.6 | 209 ± 25.4 |
| PV | 134 ± 3.3 | 207 ± 13.4 | 214 ± 4.7 | 247 ± 10.0 | 228 ± 27.0 |
| PV | 29 ± 2.0 | 39 ± 3.4 | 61 ± 7.1 | 74 ± 5.0 | 88 ± 4.8 |
| PV | 202 ± 13.4 | 218 ± 9.0 | 186 ± 11.3 | 318 ± 9.6 | 280 ± 15.0 |
Data from titrations of IGFBP-1 in the presence of 3 × 10−11 mol/L IGF-I with respect to erythroid burst formation by PV and normal progenitor cells in vitro. Data for cells of 2 normal individuals and 3 PV patients are given as the mean number of burst component colonies ± the standard error of the mean and were used to plot the curve shown in Fig 5.
DISCUSSION
We have made two main observations in the present study: (1) In vivo in patients with PV, the circulating level of IGFBP-1 was substantially and specifically elevated over the normal. (2) In vitro in cells of both PV patients and normal individuals, IGFBP-1 in the presence of IGF-I acted as a powerful stimulator of erythroid burst formation.
With RIA we found that circulating IGFBP-1 was more than fourfold higher in PV patients (37.80 ± 4.33 μg/L) than in normal individuals (9.34 ± 1.34 μg/L) or in patients with secondary erythrocytosis (9.47 ± 1.96 μg/L), whereas the plasma concentrations of IGF-I and IGFBP-3 in these patients were not significantly different from normal. Ligand blots confirmed that the plasma levels of IGFBP-1 in PV patients were increased. Although IGFBP-1 levels are regulated by a number of hormonal and metabolic factors, insulin is normally considered to be a primary physiologic regulator of IGFBP-1 in plasma.13,22 Insulin is known to depress IGFBP-1 in vivo20,23 and to inhibit its production by human fetal liver in explant culture and by the human hepatoma-derived cell line HepG2.24 Therefore, because high levels of IGFBP-1 can be associated with low insulin levels, we compared IGFBP-1 concentrations in PV versus normal at comparable levels of insulin. We showed higher (.05 > P > .02) IGFBP-1 levels in PV than in normal individuals for comparable levels of insulin, and thus ruled out low insulin in the circulation of these patients as a causative factor for the elevation of IGFBP-1 in PV. IGF-I itself has also been implicated in the regulation of IGFBP-1 levels.25 26 However, in the present study, we found plasma IGF-I levels in PV to be normal (Figs 1, 2, and 3). Thus, the elevation of IGFBP-1 appears to be related to the PV defect and is not simply due to the metabolic fluctuations to which this binding protein is susceptible. Also, the fact that circulating levels of IGFBP-1 are not elevated in patients with secondary erythrocytosis indicates that the increase is specific to PV and thus part of the PV defect, and cannot be attributed to events that nonspecifically increase the number of circulating red blood cells. Further work will be necessary to determine whether the elevation of IGFBP-1 concentration in PV is due to its increased biosynthesis, mobilization from pools, diminished clearance from the circulation, or a combination of any of these factors.
Interestingly, we found the level of IGF-I to be slightly elevated in patients with secondary erythrocytosis (237 ± 24.62) compared with normal individuals (145.00 ± 25.51; Fig 3). Because IGF-I is known to play a stimulatory role in erythropoiesis,3 it may be that the increased IGF-I in circulation is in part responsible for the observed erythrocytosis.27
IGFBP-1 has been shown to play either a stimulatory14,21,28 or an inhibitory21,28,29 role in modulating IGF-I action under different experimental conditions. In the case of MDA-231 cells, IGFBP-1 bound IGF-I and augmented its mitogenic effects. These cells normally proliferate in response to exogenous IGF-I, but exposure to IGFBP-1 in addition to IGF-I enhanced their mitogenic response by approximately twofold.30 In contrast, in the case of MG-63 osteosarcoma cells, which also proliferate in response to exogenous IGF-I, the addition of IGFBP-1 in the presence of IGF-I suppressed subsequent stimulation of DNA synthesis15; this effect was believed to be due to IGF/IGFBP-1 complex formation that effectively prevented IGF-I binding to its receptor.
We reasoned that if IGFBP-1 were stimulatory to the action of IGF-I, an elevated IGFBP-1 level could help to explain the increased IGF-I sensitivity observed in PV. The IGF-I/IGFBP-1 complex could facilitate ligand interactions with the IGF-I receptor and in such a system, less IGF-I would be required to activate the receptor. In this way, even normal levels of IGF-I could constitutively activate the IGF-I receptor.5 In contrast, if IGFBP-1 were inhibitory, it might suggest a compensatory mechanism. In PV, the hyperphosphorylated IGF-I receptor5 could result in an amplified mitogenic signal that might be interpreted by the cells as an increase in the circulating level of IGF-I. The cells could then respond by increasing the synthesis/release of IGFBP-1, in this case a putative negative regulator of IGF-I action. To distinguish between these two hypotheses, we titrated the effect of IGFBP-1 with respect to its ability to affect the number of erythroid burst component colonies under serum-free conditions in the presence of IGF-I. We found that the action of IGFBP-1 was clearly stimulatory for erythroid burst formation (Fig 5 and Table 1). IGFBP-1 increased the number of burst component colonies in cells obtained from both normal individuals and patients with PV. It also increased the sensitivity of erythroid progenitor cells in PV and normal by approximately two orders of magnitude. However, in PV, we found that a substantially lower concentration of IGFBP-1 was required for this effect. Whether it is IGFBP-1 alone or the combination of IGFBP-1 with IGF-I that is responsible for the increased erythoid burst formation seen in this work is at present under investigation.
Although our data are consistent with the notion that IGFBP-1 acts as a positive modulator of IGF-I action, the basis for the stimulatory nature of IGFBP-1 seen in the present work remains to be elucidated. However, previous work may help to shed light on this issue. Two isoforms of IGFBP-1 isolated from human amniotic fluid showed that one was able to potentiate the effects of IGF-I, whereas the other was inhibitory.21 Subsequent work has suggested a relationship between the biological activity of IGFBP-1 and its state of phosphorylation. Cultured human endometrial stromal cells from proliferative phase endometrium secrete nonphosphorylated IGFBP-1, whereas cells from the secretory nonproliferative phase endometrium secrete a heavily phosphorylated IGFBP-1.31 HepG2 cells produce an IGFBP-1 that has been used in many studies showing that it is inhibitory; this IGFBP-1 is predominantly phosphorylated,32 whereas a recombinant IGFBP-1 that has been shown to be stimulatory is nonphosphorylated.33 It is also known that phosphorylated IGFBP-1 binds IGF-I with a higher affinity than its nonphosphorylated isoform,32 thus effectively inhibiting IGF-I activity by preventing its interaction with the receptor. Nonphosphorylated IGFBP-1, which binds IGF-I less tightly, may be able to transport and release IGF-I to the receptor, thereby localizing IGF-I activity and potentiating its action. From our data it appears that, as in the case of human amniotic fluid, there are at least two isoforms of IGFBP-1 in the circulation; however, further work is required to elucidate the biological activities of these isoforms. Experiments are currently underway in our laboratory to determine the phosphorylation status of circulating IGFBP-1 in PV patients and normal individuals.
In PV, the marked increase in red blood cell mass is not due to elevated erythropoietin levels; also, as we have shown in this study, it is not due to elevated levels of free IGF-I in circulation. As our results indicate, plasma IGF-I levels in PV are not different from those in normal individuals. However, erythroid progenitor cells are hypersensitive to IGF-I in this disorder. This could be due to an intrinsic lesion in the IGF-I receptor signalling pathway, or it could be due to an extrareceptor event(s). We have shown that the level of IGFBP-1 is increased fourfold in patients with PV and that, in the presence of IGF-I, IGFBP-1 is stimulatory for erythroid burst formation. Our findings thus raise interesting new questions regarding the regulatory role of IGFBP-1 in vivo. The increase in a positive modulator of IGF-I action in PV provides an attractive mechanism to account for the heightened sensitivity of erythroid progenitor cells to IGF-I in this disorder. They also suggest that the sustained high circulating level of this positive regulator could be an important factor in the constitutively excessive erythropoiesis characteristic of PV.
ACKNOWLEDGMENT
We extend our thanks to the patients and other individuals who kindly volunteered to take part in this study and to Drs Dominick Amato and Peter Pinkerton, who referred these subjects to us and who were helpful throughout the course of the study. We also thank Denise Eskinazi and Anne Spalding for their expert technical assistance and Dr Don Redelmeier for help with the statistical analysis.
Supported by a grant from the Medical Research Council of Canada (MA3969).
Presented in part at the American Society of Hematology Meeting, Seattle, WA, December 1995 (Blood 86:151a, 1995 [abstr, suppl 1]).
Address reprint requests to Arthur Axelrad, MD, PhD, FRSC, Department of Anatomy and Cell Biology, Faculty of Medicine, University of Toronto, 1 Kings College Circle, Medical Sciences Bldg, Room 6344, Toronto, Ontario, Canada M5S 1A8.

![Fig. 1. Circulating levels of IGF-I, two key binding proteins, and insulin as determined by RIA for patients with PV and normal individuals. Data from two separate experiments (experiment no. 1 [○, •] and experiment no. 2 [□, ▪]) compare plasma levels of IGF-I in micrograms per liter (A), IGFBP-3 in milligrams per liter (B), IGFBP-1 in micrograms per liter (C), and insulin in picomoles per liter (D) for age- and sex-matched normal individuals (N) and patients with PV. Each point represents one individual, whereas the horizontal bars indicate the median value for each group. Levels of IGF-I, IGFBP-3, and insulin are normal in patients with PV, but their IGFBP-1 levels are significantly elevated.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/6/10.1182_blood.v89.6.1862/3/m_bl_0065f1.jpeg?Expires=1769080415&Signature=jQaIfvtljPw6DincMbqurDuXgRi-TTh-iIe0pIWXZ0YD3savvOFv88IjZM1DBJmbJ5rg9fHzd4bCUcEbbu7VKftqyaJ3cBUQm~9fvw1y1JGuk~j-VoPAaG4iMGWoMfaXAhrf~NwWty7-2VsOGgC80DB3XUUJ8r-~Py5Nju43R4NhJkKJZinHM2YQgymi54Ae~E8mVAq8LdP12onoONYbq1YryIwKyka-R~Qxviw6t-mbbBPVnAiR0PolH8abhKcFbA9xvJZXivqSowrU2yfqK8~lYT90uuas~n91mvRYg0X2yxVNwCE3Qu6Dvo9ZceKXjpPSsupHjarNC0x2wsWtNA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal