Abstract
The genetic polymorphisms in human glutathione S-transferases (GST) M1 and T1 have been associated with race, disease risk, and outcome of some adult cancers. Also, there are racial differences in the incidence and characteristics of childhood acute lymphoblastic leukemia (ALL). Our objectives were to compare the frequency of the null genotype for GSTM1, GSTT1, or both in children with ALL to that in healthy controls, and to determine whether GST genotype was associated with treatment outcome and prognostic factors. We studied GSTM1 and GSTT1 genotypes in somatic cell DNA from black children and white children with ALL and in 416 healthy controls, using a polymerase chain reaction technique. Ninety of 163 (55.2%) white ALL patients and 14 of 34 (41.2%) black patients were GSTM1 null, frequencies not significantly different (P = .19) than healthy controls (53.5% in whites and 27.6% in blacks), although there was a trend toward more null genotypes in black ALL patients. Twenty-three of 163 (14.1%) white ALL patients and 12 of 34 (35.3%) black ALL patients were GSTT1 null, not different (P = .34) than the frequencies in healthy controls (15.0% in whites and 24.1% in blacks). However, the frequency of the “double-null” genotype, lacking both GSTM1 and GSTT1, was higher in black patients with ALL (8 of 34 or 23.5%) than in black controls (3.9%) (P = .0005), but this was not the case in white patients with ALL (10 of 163 or 6.1%) compared to white controls (8.0%) (P = .68). In stratified analyses, the GST double-null genotype was not associated with other characteristics that might differ between whites and blacks with ALL, such as age, T-lineage immunophenotype, presenting white blood cell count, DNA index, or insurance status. The null genotype for GSTM1, GSTT1, or both was not found to be a prognostic factor for disease-free survival or probability of hematologic remission; central nervous system relapse tended to be less common in those with the GSTM1 null genotype (P = .054) . The double-null genotype for GSTM1 and GSTT1 is more common among blacks but not whites with childhood ALL. These data suggest that GST genotype, coupled with unidentified additional risk factors, may play a role in risk of childhood ALL in American blacks.
ACUTE LYMPHOBLASTIC leukemia (ALL) is the most common malignancy in childhood, constituting about 30% of all childhood cancers. Its occurrence has been linked to some environmental, maternal, and paternal characteristics and exposures, and its geographic distribution has prompted some investigators to hypothesize that the risk of common ALL in childhood may be linked to an urban affluent lifestyle.1-7
The glutathione S-transferase (GST) superfamily of enzymes catalyzes the conjugation of xenobiotics and endogenous substances with glutathione, and thereby plays a significant role in the inactivation and, occasionally, the activation of many drugs and xenobiotics.8,9 GSTs have been implicated in detoxifying mutagenic electrophilic compounds, although they more rarely have been associated with formation of toxic metabolites as well, eg, from dihaloethanes and dihalomethanes.10,11 GSTs may detoxify reactive metabolites of cytotoxic drugs such as doxorubicin and etoposide,12 and thereby contribute to drug resistance. In addition, GSTs have numerous endogenous metabolic roles, and through their reactions with lipid peroxides and DNA hydroperoxides, and their involvement in eicosanoid synthesis,13 could play a role in cellular differentiation or malignant transformation.
Two of the four cytosolic subfamilies of GSTs (α, μ or M, π, θ or T) contain members (namely, GSTM1 and GSTT1) that exhibit genetic polymorphism in their population distribution, with a large percentage of individuals displaying a homozygous deletion of the structural genes. These deletions are easily detected by polymerase chain reaction (PCR)-based tests of somatic cell DNA. The GSTM1 null genotype occurs in about 50% of whites 14-17 and about 35% of American blacks.15-17 The frequency of the GSTT1 null genotype varies from 15% to 38%17-20 in healthy volunteers, with a higher frequency among healthy blacks.17,20 The null genotypes for GSTM1 and GSTT1 are inherited independently from one another in healthy populations of whites and blacks.17 The frequency of the double-null deletion (the simultaneous deletion of both GSTM1 and GSTT1 in the same individual) does not differ by race (white v black) or sex.17
An increased frequency of GST null genotypes has been associated with several malignancies, including smoking-related cancer,21-24 stomach cancer,25 pituitary adenomas,26 bladder cancer,27 squamous cell carcinoma,22 astrocytoma,28 and myelodysplatic syndrome.29 In all cases, GST deficiency, rather than high GST activity, has been associated with an increased frequency of malignancy. However, because GST may contribute to anticancer drug resistance, deficiency in GSTM1 or GSTT1 could positively influence chemotherapeutic efficacy in some patients. Consistent with that hypothesis, lack of GSTM1 expression in leukemic lymphoblasts was associated with a higher probability of continuous remission in a study of childhood ALL30; however, somatic cell GST genotype was not determined in that30 or in other studies of childhood acute lymphocytic leukemia. In the present study, our objectives were to determine whether (1) GST genotype frequencies are different in children with ALL compared to a healthy control population, (2) GST genotypes predict treatment outcome in childhood ALL, and (3) GST genotypes are associated with selected prognostic factors.
MATERIALS AND METHODS
Chemicals.GSTM1 primers (sense primer G5-5′GAA CTC CCT GAA AAG CTA AAG C; antisense primer G6-5′GTT GGG CTC AAA TAT ACG GTG G) anneal with the GSTM1 gene at 2401-2422 and 2598-2619, respectively.31 GSTT1 primers (sense primer T1-5′TTC CTT ACT GGT CCT CAC ATC TC; antisense primer T2-5′TCA CCG GAT CAT GGC CAG CA) anneal with the GSTT1 cDNA at 469-491 and 704-723, respectively,18 and β-globin primers (sense primer GH20-5′GAA GAG CCA AGG ACA GGT AC; antisense primer PC04-5′CAA CTT CAT CCA CGT TCA CC) anneal with the β-globin gene at 61992-62011 and 62240-62259, respectively.31 All were obtained from Biosynthesis (Lewisville, TX). The nucleotide numbering for the GSTM1 gene, GSTT1 cDNA, and β-globin gene was based on GenBank (accession no. X68676 for GSTM1, X79389 for GSTT1, and U01317 for β-globin). Taq DNA polymerase was obtained from Perkin-Elmer (Norwalk, CT). Nucleotides (dATP, dTTP, dCTP, dGTP) were purchased from Promega (Madison, WI). Nusieve (3:1) agarose was purchased from FMC (Rockland, ME). 123 DNA ladder was from GIBCO-BRL (Gaithersburg, MD). An OmniGene temperature cycler (Model TR3 SM2; Woodbridge, NJ) was used.
Human subjects.All patients had ALL and received therapy at St Jude Children's Research Hospital (SJCRH) from 1985 to 1995. Over this period, 75% to 80% of our patients were referred from Tennessee, Louisiana, or states contiguous with Tennessee. Details of the criteria for ALL diagnosis, classification, immunophenotyping, and treatment regimens have been previously published.32-35 The protocol distribution of patients for the analyses contained herein are provided in Table 1. Five patients included in the analyses of GST genotypes in patients with ALL versus controls were not treated on front-line protocols. Blood samples for DNA were collected in EDTA-containing tubes in a subset of these children, after complete remission was achieved, following the provision of informed consent by the patients or their parents for pharmacogenetic studies, with approval of the Institutional Review Board. All patients with ALL from whom somatic cell DNA had been collected are included for the analyses of GST genotypes; all samples were technically evaluable. Information on the health insurance status of the patient's family at the time of their diagnosis of ALL was obtained retrospectively from the hospital's medical records department.
Number of Patients Included in Analyses of GST Genotypes in Patients With ALL by SJCRH Treatment Protocol
| Analysis . | Total n . | Total X . | Total XI . | Total XII . | Total XIIIA . | Total XIIIB . | Not Treated on a Total Protocol . |
|---|---|---|---|---|---|---|---|
| Outcome in those with DNA sample v those without; and GST as a predictor of outcome | 161 with DNA | 0 | |||||
| 525 without DNA | |||||||
| 0 | 61 | ||||||
| 280 | 75 | ||||||
| 107 | 25 | ||||||
| 138 | 0 | ||||||
| 0 | 0 | ||||||
| 0 | |||||||
| GST genotype in ALL v controls | 197 ALL | 4 | 61 | 75 | 25 | 27 | 5 |
| Predictors of double-null genotype among patients with ALL | 192 ALL | 4 | 61 | 75 | 25 | 27 | 0 |
| Analysis . | Total n . | Total X . | Total XI . | Total XII . | Total XIIIA . | Total XIIIB . | Not Treated on a Total Protocol . |
|---|---|---|---|---|---|---|---|
| Outcome in those with DNA sample v those without; and GST as a predictor of outcome | 161 with DNA | 0 | |||||
| 525 without DNA | |||||||
| 0 | 61 | ||||||
| 280 | 75 | ||||||
| 107 | 25 | ||||||
| 138 | 0 | ||||||
| 0 | 0 | ||||||
| 0 | |||||||
| GST genotype in ALL v controls | 197 ALL | 4 | 61 | 75 | 25 | 27 | 5 |
| Predictors of double-null genotype among patients with ALL | 192 ALL | 4 | 61 | 75 | 25 | 27 | 0 |
Normal control subjects were adults (18 to 60 years of age) who donated blood to a local blood bank in Memphis, TN, reported to be in good health, and not taking any medications. Race was assigned based on the subject's own assessment. The GST genotype results on this normal population (n = 416) have been previously reported.17
Preparation of DNA.DNA was extracted from whole blood with phenol/chloroform as previously described.36 DNA was dissolved in 10 mmol/L Tris-HCl–1 mmol/L EDTA (pH 8.0), stored at 4°C, and quantitated with spectrophotometry at 260 nm.
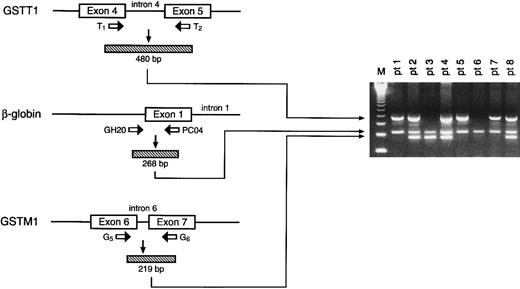
Genotyping of GSTM1 and GSTT1.A previously established PCR method17 was used to simultaneously amplify and analyze GSTM1, GSTT1, and β-globin from both healthy human subjects and children with ALL. DNA from patients with positive GSTM1, GSTT1, and β-globin alleles yielded 219-bp, 480-bp, and 268-bp products, respectively; the absence of amplifiable GSTM1 or GSTT1 (in the presence of β-globin PCR product) indicates the respective null genotype for each (Fig 1).
Depiction of strategy for amplification of GSTT1, β-globin, and GSTM1 genes (left) with resultant ethidium bromide–stained electrophoresed products from representative patient samples. The numbers 1 through 8 correspond to individual patients, with positive GSTT1, β-globin, and GSTM1 alleles yielding 480-, 268-, and 219-bp fragments, respectively; eg, patients 1, 5, and 7 are null for GSTM1, patients 2, 4, and 8 are null for neither GSTM1 nor GSTT1, patient 3 is null for GSTT1 only, and patient 6 is null for both GSTM1 and GSTT1. Absence of the PCR product indicates the null genotype. The far left lane (M) indicates the molecular-weight marker.
Depiction of strategy for amplification of GSTT1, β-globin, and GSTM1 genes (left) with resultant ethidium bromide–stained electrophoresed products from representative patient samples. The numbers 1 through 8 correspond to individual patients, with positive GSTT1, β-globin, and GSTM1 alleles yielding 480-, 268-, and 219-bp fragments, respectively; eg, patients 1, 5, and 7 are null for GSTM1, patients 2, 4, and 8 are null for neither GSTM1 nor GSTT1, patient 3 is null for GSTT1 only, and patient 6 is null for both GSTM1 and GSTT1. Absence of the PCR product indicates the null genotype. The far left lane (M) indicates the molecular-weight marker.
Statistical analysis.To determine if the population of ALL patients from whom we obtained DNA samples differed from the population of ALL patients on whom we had no DNA sample, we compared these two groups with respect to prognostic features, using Pearson chi-squared or Fisher's exact tests. Event-free-survival (EFS) was compared in those with versus those without a DNA sample using a Cox regression model, which was then stratified by treatment regimen/risk group, race, sex, age, central nervous system (CNS) leukemia status at diagnosis, white blood cell (WBC) count, and DNA index.
Fisher's exact or the Pearson chi-squared test was used to determine if any presenting features (age, sex, race, WBC count, DNA index, lineage, ploidy, CNS involvement at diagnosis, and Philadelphia chromosome positivity) were associated with GST genotype. Unstratified as well as stratified Cox regression analyses were performed to simultaneously assess the effects of GSTM1 null, GSTT1 null, and their interaction on EFS, and duration of hematologic and CNS remission. Stratification variables included treatments and risk groups as set forth by the original protocol designs and factors that were imbalanced among the GST genotype under consideration.
In a previous study we have shown that both race and sex are associated with GST genotypes.17 Thus, the comparisons of GST null genotype rates in ALL patients versus normal controls were stratified according to race and sex. The exact one-sided Mantel-Haenszel test was used separately to compare the frequency of the null genotype for GSTM1, GSTT1, and both GSTM1 and GSTT1 between patients with ALL and healthy controls. The assumption (required for the Mantel-Haenszel test) of a common odds ratio among races and sexes was tested using the Breslow-Day test for homogeneity. Where this assumption was violated, the four race-sex strata were separately analyzed using the one-sided Fisher's exact test, using a conservative Bonferroni's adjustment of 0.05/4 = 0.0125 to declare statistical significance. After the analysis, the strata were grouped according to similarities of the estimated odds ratios and standard errors, and the Mantel-Haenszel test was performed in each racial group.
The Mantel-Haenszel test was also used to test for associations between pretreatment prognostic factors and GST double-null genotype, stratified by race. Prognostic factors tested were age at diagnosis (<1 or >10 years v ≥1 and ≤10 years), lymphoblast CD10 expression (<20% v ≥20% in B-lineage ALL only), lymphoblast DNA index (<1.16 or >1.6 v ≥1.16 and ≤1.6), sex, blast cell immunophenotype (T- v B-lineage), and WBC count at diagnosis (<50,000 cells/μL v >50,000 cells/μL). In addition, we examined whether insurance status (traditional insurance, TennCare/Medicaid, or noninsured) varied with GST genotype.
RESULTS
Patient characteristics and GST as a possible prognostic factor.To determine whether the patients from whom DNA was available differed from the entire population of children in our front-line treatment protocols for ALL, we compared EFS in the 161 children with DNA samples versus the 525 patients without DNA samples enrolled on SJCRH Studies Total XI, XII, and XIIIA. The overall 10-year EFS (80%) was significantly higher in the 161 children from whom a DNA sample was available, compared with 60% in the other 525 patients from whom no DNA sample was available (unstratified log-rank test, P = .0002) The differences in EFS persisted after adjustment for treatment and several prognostic factors including age, sex, race, WBC count, DNA index, lineage, ploidy, CNS involvement at diagnosis, and the presence of the Philadelphia chromosome (stratified log-rank test P = .052). Because our pharmacogenetic studies did not begin until after Total XI was already well under way (1988), and even after that time informed consent was often obtained after patients had survived their first few months on therapy, it was not unexpected that the population of patients for whom blood DNA was available was biased toward survivors. However, in a multivariate analysis to determine whether any known prognostic features differed between the two groups (DNA sample v no sample), no factors were identified. Thus, although the group available for GST genotyping has a better prognosis than the other patients treated using the same protocols, this population of patients does not differ from the general referral population of children with ALL at SJCRH with regard to known prognostic features.
The subset of patients who were included in the analysis of GST genotype versus outcome of ALL is indicated in Table 1; patients from Total XIIIB were excluded from the outcome analysis because of short follow-up. There was no significant effect on EFS of the four different GSTM1 and GSTT1 genotype combinations (GSTM1 non-null + GSTT1 non-null, GSTM1 null + GSTT1 non-null, GSTM1 non-null + GSTT1 null, or GSTM1 null + GSTT1 null; P = .311). Stratified P values for the overall effects of GSTMI or GSTTI null genotypes on EFS, duration of hematologic remission, and time to isolated CNS relapse are .658, .807, and .193, respectively. Subset analyses showed that the probability of CNS–relapse-free survival at 5 years tended to be higher in those with the GSTM1 null genotype (.98; 95% confidence interval [CI] 0.94 to 1.0) versus those who were not null for GSTM1 (.90; 95% CI = 0.83 to 0.97) (two-sided P value = .0535).
GST genotypes in children with ALL versus healthy controls.DNA was available for GST genotyping from a total of 163 whites and 34 blacks with ALL (Table 1). Stratifying for sex and race, the frequency of the GSTM1 null genotype did not significantly differ between ALL patients and healthy controls (Tables 2 and 3). However, there was a trend for a higher frequency of the GSTM1 null genotype in black ALL patients (41.2%) than in black healthy controls (27.6%), particularly in black male children with ALL (45.5%), compared with normal male blacks (22.3%).
GSTM1 Null, GSTT1 Null, and GSTM1/GSTT1 Null Genotypes in Healthy Controls and in ALL Patients
| Genotype . | No. (%) . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | Whites . | Blacks . | . | . | . | . | ||||
| . | Male . | Female . | Total . | Male . | Female . | Total . | . | . | . | . |
| GSTM1 null | ||||||||||
| Control | 55/111 (49.5) | 59/102 (57.8) | 114/213 (53.5) | 23/103 (22.3) | 33/100 (33.0) | 56/203 (27.6) | ||||
| ALL | 43/85 (50.6) | 47/78 (60.3) | 90/163 (55.2) | 10/22 (45.5) | 4/12 (33.3) | 14/34 (41.2) | ||||
| GSTT1 null | ||||||||||
| Control | 17/111 (15.3) | 15/102 (14.7) | 32/213 (15.0) | 28/103 (27.2) | 21/100 (21.0) | 49/203 (24.1) | ||||
| ALL | 14/85 (16.5) | 9/78 (11.5) | 23/163 (14.1) | 8/22 (36.4) | 4/12 (33.3) | 12/34 (35.3) | ||||
| GSTM1 null + GSTT1 null | ||||||||||
| Control | 10/111 (9.0) | 7/102 (6.9) | 17/213 (8.0) | 4/103 (3.9) | 4/100 (4.0) | 8/203 (3.9) | ||||
| ALL | 5/85 (5.9) | 5/78 (6.4) | 10/163 (6.1) | 5/22 (22.7) | 3/12 (25.0) | 8/34 (23.5) | ||||
| Genotype . | No. (%) . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | Whites . | Blacks . | . | . | . | . | ||||
| . | Male . | Female . | Total . | Male . | Female . | Total . | . | . | . | . |
| GSTM1 null | ||||||||||
| Control | 55/111 (49.5) | 59/102 (57.8) | 114/213 (53.5) | 23/103 (22.3) | 33/100 (33.0) | 56/203 (27.6) | ||||
| ALL | 43/85 (50.6) | 47/78 (60.3) | 90/163 (55.2) | 10/22 (45.5) | 4/12 (33.3) | 14/34 (41.2) | ||||
| GSTT1 null | ||||||||||
| Control | 17/111 (15.3) | 15/102 (14.7) | 32/213 (15.0) | 28/103 (27.2) | 21/100 (21.0) | 49/203 (24.1) | ||||
| ALL | 14/85 (16.5) | 9/78 (11.5) | 23/163 (14.1) | 8/22 (36.4) | 4/12 (33.3) | 12/34 (35.3) | ||||
| GSTM1 null + GSTT1 null | ||||||||||
| Control | 10/111 (9.0) | 7/102 (6.9) | 17/213 (8.0) | 4/103 (3.9) | 4/100 (4.0) | 8/203 (3.9) | ||||
| ALL | 5/85 (5.9) | 5/78 (6.4) | 10/163 (6.1) | 5/22 (22.7) | 3/12 (25.0) | 8/34 (23.5) | ||||
Comparison of GST Null Genotypes in Children With ALL Versus Healthy Controls
| GST Genotype . | Racial Grouping . | Odds Ratio (SE) ALL v Control . | P Value . | 95% Lower Bounds for CI for Odds Ratio3-150 . |
|---|---|---|---|---|
| GSTM1 null, GSTT1 non-null | Whites and blacks3-151 | 1.20 (0.2) | .19 | 0.87 |
| GSTT1 null, GSTM1 non-null | Whites and blacks3-151 | 1.12 (0.25) | .34 | 0.74 |
| GSTM1 null and GSTT1 null | Whites3-152 | 0.75 (0.36) | .68 | 0.35 |
| Blacks3-152 | 7.36 (1.71) | .0005 | 2.61 |
| GST Genotype . | Racial Grouping . | Odds Ratio (SE) ALL v Control . | P Value . | 95% Lower Bounds for CI for Odds Ratio3-150 . |
|---|---|---|---|---|
| GSTM1 null, GSTT1 non-null | Whites and blacks3-151 | 1.20 (0.2) | .19 | 0.87 |
| GSTT1 null, GSTM1 non-null | Whites and blacks3-151 | 1.12 (0.25) | .34 | 0.74 |
| GSTM1 null and GSTT1 null | Whites3-152 | 0.75 (0.36) | .68 | 0.35 |
| Blacks3-152 | 7.36 (1.71) | .0005 | 2.61 |
Only lower bounds estimated because comparisons were based on one-sided a priori assumptions.
Because the assumption of homogeneity of odds ratios across races was met, the Mantel-Haenszel test was used to test for the overall difference between ALL and controls.
Because the assumption of homogeneity of odds ratios across races was not met (ie, Breslow-Day test P value = .0036), the Fisher's exact test using a Bonferroni's adjustment was used to compare ALL v controls in individual race/disease strata. By race and sex, the odds ratios (SE) for children with ALL v controls was 0.63 (0.69) in white males (P = .70), 0.93 (0.58) in white females (P = .58), 7.28 (2.28) in black males (P = .0085), and 8.00 (2.80) in black females (P = .0261).
Adjusting for sex and race, there was no significant difference in the frequency of the GSTT1 null genotype between ALL patients and healthy controls (Tables 2 and 3). However, again the GSTT1 null genotype tended to be more common among black ALL patients (35.3%) than among black controls (24.1%).
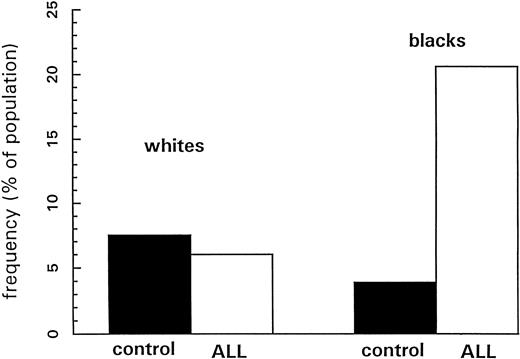
For comparing the double-null genotype frequencies between ALL patients and healthy controls, the common odds ratio assumption for the four race-sex strata required for the Mantel-Haenszel test was rejected by the Breslow-Day test of homogeneity (P = .0036). Thus, Fisher's exact test was used for each stratum, and the results are summarized in Table 3. The double-null deletion of GSTM1 and GSTT1 among blacks with ALL was significantly more common than among normal control blacks (23.5% v 3.9%, P = .0005) (Fig 2). There was no difference in the frequency of the double-null genotype between whites with ALL and control whites (P = .6837).
Frequencies of the “double-null” genotype for GSTM1 and GSTT1 in whites (left) and blacks (right); normal healthy controls depicted in solid bars, and children with ALL depicted with open bars. P values for differences between children with ALL and normal controls are .7 for whites and .0005 for blacks.
Frequencies of the “double-null” genotype for GSTM1 and GSTT1 in whites (left) and blacks (right); normal healthy controls depicted in solid bars, and children with ALL depicted with open bars. P values for differences between children with ALL and normal controls are .7 for whites and .0005 for blacks.
Relationship of patient-related factors and GST double-null genotype.The finding that the double-null GST genotype was higher in blacks but not whites with ALL prompted an analysis of whether any other patient or ALL-related factors (besides race) were correlated with the occurrence of the double-null GST genotype. There was no independent significant association between any patient factors (other than race) and GST double-null genotype, although two factors, WBC count and DNA index, tended to be associated with the GST double-null genotype (P = .141 and P = .121, respectively). Sixteen of 18 patients with the double-null genotype (89%) compared to 130 of 170 (76%) patients without the double-null genotype had unfavorable DNA indices (<1.16 or >1.6); 16 of 18 double-null genotype patients (89%) compared to 126 of 174 (72%) patients without the double-null genotype had low WBC counts (>50,000/μL) at diagnosis.
DISCUSSION
ALL is the most common childhood malignancy, is relatively uncommon in older age groups,2,37-39 and is more common in whites than in blacks.38 Compared to white children with ALL, black children with this disease are more likely to have a high WBC count, T-cell immunophenotype, CD10− B-lineage immunophenotype, and nonhyperdiploid B-progenitor ALL.3,4,38,40 Epidemiologic studies have shown an elevated risk of childhood leukemias associated with maternal drug use and parental occupational exposure to pesticides.1,2,4 Possible links between the risk for ALL and socio-economic or infectious factors3 and inducibility of the drug metabolizing enzyme CYP1A1 have also been hypothesized.7 Together, these data support a possible genetic and/or environmental influence on the risk of childhood ALL. In this regard, differences in the distribution of a group of protective enzymes, the glutathione S-transferases, have been shown to be related to cancer prevalence in adults.21-29 This background provided the rationale for our comparison of GST genotypes in children with ALL versus controls; this is the first report of GST genotypes in childhood ALL. Because of substantial differences in GST genotype distributions among whites and blacks,15-17,20 27 it was crucial that our study control for race when comparing children with ALL to normal healthy controls. Our finding that blacks with ALL but not whites with ALL have an overrepresentation of the GST double-null genotype is consistent with blacks having an exposure that differs from whites, and/or with blacks having an additional genetic trait that predisposes to the development of ALL when combined with the GST double-null genotype.
The fact that the GST double-null genotype was more frequent in blacks, but not whites, with ALL led us to question whether there were other variables that were more common in blacks than in whites with ALL, and whether any of these variables were associated with the double-null genotype. Whether the blacks in our study had different environmental exposures than the whites is not known, because epidemiologic data were not collected. Because we hypothesized that socioeconomic status might be a surrogate for more leukemogenic environmental exposures, and the only data available to us possibly related to economic status was the insurance classification for patients, we also assessed whether insurance status differed between whites and blacks, and then, more importantly, assessed whether lack of insurance was independently predictive of the GST double-null genotype. Consistent with other reports,40-42 black children in our study had significantly higher initial leukocyte counts, fewer hyperdiploid cases, more T-cell immunophenotype cases, and more CD10− B-lineage cases of ALL. However, none of these features was independently predictive of the GST double-null genotype, after adjusting for race. We speculate that some environmental exposures and/or genetic factors more common in blacks predispose to development of childhood ALL when combined with the GST double-null genotype. Such a race-specific alteration of drug-metabolizer genotype prevalence has also been reported for another enzyme, CYP1A1, in blacks but not whites with breast cancer.43
We recognize that blacks are at a lower, not higher, risk than whites for childhood ALL.3,38,44 Moreover, a larger proportion of blacks with ALL develop T-cell immunophenotype, CALLA−, unfavorable-prognosis ALL than whites,40-42 but it is because of an underrepresentation of B-progenitor, better-prognosis ALL.3,38,42 It cannot be stated that an overrepresentation of the GST double-null genotype could explain ALL risk in blacks because healthy blacks do not have a higher prevalence of the GST double-null genotype than healthy whites, and even the risk of T-cell ALL in blacks is not higher than the risk in whites.3,38 42 Thus, these data suggest that there must be an additional factor that interacts with the GST double-null genotype to put blacks with that genotype at higher risk than whites.
It is noteworthy that only the deficiency of both GSTM1 and GSTT1 was more frequent in black children with ALL. Because of possible overlapping substrate specificities among different GSTs, it is possible that only the deletion of two GSTs confers a profound enough loss of function to predispose to leukemogenesis. To our knowledge, this is the first study linking the deletion of both polymorphic GSTs to a human cancer.
The null genotype for GSTM1 or GSTT1 was not predictive of ALL outcome in our study. There could be several reasons for this finding. First, although there were no differences in known prognostic features in those in whom we had DNA samples versus those in whom we did not, there were fewer failures in population from whom DNA samples were available for GST genotyping; thus, identification of prognostic features in this subgroup may have been difficult because of fewer adverse events. Also, it is possible that the treatment given was not heavily dependent on GSTs for its inactivation. Finally, GST null genotypes may have covaried with a prognostic variable (such as ploidy45 46 ), thus obscuring its possible impact on prognosis. In fact, the occurrence of the double-null GST genotype did tend to cosegregate with unfavorable ploidy and favorable presenting leukocyte count in our patients.
These data are the first characterization of GSTM1 and GSTT1 null genotypes in children with ALL. The finding of an overrepresentation of the double-null GST genotype in black, but not white, patients with ALL suggests that epidemiologic studies of childhood ALL should carefully examine whether differences in environmental exposures or other polymorphic genes exist between blacks and whites, and provide further evidence that drug metabolizing genotyping and disease association data obtained in white populations should not be extrapolated to other racial groups.
ACKNOWLEDGMENT
We thank Pamela McGill, Anthony Tuggle, and Amy Atkinson for their excellent technical assistance; Sheri Ring, Lisa Walters, and Janet Fry for patient recruitment; and Patricia Harrison for her statistical programming. We also thank Drs Sally Pemble and Brian Ketterer, University College, London, UK, for sharing GSTT1 genomic structural information.
Supported by National Institutes of Health Grants No. CA51001 and CA20180, Cancer Center CORE Grant No. CA21765, a Center of Excellence grant from the State of Tennessee, and American Lebanese Syrian Associated Charities (ALSAC).
Address reprint requests to Mary V. Relling, Pharm D, St Jude Children's Research Hospital, 332 N Lauderdale, Memphis, TN 38101.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal