Abstract
Patients with myelodysplastic syndrome (MDS) have ineffective in vivo and in vitro erythropoiesis, characterized by an impaired response to erythropoietin (Epo). We examined proliferation and maturation of MDS marrow cells in response to Epo in more detail. Epo-dependent DNA synthesis as well as induction of GATA-1 binding activity in marrow cells from 15 MDS cases were severely reduced as compared with normal bone marrow (NBM). Additionally, the appearance of morphologically identifiable erythroid cells was decreased in MDS cell cultures. These data indicate that both the Epo-dependent proliferation as well as the differentiation induction by Epo is suppressed. To study more upstream events of the Epo signal transduction route we investigated activation of the signal transducer and activator of transcription (STAT) 5. In all 15 MDS samples tested, STAT5 activation was absent or greatly suppressed in response to Epo. In contrast, interleukin-3 induced a normal STAT5 response in MDS cells. Further, in MDS the subset of CD71+ BM cells that is phenotypically similar to Epo-responsive cells in normal marrow, was present. We conclude that the Epo response in MDS is disturbed at an early point in the Epo receptor (EpoR) signal transduction pathway.
THE MYELODYSPLASTIC syndromes (MDS) constitute a heterogeneous group of disorders of the hematopoietic pluripotent stem cell, characterized by ineffective hematopoiesis.1 In the majority of cases anemia is an early and prominent clinical symptom. In a considerable number of cases the disease progresses to acute leukemia. Bone marrow (BM) biopsy samples from MDS patients generally show normocellularity or hypercellularity with active erythropoiesis. When BM cells from MDS patients are cultured in vitro in the presence of erythropoietin (Epo), the formation of erythroid colonies is reduced as compared with colony formation by cells from normal marrow.2-6 Ineffective erythropoiesis is not due to a lack of Epo. Patients with MDS have normal or elevated serum levels of Epo,7,8 and treatment with pharmacological doses of Epo only infrequently improves the anemia significantly.9 To elucidate the mechanism of impaired erythropoiesis in MDS, we set out to investigate the role of the Epo receptor (EpoR) and its signal transduction.
Epo, the major regulator of erythropoiesis, is involved in survival, proliferation, and differentiation of erythroid progenitors. The EpoR is expressed at relatively low levels on BFU-E (burst-forming units-erythroid, erythroid progenitors), but expression increases with erythroid maturation toward late erythroblasts. EpoRs are lost on more mature erythroid cells.10-13 The EpoR is a member of the hemopoietin and cytokine receptor super-family.14,15 It is currently assumed that the receptor forms a homodimer after ligand binding, even though some reports would suggest the possible involvement of another receptor chain.16-21 Dimerization is essential for receptor function.22-24 Upon binding of Epo, the Janus kinase JAK2 is rapidly activated via the membraneproximal region of the cytoplasmic domain of the receptor.25-27 JAK2 activation leads to the tyrosine-phosphorylation of several proteins, including the EpoR itself.28-31 Recent data have shown that one of the activated proteins is STAT5.32,33 Two forms of STAT5 (STAT5A and STAT5B) derived from two different genes have been described, which are highly homologous.34 Preliminary evidence indicates that STAT5 is involved in proliferation signaling from the interleukin-3 (IL-3) receptor.35 Further, it has been suggested that STAT5 may inhibit Epo-induced differentiation.36
One of the specific events during erythroid differentiation is the expression of the transcription factor GATA-1.37 GATA-1 upregulates the expression of erythroid-specific genes, including the gene for EpoR and the GATA-1 gene. GATA-1 expression levels are low in progenitor cells, but increase dramatically when the cells are induced to differentiate along the erythroid lineage.38,39 During differentiation to myeloid cells, expression of GATA-1 is shut off.40 When erythroid progenitors derived from normal blood are cultured in the presence of Epo, GATA-1 induction can be shown at day 3.38,41 Thus, EpoRs may be upregulated by GATA-1, whereas activation of EpoR may enhance GATA-1 expression.42
Recently we have shown that EpoRs are present in normal numbers on marrow cells of patients with MDS, and that the ligand binding capacity of the receptor is intact.43 Different splice variants of the receptor have been described.44,45 In MDS cases, we have not found aberrant splice variants of the EpoR.43 In this report we further delineate the consequences of Epo stimulation of MDS marrow. We show that the percentages of erythroid cells increase in Epo-stimulated normal BM (NBM) cultures, but not in similar cultures of MDS marrow. In contrast to NBM, there is hardly any induction of GATA-1 binding activity by Epo in MDS marrow. Furthermore, in most MDS cases Epo failed to activate STAT5 in marrow cells, although in these cells STAT5 can readily be activated by IL-3. Finally, we show that the inability of Epo to activate STAT5 is not caused by the absence of the relevant cells in MDS. The subpopulation capable of Epo-specific STAT5 activation in NBM has a distinct immunophenotype, and these cells are present in MDS marrow.
MATERIALS AND METHODS
Patient material.BM cells were collected from 21 patients with MDS. Patients were classified according to the criteria of the French-American-British (FAB) group46 as refractory anemia (RA, n = 3), refractory anemia with ring sideroblasts (RARS, n = 1), refractory anemia with excessive blasts (RAEB, n = 5), refractory anemia with excessive blasts in transformation (RAEB-t, n = 6), and chronic myelomonocytic leukemia (CMML, n = 6). NBM was obtained from BM transplantation donors after informed consent. Mononuclear cells were collected, and cryopreserved as described previously.47
Patient Characteristics and Erythroid Colony Formation
| Patient . | Age/Sex . | Karyotype . | FAB Subtype . | BFU-E* . |
|---|---|---|---|---|
| 1 | 79/M | 46,XY | RA | 40 |
| 2 | 80/M | 46,XY | RA | 0 |
| 3 | 80/F | 46,XX | RA | 40 |
| 4 | 38/M | 46,XY | RARS | 225 |
| 5 | 69/M | 46,XY | RAEB | 0 |
| 6 | 33/F | 46,XX t(3,12),del 7 | RAEB | 0 |
| 7 | 67/M | 47,XY,+8(62%);46,XY(38%) | RAEB | 0 |
| 8 | 63/M | 46,XY | RAEB | 8 |
| 9 | 67/F | 47,XX,+8(91%);46,XX(9%) | RAEB | 5 |
| 10 | 67/M | 46,XY,del(5)(q13q35),−7,+8,−16, add19(?p or q13),−20, +R(95%);46,XY(5%) | RAEB-t | 10 |
| 11 | 83/M | NA | RAEB-t | 0 |
| 12 | 68/M | 46,XY(3%);44-48,XY,−4,−16, −18(50%); 45-49,XY,−4,−16, −18,5q-,7q-,13q-(47%) | RAEB-t | NA |
| 13 | 68/M | 46,XY(90%);47-48,XY,+8(10%) | RAEB-t | 18 |
| 14 | 27/M | 46,XY | RAEB-t | 9 |
| 15 | 42/M | 46,XY | RAEB-t | 0 |
| 16 | 64/M | 46,XY(9%)46,XY,9q-(91%) | CMML | 0 |
| 17 | 76/M | 47,XY,+8 | CMML | 45 |
| 18 | 62/M | 46,XY | CMML | 5 |
| 19 | 60/M | 46,XY,inv4(p15.3q11)c | CMML | 15 |
| 20 | 46/M | 46,XY | CMML | 18 |
| 21 | 67/M | 46,XY | CMML | 3 |
| Patient . | Age/Sex . | Karyotype . | FAB Subtype . | BFU-E* . |
|---|---|---|---|---|
| 1 | 79/M | 46,XY | RA | 40 |
| 2 | 80/M | 46,XY | RA | 0 |
| 3 | 80/F | 46,XX | RA | 40 |
| 4 | 38/M | 46,XY | RARS | 225 |
| 5 | 69/M | 46,XY | RAEB | 0 |
| 6 | 33/F | 46,XX t(3,12),del 7 | RAEB | 0 |
| 7 | 67/M | 47,XY,+8(62%);46,XY(38%) | RAEB | 0 |
| 8 | 63/M | 46,XY | RAEB | 8 |
| 9 | 67/F | 47,XX,+8(91%);46,XX(9%) | RAEB | 5 |
| 10 | 67/M | 46,XY,del(5)(q13q35),−7,+8,−16, add19(?p or q13),−20, +R(95%);46,XY(5%) | RAEB-t | 10 |
| 11 | 83/M | NA | RAEB-t | 0 |
| 12 | 68/M | 46,XY(3%);44-48,XY,−4,−16, −18(50%); 45-49,XY,−4,−16, −18,5q-,7q-,13q-(47%) | RAEB-t | NA |
| 13 | 68/M | 46,XY(90%);47-48,XY,+8(10%) | RAEB-t | 18 |
| 14 | 27/M | 46,XY | RAEB-t | 9 |
| 15 | 42/M | 46,XY | RAEB-t | 0 |
| 16 | 64/M | 46,XY(9%)46,XY,9q-(91%) | CMML | 0 |
| 17 | 76/M | 47,XY,+8 | CMML | 45 |
| 18 | 62/M | 46,XY | CMML | 5 |
| 19 | 60/M | 46,XY,inv4(p15.3q11)c | CMML | 15 |
| 20 | 46/M | 46,XY | CMML | 18 |
| 21 | 67/M | 46,XY | CMML | 3 |
Abbreviation: NA, not available.
Colonies per 105 cells.
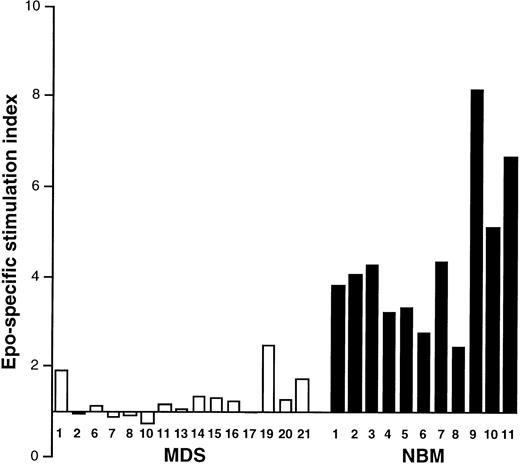
Epo-induced activation of DNA synthesis in MDS and NBM. Results are given from experiments in 15 MDS patients (□, numbers correspond to Table 1) and 11 NBM samples (▪). Epo was added for 3 days.
Epo-induced activation of DNA synthesis in MDS and NBM. Results are given from experiments in 15 MDS patients (□, numbers correspond to Table 1) and 11 NBM samples (▪). Epo was added for 3 days.
Fraction of Erythroid Cells Recovered From Epo-Stimulated Cultures of Normal and MDS Marrow
| Growth Factor Added . | NBM . | RA . | RAEB . | RAEB-t . | CMML . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | 1 . | 2 . | 3 . | 1* . | 2* . | 6* . | 7* . | 8* . | 10* . | 11* . | 13* . | 14* . | 15* . | 17* . | 21* . |
| — | 1.2 | 0.7 | 0.1 | 1.1 | 0.1 | 0 | NA | 0.2 | 0.2 | 0 | 0.4 | 0.4 | 0.5 | 0.1 | 0.1 |
| Epo | 2.7 | 2.5 | 0.9 | 0.6 | 0.2 | 0.2 | 0 | 0.4 | 0.1 | 0.5 | 0.9 | 0.4 | 0.6 | 0 | 0.7 |
| Epo + IL-3 | 5.3 | 8.5 | 1.6 | 0.5 | 0.1 | 0 | 0.1 | 0.2 | 0.2 | 0 | 0.9 | 0.4 | 0.2 | 0.1 | 0.4 |
| Epo + SCF | 9.6 | 3.0 | 2.0 | 0.7 | 0.1 | 0.5 | 0.1 | 0.1 | 0.1 | 0 | 1.1 | 0.3 | 0.2 | 0 | 0.2 |
| IL-3 | 1.2 | 0.8 | 0.8 | 0.7 | 0.1 | 0.8 | 0 | 0.1 | 0 | 0 | 0.2 | 0.1 | 0.5 | 0 | 0.5 |
| SCF | 1.2 | 0.6 | 0.7 | 0.5 | 0.1 | 0.3 | NA | 0.1 | 0.1 | 0 | 0.5 | 0.1 | 0.5 | 0 | 0.7 |
| Growth Factor Added . | NBM . | RA . | RAEB . | RAEB-t . | CMML . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | 1 . | 2 . | 3 . | 1* . | 2* . | 6* . | 7* . | 8* . | 10* . | 11* . | 13* . | 14* . | 15* . | 17* . | 21* . |
| — | 1.2 | 0.7 | 0.1 | 1.1 | 0.1 | 0 | NA | 0.2 | 0.2 | 0 | 0.4 | 0.4 | 0.5 | 0.1 | 0.1 |
| Epo | 2.7 | 2.5 | 0.9 | 0.6 | 0.2 | 0.2 | 0 | 0.4 | 0.1 | 0.5 | 0.9 | 0.4 | 0.6 | 0 | 0.7 |
| Epo + IL-3 | 5.3 | 8.5 | 1.6 | 0.5 | 0.1 | 0 | 0.1 | 0.2 | 0.2 | 0 | 0.9 | 0.4 | 0.2 | 0.1 | 0.4 |
| Epo + SCF | 9.6 | 3.0 | 2.0 | 0.7 | 0.1 | 0.5 | 0.1 | 0.1 | 0.1 | 0 | 1.1 | 0.3 | 0.2 | 0 | 0.2 |
| IL-3 | 1.2 | 0.8 | 0.8 | 0.7 | 0.1 | 0.8 | 0 | 0.1 | 0 | 0 | 0.2 | 0.1 | 0.5 | 0 | 0.5 |
| SCF | 1.2 | 0.6 | 0.7 | 0.5 | 0.1 | 0.3 | NA | 0.1 | 0.1 | 0 | 0.5 | 0.1 | 0.5 | 0 | 0.7 |
Cells were incubated for 72 hours in the presence of the indicated growth factors. Epo, 1 U/mL; IL-3, 2.5 ng/mL; SCF, 100 ng/mL.
Abbreviation: NA, not available.
Case of MDS (see Table 1).
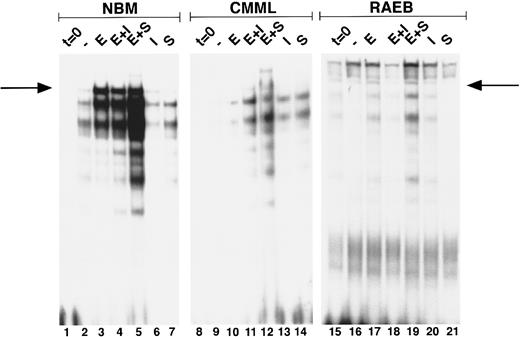
Induction of GATA-binding in MDS and NBM. Cells were obtained and extracted at t = 0 (lanes 1, 8, 15), and after culture for 3 days with no factor (lanes 2, 9, 16) Epo (lanes 3, 10, 17), Epo + IL-3 (lanes 4, 11, 18) Epo + SCF (lanes 5, 12, 19), IL-3 (lanes 6, 13, 20), and SCF (lanes 7, 14, 21). Nuclear extracts of equivalent amounts of marrow cells from two MDS patients (no. 19, CMML; and no. 7, RAEB) and one NBM were incubated with the β-globin probe and run on a nondenaturing polyacrylamide gel. Position of the GATA-1–probe complex (as determined from co-runs with extracts from GATA-1–expressing cell lines) is indicated with an arrow. Lower bands are caused by binding of GATA-1 proteolytic fragments generated during culture and sample preparation. Lanes 15 through 21 are relatively overexposed as compared with lanes 1 through 14.
Induction of GATA-binding in MDS and NBM. Cells were obtained and extracted at t = 0 (lanes 1, 8, 15), and after culture for 3 days with no factor (lanes 2, 9, 16) Epo (lanes 3, 10, 17), Epo + IL-3 (lanes 4, 11, 18) Epo + SCF (lanes 5, 12, 19), IL-3 (lanes 6, 13, 20), and SCF (lanes 7, 14, 21). Nuclear extracts of equivalent amounts of marrow cells from two MDS patients (no. 19, CMML; and no. 7, RAEB) and one NBM were incubated with the β-globin probe and run on a nondenaturing polyacrylamide gel. Position of the GATA-1–probe complex (as determined from co-runs with extracts from GATA-1–expressing cell lines) is indicated with an arrow. Lower bands are caused by binding of GATA-1 proteolytic fragments generated during culture and sample preparation. Lanes 15 through 21 are relatively overexposed as compared with lanes 1 through 14.
Erythroid colony culture.Erythroid colony formation was assayed in methylcellulose cultures supplemented with 1 U/mL Epo and 2.5 ng/mL IL-3 as previously described.2
DNA synthesis assay.NBM and MDS cells were incubated overnight (1 to 2 × 106 cells/mL) in liquid medium (Iscove's modified Dulbecco's medium, supplemented with bovine serum albumin [BSA], transferrin, lecithin, sodium selenite, and β-mercaptoethanol, and 30% fetal calf serum48 ). The next morning, cells were diluted to 2 × 105 cells/mL and cultured in the presence or absence of Epo (1 U/mL). Irradiated cells were used as background controls. After 3 days, 3H-TdR (0.1 μCi/well) was added for 8 hours. Incorporation was measured by liquid scintillation counting as previously described.49 Cells were procured on nitrocellulose filters and incorporated label was counted in a β-plate scintillation counter (LKB, Bronna, Sweden). To compensate for spontaneous incorporation, results were expressed as the Epo-specific stimulation index, obtained by dividing the counts incorporated in the presence of Epo by the counts incorporated in medium alone.
GATA-1 induction.NBM and MDS cells were incubated overnight in liquid medium without growth factor at a cell concentration of 0.5 to 1.0 × 106 cells/mL. The next day the following combinations of growth factors were added: Epo (1 U/mL), IL-3 (2.5 ng/mL), stem cell factor (SCF ) (100 ng/mL), Epo + IL-3, and Epo + SCF, or none. At the time of harvest, cells were counted, cytospins were made, and the bulk of cells was used to isolate nuclear proteins. Nuclear extracts were prepared as described.50 In brief, cells were collected by centrifugation, resuspended in buffer A (10 mmol/L HEPES [pH 7.8], 10 mmol/L KCl, 0.1 mmol/L EDTA, 0.1 mmol/L EGTA, 1 mmol/L dithiothreitol, 1% Pefabloc SC [Boehringer, Germany], 50 μg/mL aprotinin, 50 μg/mL leupeptin, 50 μg/mL bacitracin, and 50 μg/mL iodoacetamide) and incubated for 10 minutes on ice. Nonidet P-40 (Sigma, St Louis, MO) was added to a final concentration of 0.6%, and nuclei were spun down for 1 minute at 10,000g. Nuclear proteins were extracted by addition of buffer C (20 mmol/L HEPES [pH 7.8], 0.4 mol/L NaCl, 1 mmol/L EDTA, 1 mmol/L EGTA, 1% Pefabloc SC, 50 μg/mL aprotinin, 50 μg/mL leupeptin, 50 μg/mL bacitracin, and 50 μg/mL iodoacetamide), and rocking incubation for 15 minutes at 4°C. Extracts were cleared by 5 minutes of centrifugation at 15,000 rpm. The presence of GATA-binding proteins in the nuclear extracts was determined by electrophoretic mobility shift assay (EMSA), using a synthetic oligonucleotide probe from the β-globin region, containing the GATA-binding consensus sequence. Three to 5 μL of extract was incubated for 20 to 30 minutes with 0.2 ng of 32P-labeled (10,000 to 20,000 cpm) double-stranded oligonucleotide, in the presence of excess aspecific competitor [2 μg poly(dI-dC)] in 20 μL binding buffer (10 mmol/L Tris hydrochloride [pH 7.6], 100 mmol/L KCl, 5 mmol/L MgCl2 , 1 mmol/L EDTA, 12.5% glycerol, 1 mmol/L dithiothreitol) and then separated on a 8% polyacrylamide gel in 1× Tris-Borate/EDTA electrophoresis buffer (TBE).
STAT-protein activation.After overnight incubation in serum free medium,49 NBM or MDS cells were procured, counted, and 2 to 5 × 106 cells were incubated for the desired time in the absence or presence of growth factor (Epo: 100 U/mL, IL-3: 2.5 μg/mL). Nuclear extracts were made as previously described,51,52 leading to 20 μL final extract. EMSA was performed in the presence of excess aspecific competitor [poly(dI-dC)] on 3 μL extract, and probe-protein complexes were separated at 4°C on a 5% polyacrylamide gel in 0.25× TBE, containing 5% glycerol. For supershift assays, specific antibodies were added for 1 hour before the incubation with probe. Antibodies used were against sheep STAT5,53 and antibodies against human STAT5A and STAT5B, respectively. STAT5 was detected using a synthetic oligonucleotide probe from the β-casein promoter (5-AGATTTCTAGGAATTCAATCC-3′ ).54
Analysis of morphology.Differential counts were made on cytospins of 1 to 4 × 104 cells, stained with May-Grünwald-Giemsa. The total amount of cells in the erythroid compartment of a given sample was then obtained by multiplying the absolute cell number with the percentage of erythroid cells. The fraction of recovered erythroid cells was calculated by dividing the total erythroid cells in a sample by the total amount of erythroid cells at t = 0.
Sorting and FACScan analysis.Thirty to 60 × 106 NBM cells were labeled in a three-step procedure, 30 minutes per incubation, in phosphate-buffered saline containing 1% BSA and 1% preimmune rabbit serum (PBR). First step, mouse anti-human glycophorin A (GPA) monoclonal antibody; second step, rabbit anti-mouse Ig conjugated with RPE (RAM-PE) (Dako, Glostrup, Denmark); third step, mouse anti-transferrin receptor-FITC (CD71-FITC) (Becton Dickinson, San Jose, CA). In between, cells were washed twice in PBR. As controls, 1 × 106 cells were labeled with CD71-FITC or GPA/RAM-PE. Finally, cells were resuspended in PBR, and sorted on a FACSvantage (Becton Dickinson). Analysis of data was done with the Lysis software program (Becton Dickinson). After sorting, cells collected in all four windows were counted, and cytospins were made. The bulk of cells was divided in three portions, and incubated for 15 minutes with no factor, Epo (100 U/mL), or IL-3 (2.5 μg/mL). Extracts were made as described above, leading to 10 μL final volume. EMSA was performed with 5 to 7 μL extract, in the same way as described above.
For analysis of transferrin receptor- and glycophorin A-expression, 1 to 2 × 106 cells from NBM and MDS patients were labeled in the same way with CD71-FITC and GPA/RAM-PE. Samples were analyzed on a FACScan (Becton Dickinson). Analysis of data was done with the Lysis software program. Windows were designed on NBM samples, and also used for the analysis of MDS patient material.
RESULTS
Erythroid colony formation and DNA synthesis in response to Epo.The clinical data and in vitro colony formation of the patients with MDS are listed in Table 1. All MDS patients except for case 4 showed impaired erythroid colony formation after Epo stimulation (mean, 22 ± 50 colonies/105 cells). For NBM, the numbers of erythroid colonies under identical conditions varied between 105 and 1,115 per 105 cells (mean, 454 ± 281; n = 11). Because erythroid colony formation is the result of both proliferation and differentiation of progenitor cells, an attempt was made to assess these processes separately. The proliferative response to Epo was measured at day 3 using the 3H-TdR assay. As can be seen in Fig 1, Epo-specific activation of DNA synthesis was suppressed in all MDS samples as compared with NBM.
Erythroid differentiation and GATA-1 induction.To investigate the early stages of erythroid differentiation in MDS more closely, we analyzed the appearance of morphologically identifiable erythroid cells. NBM cultures stimulated with Epo, Epo + IL-3, or Epo + SCF contained increased fractions of erythroid cells at day 3 as compared to control cultures with no factor, IL-3, or SCF (Table 2), indicating that Epo is necessary for induction of erythroid differentiation in these cells. In MDS cases Epo did not stimulate an expansion of the fraction of erythroid cells (Table 2). In addition, we assessed the induction of GATA-1 after Epo exposure of BM cells. Nuclear extracts of equal cell numbers obtained at day 0 and day 3 were assayed for the presence of GATA-1 protein (Fig 2). In NBM cells, GATA-binding protein was detectable after 3 days in cultures that contained Epo (Fig 2, lanes 3, 4, and 5). Under the same conditions GATA-1 was only minimally upregulated in certain MDS cases (Fig 2, CMML) (6 of 15 cases), whereas in other cases GATA-1 was not induced (Fig 2, RAEB) (9 of 15 cases; see also Table 3).
Summary of Results
| FAB Subtype . | Patient No. . | EpoR Analysis . | 3H-Thymidine Incorporation on Epo . | STAT5 Activation on Epo . | GATA-1 Activation on Epo . |
|---|---|---|---|---|---|
| RA | 1 | — | Low | Low pos/no | No |
| 2 | — | No | No | No | |
| 3 | Normal | — | — | — | |
| RARS | 4 | Normal | — | — | — |
| RAEB | 5 | Normal | — | — | — |
| 6 | — | No | No | No | |
| 7 | — | No | No | No | |
| 8 | Normal | No | No | No | |
| 9 | Normal | — | — | — | |
| RAEB-t | 10 | — | No | No | No |
| 11 | — | No | Low pos | No | |
| 12 | Normal | — | — | — | |
| 13 | Normal | No | No | Low | |
| 14 | Normal | No | No | Low | |
| 15 | — | No | No | Low | |
| CMML | 16 | Normal | No | Low pos/no | Low |
| 17 | — | No | No | No | |
| 18 | Normal | — | — | — | |
| 19 | — | Low | Low pos/no | Low | |
| 20 | — | No | No | No | |
| 21 | — | Low | No | Low |
| FAB Subtype . | Patient No. . | EpoR Analysis . | 3H-Thymidine Incorporation on Epo . | STAT5 Activation on Epo . | GATA-1 Activation on Epo . |
|---|---|---|---|---|---|
| RA | 1 | — | Low | Low pos/no | No |
| 2 | — | No | No | No | |
| 3 | Normal | — | — | — | |
| RARS | 4 | Normal | — | — | — |
| RAEB | 5 | Normal | — | — | — |
| 6 | — | No | No | No | |
| 7 | — | No | No | No | |
| 8 | Normal | No | No | No | |
| 9 | Normal | — | — | — | |
| RAEB-t | 10 | — | No | No | No |
| 11 | — | No | Low pos | No | |
| 12 | Normal | — | — | — | |
| 13 | Normal | No | No | Low | |
| 14 | Normal | No | No | Low | |
| 15 | — | No | No | Low | |
| CMML | 16 | Normal | No | Low pos/no | Low |
| 17 | — | No | No | No | |
| 18 | Normal | — | — | — | |
| 19 | — | Low | Low pos/no | Low | |
| 20 | — | No | No | No | |
| 21 | — | Low | No | Low |
These results indicate that MDS marrow cells show an impaired Epo response, both with regard to proliferation, as evident from the activation of DNA synthesis, and differentiation, as apparent from GATA-1–induction and evaluation of morphology.
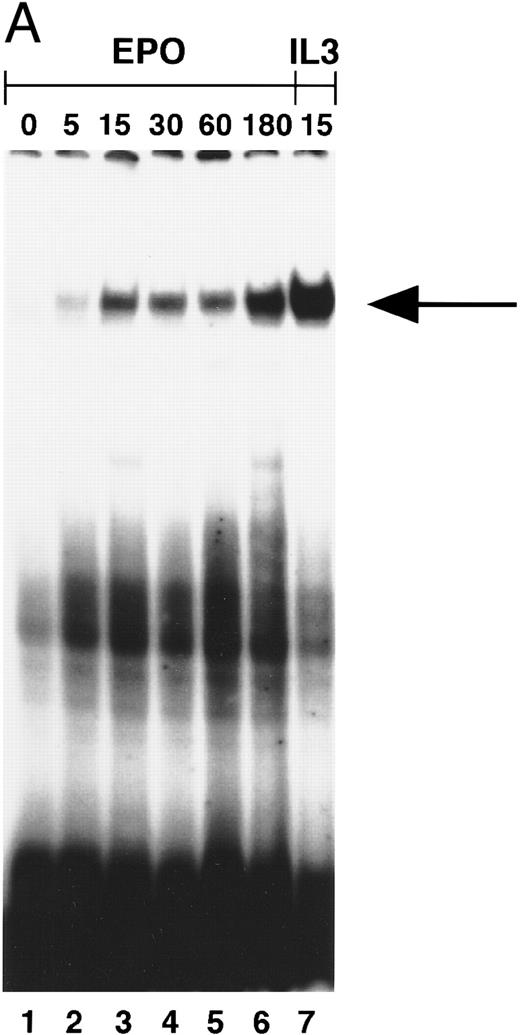
STAT5 activation in NBM.Induction of STAT5 DNA-binding by Epo was assessed in EMSA using the β-casein probe, which specifically recognizes activated STAT5.33 In a time-course experiment, the probe-protein complex was already detectable at 5 minutes, and persisted for 3 hours after stimulation of NBM cells (Fig 3A). Also, incubation with IL-3 (15 minutes) induced the activation of a STAT5 DNA-binding activity (Fig 3A, lane 7). The protein-probe complex was not present in extracts from cells stimulated for 15 minutes without factor (Fig 3B, lane 1). In all NBM samples tested (n = 12) Epo-specific induction of STAT5 DNA-binding was detected. To confirm that the protein in the complex was indeed STAT5, extracts were incubated with different antibodies against STAT5 before the addition of probe. With all three antibodies, a partial (Fig 3B, lanes 3 and 4) or complete (lane 5) supershift was detected, indicating that all complexes contain STAT5 protein. The complete supershift with the α-STAT5B antibody (lane 5) suggests that STAT5B is the predominant Epo-activated form. Control incubations with antibodies against STAT-1 or STAT-3 failed to supershift the complex (not shown). The m67 probe (5′-CATTTCCCGTAAATC-3′ ), a high-affinity mutant of the sis-inducible element of the human c-fos gene,55 was used to assay extracts for the activation of other STAT-proteins. Using this probe, no Epo-induced DNA binding could be detected (not shown).
(A) Activation of β-casein probe-binding proteins in NBM at different time points. Cells were obtained and extracted immediately (lane 1) or after incubation with Epo for 5 (lane 2), 15 (lane 3), 30 (lane 4), 60 (lane 5), or 180 (lane 6) minutes, or after incubation for 15 minutes with IL-3 (lane 8). Nuclear extracts of equivalent amounts of NBM cells incubated with the β-casein probe were run on a nondenaturing polyacrylamide gel. Arrow indicates position of STAT5 complex. (B) Identification of STAT-binding proteins activated by Epo in NBM cells. Nuclear extracts obtained from nonstimulated cells (lane 1) or from cells stimulated for 15 minutes with Epo (lanes 2 through 5) were incubated with the β-casein probe in the absence (lanes 1 and 2) or presence (lanes 3 through 5) of α-STAT5 antibodies. α5-1 (lane 3), α-sheep STAT5; α5-2 (lane 4), α-human STAT5A; α5-3 (lane 5), α-human STAT5B. Lower arrow indicates position of STAT5 complex, higher arrow the position of antibody-containing complexes.
(A) Activation of β-casein probe-binding proteins in NBM at different time points. Cells were obtained and extracted immediately (lane 1) or after incubation with Epo for 5 (lane 2), 15 (lane 3), 30 (lane 4), 60 (lane 5), or 180 (lane 6) minutes, or after incubation for 15 minutes with IL-3 (lane 8). Nuclear extracts of equivalent amounts of NBM cells incubated with the β-casein probe were run on a nondenaturing polyacrylamide gel. Arrow indicates position of STAT5 complex. (B) Identification of STAT-binding proteins activated by Epo in NBM cells. Nuclear extracts obtained from nonstimulated cells (lane 1) or from cells stimulated for 15 minutes with Epo (lanes 2 through 5) were incubated with the β-casein probe in the absence (lanes 1 and 2) or presence (lanes 3 through 5) of α-STAT5 antibodies. α5-1 (lane 3), α-sheep STAT5; α5-2 (lane 4), α-human STAT5A; α5-3 (lane 5), α-human STAT5B. Lower arrow indicates position of STAT5 complex, higher arrow the position of antibody-containing complexes.
STAT5 activation in MDS marrow cells.We then examined whether STAT5 was activated after Epo stimulation in marrow cells of 15 MDS cases. The results of these studies are summarized in Table 3. A representative experiment including 5 MDS cases is shown in Fig 4A. No or minimal STAT5 activation in response to Epo was seen in these cases, whereas IL-3 activated STAT5 normally.
(A) STAT5 activation in MDS patients cells after incubation with Epo. Equivalent amounts of cells were obtained and extracted after 15 minutes of incubation without factor (lanes 1, 4, 7, 10, 13, 16), with IL-3 (lanes 2, 5, 8, 11, 14, 17), or with Epo (lanes 3, 6, 9, 12, 15, 18). Marrow cells used were obtained from patient 8 (lanes 1 through 3), patient 13 (lanes 4 through 6), patient 2 (lanes 7 through 9), patient 1 (lanes 10 through 12), patient 19 (lanes 13 through 15), patient 7 (lanes 16 through 18); patient numbers correlate to case numbers in Table 1. Nuclear extracts were incubated with the β-casein probe. Arrow indicates position of STAT5 complex. (B) Activation of STAT5 in MDS at different time points. Cells from two patients with MDS (patient 13, lanes 1 through 7; and patient 8, lanes 8 through 14) were obtained and extracted after no stimulation (lanes 1 and 8), or after incubation with Epo for 5 (lanes 2 and 9), 15 (lanes 3 and 10), 30 (lanes 4 and 11), 60 (lanes 5 and 12), or 180 minutes (lanes 6 and 13), or after incubation for 15 minutes with IL-3 (lanes 7 and 14). The probe used is the β-casein probe, and the film was relatively overexposed. Arrow indicates position of STAT5 complex.
(A) STAT5 activation in MDS patients cells after incubation with Epo. Equivalent amounts of cells were obtained and extracted after 15 minutes of incubation without factor (lanes 1, 4, 7, 10, 13, 16), with IL-3 (lanes 2, 5, 8, 11, 14, 17), or with Epo (lanes 3, 6, 9, 12, 15, 18). Marrow cells used were obtained from patient 8 (lanes 1 through 3), patient 13 (lanes 4 through 6), patient 2 (lanes 7 through 9), patient 1 (lanes 10 through 12), patient 19 (lanes 13 through 15), patient 7 (lanes 16 through 18); patient numbers correlate to case numbers in Table 1. Nuclear extracts were incubated with the β-casein probe. Arrow indicates position of STAT5 complex. (B) Activation of STAT5 in MDS at different time points. Cells from two patients with MDS (patient 13, lanes 1 through 7; and patient 8, lanes 8 through 14) were obtained and extracted after no stimulation (lanes 1 and 8), or after incubation with Epo for 5 (lanes 2 and 9), 15 (lanes 3 and 10), 30 (lanes 4 and 11), 60 (lanes 5 and 12), or 180 minutes (lanes 6 and 13), or after incubation for 15 minutes with IL-3 (lanes 7 and 14). The probe used is the β-casein probe, and the film was relatively overexposed. Arrow indicates position of STAT5 complex.
To exclude the possibility that the induction of STAT5 in MDS was quantitatively normal, but delayed, cells of two patients were incubated with Epo for prolonged time periods. Even after incubation with Epo for 3 hours, STAT5 activation could not be detected, whereas the IL-3–induced signal was maximal at 15 minutes (Fig 4B).
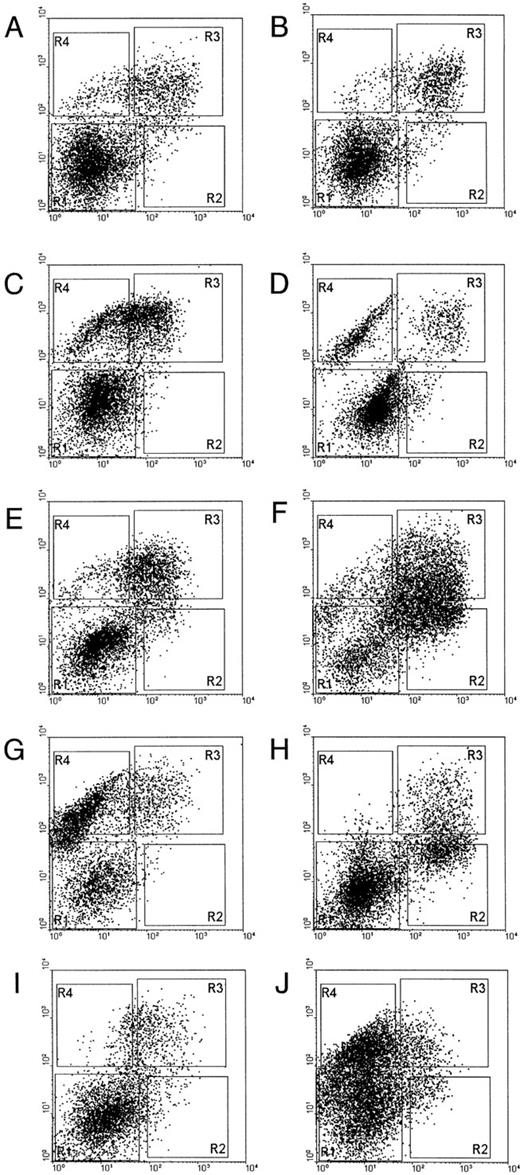
Epo-induced STAT5 activation in subpopulations of NBM cells.To define the subpopulation of cells in which STAT5 is activated in response to Epo, and to verify the presence of such a population in MDS, marrow cells were analyzed for their expression of CD71 and GPA. These markers distinguish successive stages of erythroid differentiation: early erythroblasts are CD71 (transferrin receptor)-positive, but glycophorin A (GPA)-negative (CD71+GPA−), normoblasts have acquired GPA (CD71+GPA+), and more mature red blood cells have lost the CD71-marker (CD71−GPA+).56 NBM cells were sorted into four fractions: R1 = CD71−GPA−, R2 = CD71+GPA−, R3 = CD71+GPA+, and R4 = CD71−GPA+ (Fig 5A). Morphological analysis of the different fractions confirmed the previously established correlation between cytological stage and cell-surface phenotype (not shown). Sorted fractions were incubated with IL-3, Epo, or without factor. The CD71−GPA− fraction shows clear STAT5 activation with IL-3 (Fig 5B, lane 5), but not with Epo (Fig 5B, lane 6). The CD71+GPA− subpopulation of cells shows both IL-3–mediated (Fig 5B, lane 8) as well as Epo-mediated STAT5 activation (Fig 5B, lane 9). In CD71+GPA+ cells STAT5 is inducible with Epo, but not with IL-3 (Fig 5B, lanes 10 through 12). Finally, more mature CD71−GPA+ cells show no STAT5 activation after Epo stimulation (lanes 13 through 15).
(A) Flow cytometric analysis of sorted NBM cells. CD71− and GPA− cells (rectangle 1), CD71+ and GPA− cells (rectangle 2), CD71+ and GPA+ cells (rectangle 3), CD71− and GPA+ cells (rectangle 4). (B) STAT5 activation in NBM cells sorted for CD71 and GPA expression. Cells sorted according to the windows in (A) (0, unsorted cells [lanes 1 through 3]; 1, CD71− and GPA− cells [lanes 4 through 6]; 2, CD71+ and GPA− cells [lanes 7 through 9]; 3, CD71+ and GPA+ cells [lanes 10 through 12]; and 4, CD71− and GPA+ cells [lanes 13 through 15]) were obtained and extracted after 15 minutes of incubation without factor (lanes 1, 4, 7, 10, 13), with IL-3 (lanes 2, 5, 8, 11, 14), or with Epo (lanes 3, 6, 9, 12, 15). Nuclear extracts were incubated with the β-casein probe and analyzed for activation of STAT5 on a nondenaturing polyacrylamide gel. Arrow indicates position of STAT5 complex.
(A) Flow cytometric analysis of sorted NBM cells. CD71− and GPA− cells (rectangle 1), CD71+ and GPA− cells (rectangle 2), CD71+ and GPA+ cells (rectangle 3), CD71− and GPA+ cells (rectangle 4). (B) STAT5 activation in NBM cells sorted for CD71 and GPA expression. Cells sorted according to the windows in (A) (0, unsorted cells [lanes 1 through 3]; 1, CD71− and GPA− cells [lanes 4 through 6]; 2, CD71+ and GPA− cells [lanes 7 through 9]; 3, CD71+ and GPA+ cells [lanes 10 through 12]; and 4, CD71− and GPA+ cells [lanes 13 through 15]) were obtained and extracted after 15 minutes of incubation without factor (lanes 1, 4, 7, 10, 13), with IL-3 (lanes 2, 5, 8, 11, 14), or with Epo (lanes 3, 6, 9, 12, 15). Nuclear extracts were incubated with the β-casein probe and analyzed for activation of STAT5 on a nondenaturing polyacrylamide gel. Arrow indicates position of STAT5 complex.
Epo-responsive subpopulation in MDS.We then analyzed the expression pattern of CD71 and GPA for eight different MDS patient marrow cells (Fig 6). Although the patterns of expression of these surface markers are more heterogeneous among MDS cases, in all instances considerable numbers of cells with CD71+GPA− and CD71+GPA+ expression were present. This indicates that the reactive erythroid progenitor cell subset has not been eliminated in MDS, and thus a quantitative lack of these cells does not explain the reduced Epo-induced STAT5 activation in MDS.
Flow cytometric analysis of NBM and MDS patient marrow cells. Marrow cells were labeled with CD71-FITC (horizontal axis) and GPA/RAM-PE (vertical axis). (A and B), NBM samples; (C), patient 13; (D), patient 17; (E), patient 8; (F ), patient 1; (G), patient 2; (H), patient 6; (I), patient 16; (J), patient 7. Patient numbers correlate to case numbers in Table 1.
Flow cytometric analysis of NBM and MDS patient marrow cells. Marrow cells were labeled with CD71-FITC (horizontal axis) and GPA/RAM-PE (vertical axis). (A and B), NBM samples; (C), patient 13; (D), patient 17; (E), patient 8; (F ), patient 1; (G), patient 2; (H), patient 6; (I), patient 16; (J), patient 7. Patient numbers correlate to case numbers in Table 1.
DISCUSSION
In this study, we have analyzed the deficient erythroid maturation in MDS in response to Epo as compared with NBM. Epo-induced DNA synthesis was considerably reduced in all cases of MDS tested. Stimulation of maturation by Epo was also impaired in MDS as assessed by the induction of GATA-binding proteins, and the appearance of erythroid cells in culture. As a first possible mechanism, we looked for mutations in the EpoR. For instance, mutations in the JAK2 binding domain of the EpoR27 57 lead to abrogation of the Epo-mediated mitogenic response. However, screening of the primary sequence of the EpoR of a number of Epo-unresponsive MDS cases (n = 10) did not provide evidence for an abnormal EpoR structure as a general mechanism of impaired Epo responsiveness (see Table 3, and data not shown).
The relation between Epo stimulation and the role of GATA-1 is complex, and the hierarchy of cellular events during erythroid development has not been fully elucidated. However, it is clear that upregulation of GATA-1 expression precedes the increase of EpoR mRNA during erythroid differentiation.39 Studies with GATA-1− mouse embryonic stem cells have shown that GATA-1 is essential for erythroid cell development.58,59 GATA-1− precursors are arrested at the proerythroblast stage and undergo premature cell death. A low level of GATA-1, present in early progenitors,39,40,60 may induce a low level of EpoR expression. A GATA-1 binding site is present in the EpoR promoter, and this site is involved in high-level tissue specific expression of the EpoR.61,62 Studies in knock-out mice with a deleted gene of either the EpoR or Epo have shown that Epo is not necessary for erythroid commitment, but it is essential for the proliferation and differentiation of committed progenitors.63 Epo stimulation of normal erythroid progenitors leads to an upregulation of GATA-1 expression (Fig 2 38,41). Since we have shown that normal levels of EpoR, capable of binding their natural ligand, are present on MDS cells,43 it is less likely that a primary GATA-1 block and suppression of Epo-R expression during differentiation play a dominant role in the reduced erythropoiesis.
A lack of STAT5 activation after Epo stimulation was apparent in 11 of 15 MDS patients, and it was low in 4 of 15 patients. In contrast, activation of STAT5 by Epo could always be detected in NBM cells. Although it has been reported that Epo can activate STAT1 in certain cell lines,64 our studies in primary BM cells show only activation of STAT5 by Epo. Extended incubation times showed that the response is detectable after 5 minutes, it is maximal at 15 minutes, and it remains up to 3 hours. At none of these time points could STAT5 activation be detected in MDS. Recent data using dominant-negative mutants of STAT535 and anti-sense approaches36 underscore the importance of STAT5 for mediating proliferation signals of the IL-3 receptor and the EpoR, respectively. In our studies, reduced or absent STAT5 activation correlated with an impairment of DNA synthesis and with inhibited erythroid colony-forming capacity of MDS marrow in all instances (see Table 3).
The Epo-mediated STAT5 activation could be assigned to a specific subpopulation of erythroid cells expressing high levels of CD71+. CD71+ cells were present in considerable numbers in all MDS samples tested. Thus, paucity of cells with the Epo-responsive phenotype cannot explain the abnormal Epo response. Therefore, we conclude that the reduced Epo-induced STAT5 response in MDS must result from defective signaling of the EpoR at an early level.
The experiments reported here are a first step toward understanding at which level the cascade of the Epo signaling is disturbed in MDS. In this report we have used STAT5 and GATA1 as monitors for the activation of the EpoR to localize the level at which this response is inhibited. The nature of these studies is descriptive, as is frequently the case when primary patient samples are being investigated. Nevertheless, the studies cannot be regarded purely phenomenologic, because it has been established that inhibition of STAT5 and GATA1 interferes with Epo-induced cell proliferation and survival.35,36,50,59 In contrast, this has as yet not been reported for Epo-induced MAP kinase and P13K activation. We do not suggest that the inhibited Epo response is an effect specific of STAT5. Failure to activate STAT5, for instance, could be related to a deficiency in JAK2 activation. Activation of JAK2 may also be necessary for the activation of the ras-pathway,65 so that multiple pathways downstream of JAK2 in MDS could be deficient, including the ras/MAPK66,67 and PI3K-routes.68-71 Measurements of the activation of these molecules by immunoprecipitation procedures are hampered by the limited amount of patient material available. Although our data suggest that the inhibited STAT5 response is a crucial step in the arrest of mitogenesis of erythropoietic cells, thus leading to suppressed erythropoiesis, other mechanisms interfering with Epo responsiveness should be considered as well. These may involve additional molecules known to become activated after stimulation with Epo. Notably, the tyrosine phosphatase Syp (SHPTP1D)72 is tyrosine-phosphorylated and associates with the EpoR after Epo stimulation. For the prolactin receptor it has been shown that Syp has a positive effect on signal transduction.73 In analogy, inability to activate Syp could lead to an impaired Epo response. Alternatively, overactivation of another tyrosine phosphatase, hematopoietic cell phosphatase (HCP, PTP1C), could downregulate the Epo-mediated signal,74 leading to a reduced Epo response. In addition, the activation of receptors that act upstream in developmental pathways may interfere with the function of more downstream receptors.75 All of these signaling pathways deserve a careful analysis in future studies dealing with the disturbed erythroid development in MDS.
ACKNOWLEDGMENT
The authors thank Dr M.M. von Lindern for helpful discussions; Dr A.W. Wognum for help with the FACSvantage; A. Boudewijn for the gift of anti-GPA mouse monoclonal; K. van Rooyen for photographic assistance and figure artwork; Dr J. Philipsen, Department of Cell Biology, for the gift of the GATA-binding probe; Dr Groner, Freiburg, Germany for the gift of anti-STAT5 antibody; and Dr T. Decker, Vienna, Austria, for the kind gift of anti-STAT5A and anti-STAT5B antibodies.
Supported by the Dutch Cancer Society “Koningin Wilhelmina Fonds.”
Address reprint requests to Bob Löwenberg, MD, PhD, Department of Hematology, Dr Daniel Den Hoed Cancer Center, PO Box 5201, 3008 AE Rotterdam, The Netherlands.




![Fig. 5. (A) Flow cytometric analysis of sorted NBM cells. CD71− and GPA− cells (rectangle 1), CD71+ and GPA− cells (rectangle 2), CD71+ and GPA+ cells (rectangle 3), CD71− and GPA+ cells (rectangle 4). (B) STAT5 activation in NBM cells sorted for CD71 and GPA expression. Cells sorted according to the windows in (A) (0, unsorted cells [lanes 1 through 3]; 1, CD71− and GPA− cells [lanes 4 through 6]; 2, CD71+ and GPA− cells [lanes 7 through 9]; 3, CD71+ and GPA+ cells [lanes 10 through 12]; and 4, CD71− and GPA+ cells [lanes 13 through 15]) were obtained and extracted after 15 minutes of incubation without factor (lanes 1, 4, 7, 10, 13), with IL-3 (lanes 2, 5, 8, 11, 14), or with Epo (lanes 3, 6, 9, 12, 15). Nuclear extracts were incubated with the β-casein probe and analyzed for activation of STAT5 on a nondenaturing polyacrylamide gel. Arrow indicates position of STAT5 complex.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/5/10.1182_blood.v89.5.1690/3/m_bl_0039f5a.jpeg?Expires=1765363056&Signature=3ZRkQ6LDDIheW4X2hVvpV-UUrtBlTvls87MoAa6EpsixOlkA8tGW9~IGRH2O80Ksze4ve0TuZxCwry5F3hcJu7EfLOqzqaS0r23QJpKfH49Dj7MQwkYzi4WXXfOeIWW3uHMQq3V~Foz9YpbiX7pk~oKOaa5odYHnNxiZrcXTAhhKnRMVgn7jJnu76qn3~s5auBYJYIhBw8MZIUCBIL1NTDMAx080F3sb4Bl8YESzeX1iK5dBJerrgvd6DCcahGL1JlRygCy7HmBQZOnNovOmE9-yVUBcnZKxkrQbhJe19x65ey0y7GvaOg~FFx9U0HLYvxvJLGQpyh3wP-211Vp4Ww__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. (A) Flow cytometric analysis of sorted NBM cells. CD71− and GPA− cells (rectangle 1), CD71+ and GPA− cells (rectangle 2), CD71+ and GPA+ cells (rectangle 3), CD71− and GPA+ cells (rectangle 4). (B) STAT5 activation in NBM cells sorted for CD71 and GPA expression. Cells sorted according to the windows in (A) (0, unsorted cells [lanes 1 through 3]; 1, CD71− and GPA− cells [lanes 4 through 6]; 2, CD71+ and GPA− cells [lanes 7 through 9]; 3, CD71+ and GPA+ cells [lanes 10 through 12]; and 4, CD71− and GPA+ cells [lanes 13 through 15]) were obtained and extracted after 15 minutes of incubation without factor (lanes 1, 4, 7, 10, 13), with IL-3 (lanes 2, 5, 8, 11, 14), or with Epo (lanes 3, 6, 9, 12, 15). Nuclear extracts were incubated with the β-casein probe and analyzed for activation of STAT5 on a nondenaturing polyacrylamide gel. Arrow indicates position of STAT5 complex.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/5/10.1182_blood.v89.5.1690/3/m_bl_0039f5b.jpeg?Expires=1765363056&Signature=BK7kDop1WHBqE38HkL~i2zrKUzpP4uI1sGZXmg0ZlwJTLP05c1riFgZbtNxAmNB3DnzL8YgC1zotnRXLZ-V7soneVniLscvBmrhRvQGTopczHO6bESE55fWx7T1Fd27XBahAR9m1xL~C2OJQemZEpJene-zV-KqwGhuHH9iUAoj6CuEg0M9hYiwOHqU6JL09LTrrMZAWvmyxhx3L4KcHiBavnFNxaqo5TJXWyNk5GnR7UhQ6akQTMTjuPD4tAueOVdRFD8k0dctm3ulCziBZrlAcJ0yJl2se-0lZMCkGDwHEqCyXj3u~BY9WmfBM7rDOJRojw~2riNSlglnIb1IUPg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal