Abstract
The non-Hodgkin's lymphoma (NHL) subset commonly referred to as large cell lymphoma (LCL) has historically been characterized by its marked cytological, immunological, and clinical heterogeneity. One potential defining feature of these lymphomas, the t(2; 5)(p23; q35), occurs in 25% to 30% of anaplastic LCLs and is also found in cases with diffuse large cell or immunoblastic morphology. We recently identified nucleophosmin (NPM ) and anaplastic lymphoma kinase (ALK ) as the genes on chromosomes 5 and 2, respectively, that are juxtaposed by this translocation. To provide a complementary approach to the use of classical cytogenetics or polymerase chain reaction-based methods for the detection of this abnormality, we have developed a two-color fluorescent in situ hybridization (FISH) assay for the t(2; 5) that may be used for the analysis of both interphase nuclei and metaphase chromosomes. Three overlapping chromosome 5 cosmid clones located immediately centromeric to the NPM gene locus and an ALK P1 clone located telomeric to the chromosome 2 breakpoint were labeled with digoxigenin or biotin, respectively, and used to visualize the derivative chromosome 5 produced by the t(2; 5), evident as juxtaposed or overlapping red and green fluorescent signals. This NPM-ALK FISH assay was initially validated by analysis of a series of cytogenetically characterized cell lines, with the presence of the der(5) chromosome showed specifically only in those lines known to contain the t(2; 5). The assay was then applied in a blinded fashion to a series of eight cytogenetically t(2; 5)-positive clinical specimens and seven known t(2; 5)-negative cases, including three NHL and four Hodgkin's disease biopsy samples. Whereas the t(2; 5)-negative cases were negative by FISH, all eight t(2; 5)-positive cases were positive. One additional case, initially thought to be positive for the translocation by cytogenetics, was proven to not be a classic t(2; 5) by interphase and metaphase FISH. These data indicate that the FISH assay described is a highly specific and rapid test that should prove to be a useful adjunct to the currently available methods for detection of the t(2; 5).
THE IDENTIFICATION of genetic abnormalities specific to given malignancies is a prerequisite for the recognition of biologic subtypes that may have characteristic prognostic features or therapeutic responses. Perhaps the best example of the rapidly evolving genetic characterization of malignant disease is provided by the analysis of the chromosomal abnormalities unique to different subsets of non-Hodgkin's lymphoma (NHL). The NHL subset referred to as large cell lymphoma (LCL),1 which comprises ∼25% and 40% of NHL in children and adults, respectively, has until recently been poorly characterized from a molecular genetic standpoint. Over the past 5 years, a number of cytogenetic studies have identified a t(2; 5)(p23; q35) chromosomal rearrangement, frequently as the only identifiable abnormality, in a high percentage of LCLs.2-8
Although the t(2; 5) is found in LCL of diffuse mixed cell, diffuse large cell, and immunoblastic histologies,8,9 the majority of cases containing the translocation are of the anaplastic large cell type.10,11 These anaplastic large cell lymphomas (ALCL) are often referred to as Ki-1+ lymphomas because of their frequent, although not invariant, expression of the CD30 antigen, which is recognized by the Ki-1 and Ber-H2 monoclonal antibodies12 and is a transmembrane receptor for a ligand related to tumor necrosis factor.13-15 The ALCL/Ki-1+ lymphomas are heterogeneous with regard to cell lineage with the majority of cases being of T-cell phenotype (75%), although occasional cases are either non-T/non-B (10%) or express B-cell restricted antigens (15%). Clinically, these lymphomas typically behave as aggressive, high-grade tumors that are frequently associated with extranodal involvement including skin, bone, soft tissue, gastrointestinal tract, and lung. Although several studies have suggested that ALCL/Ki-1+ lymphomas containing the t(2; 5) may have a better overall survival compared with other large cell subtypes, 20% to 30% of these patients eventually succumb to their disease despite aggressive therapeutic intervention.16-24 It should be emphasized that neither anaplastic morphology nor the expression of CD30 accurately predict the presence of the t(2; 5). For example, in a series of 70 cases of LCL analyzed for the t(2; 5) by reverse transcriptase-polymerase chain reaction (RT-PCR), our group identified the translocation in only slightly greater than half of the 28 cases with anaplastic morphology and only 59% of CD30-expressing cases.9 Thus, t(2; 5)-containing LCLs appear to comprise a distinct genetic subgroup of NHL that can have variable histologic and immunophenotypic features.
We have recently cloned the t(2; 5),25,26 demonstrating it to involve the gene loci encoding nucleophosmin (NPM), a highly-conserved RNA-binding nucleolar phosphoprotein27-30 on chromosome 5, and anaplastic lymphoma kinase (ALK), a novel receptor tyrosine kinase of the insulin receptor subfamily on chromosome 2. The translocation produces a fusion of these genes on the der(5) chromosome that consists of the N-terminal end of NPM linked in-frame to the intracellular kinase domain of ALK. Whereas NPM is expressed ubiquitously at high levels, the normal expression of ALK is restricted to neural tissues and is important for normal neural development and function (S.W. Morris, manuscript submitted). Thus, the t(2; 5) results in the transcription of ALK driven off the strong NPM gene promoter, leading to the inappropriate expression and constitutive activation of a truncated ALK protein in lymphoid cells.25,31 32 The potential ALK-NPM fusion mRNA and protein encoded on the der(2) chromosome produced by the translocation is not expressed.
NPM-ALK Fish Assay of Lymphoma Biopsy Samples
| Accession No. . | Date of Specimen . | Tissue . | Diagnosis . | FISH Results* . | Karyotype . |
|---|---|---|---|---|---|
| t(2; 5) Positive Cases | |||||
| 931762 | 02/11/93 | LN | ALCL (Indeterminant) | + | 46,XX,t(2; 5)(p23;q35)[3]/92,XXXX,t(2; 5)(p23;q35)x2[17] |
| 100/150 = 66% | |||||
| 910490 | 09/07/90 | LN | ALCL (T cell) | + | 91,XXXX,+i(1)(q10),t(2; 5)(p23;q35)x2,−6,−9,−10, −11,+12,−13,del(17)(p11),add(19)(q13),+add(20) (p13),+del(20)(q12),−21,−22,+mar1,+mar2[17]/46,XX[3] |
| 932394 | 04/28/93 | LN | ALCL (T cell) | + | 91,XXYY,i(1q),−2,t(2; 5)(p23;q35)x2,−4,+5,+6,−7x2, −11,add(15)(p11),−17,−21,+mar1,+mar2, +mar3x2[19]/46,XY[2] |
| 930926 | 10/29/92 | LN | Immunoblastic lymphoma (T cell) | + | 47,XY,+X,t(2; 5)(p23;q35)[4]/46,XY[3] |
| 47/100 = 47% | |||||
| 911489 | 01/21/91 | LN | Diffuse LCL (T cell) | + | 46,XY,t(2; 5)(p23;q35)[9]/45,X,−Y,t(2; 5)(p23;q35)[5]/46,XY[6] |
| 931737 | 02/09/93 | BM | Diffuse LCL (T cell) | + | 47,XY,t(2; 5)(p23;q35),+7[2]/46,XY[18] |
| 24/150 = 16% | |||||
| 922873 | 06/18/92 | LN | Diffuse LCL (T cell) | + | 47,XY,t(2; 5)(p23;q35),+7[20] |
| 932499 | 05/13/93 | LN | Follicular mixed cell lymphoma (B cell) | + | 50,XX,t(2; 5)(p23;q35),−3,+del(3)(p25)x3,del(13) (q22),+18,+22[cp5]/46,XX[12] Control Cases |
| 36/150 = 24% | |||||
| 870797 | 12/10/86 | LN | HD | − | 46,XY[20] |
| 14/150 = 9% | |||||
| 911100 | 11/20/90 | LN | HD | − | 46,XX[1] |
| 922401 | 04/23/92 | LN | HD | − | 46,XY[20] |
| 9/100 = 9% | |||||
| 932553 | 05/17/93 | LN | HD | − | 46,XY[20] |
| 871261 | 03/11/87 | LN | Follicular mixed cell lymphoma (B cell) | − | 46,XX,t(14; 18)(q32;q21)[2]/46,XX[1] |
| 930808 | 10/14/92 | LN | Follicular mixed cell lymphoma (B cell) | − | 46,XX,inv(9)(p24q32),t(14; 18)(q32;q21)[4]/46,XX[10] |
| 7/100 = 7% | |||||
| 921332† | 12/10/91 | LN | Diffuse LCL (B cell) | − | 87,XX,−Yx2,−1,dup(1)(q21q25),del(2)(p23),+del(2) (p23),−4,−6,+7x2,−9,−11,−17[10]/46,XY[3] |
| 4/100 = 4% |
| Accession No. . | Date of Specimen . | Tissue . | Diagnosis . | FISH Results* . | Karyotype . |
|---|---|---|---|---|---|
| t(2; 5) Positive Cases | |||||
| 931762 | 02/11/93 | LN | ALCL (Indeterminant) | + | 46,XX,t(2; 5)(p23;q35)[3]/92,XXXX,t(2; 5)(p23;q35)x2[17] |
| 100/150 = 66% | |||||
| 910490 | 09/07/90 | LN | ALCL (T cell) | + | 91,XXXX,+i(1)(q10),t(2; 5)(p23;q35)x2,−6,−9,−10, −11,+12,−13,del(17)(p11),add(19)(q13),+add(20) (p13),+del(20)(q12),−21,−22,+mar1,+mar2[17]/46,XX[3] |
| 932394 | 04/28/93 | LN | ALCL (T cell) | + | 91,XXYY,i(1q),−2,t(2; 5)(p23;q35)x2,−4,+5,+6,−7x2, −11,add(15)(p11),−17,−21,+mar1,+mar2, +mar3x2[19]/46,XY[2] |
| 930926 | 10/29/92 | LN | Immunoblastic lymphoma (T cell) | + | 47,XY,+X,t(2; 5)(p23;q35)[4]/46,XY[3] |
| 47/100 = 47% | |||||
| 911489 | 01/21/91 | LN | Diffuse LCL (T cell) | + | 46,XY,t(2; 5)(p23;q35)[9]/45,X,−Y,t(2; 5)(p23;q35)[5]/46,XY[6] |
| 931737 | 02/09/93 | BM | Diffuse LCL (T cell) | + | 47,XY,t(2; 5)(p23;q35),+7[2]/46,XY[18] |
| 24/150 = 16% | |||||
| 922873 | 06/18/92 | LN | Diffuse LCL (T cell) | + | 47,XY,t(2; 5)(p23;q35),+7[20] |
| 932499 | 05/13/93 | LN | Follicular mixed cell lymphoma (B cell) | + | 50,XX,t(2; 5)(p23;q35),−3,+del(3)(p25)x3,del(13) (q22),+18,+22[cp5]/46,XX[12] Control Cases |
| 36/150 = 24% | |||||
| 870797 | 12/10/86 | LN | HD | − | 46,XY[20] |
| 14/150 = 9% | |||||
| 911100 | 11/20/90 | LN | HD | − | 46,XX[1] |
| 922401 | 04/23/92 | LN | HD | − | 46,XY[20] |
| 9/100 = 9% | |||||
| 932553 | 05/17/93 | LN | HD | − | 46,XY[20] |
| 871261 | 03/11/87 | LN | Follicular mixed cell lymphoma (B cell) | − | 46,XX,t(14; 18)(q32;q21)[2]/46,XX[1] |
| 930808 | 10/14/92 | LN | Follicular mixed cell lymphoma (B cell) | − | 46,XX,inv(9)(p24q32),t(14; 18)(q32;q21)[4]/46,XX[10] |
| 7/100 = 7% | |||||
| 921332† | 12/10/91 | LN | Diffuse LCL (B cell) | − | 87,XX,−Yx2,−1,dup(1)(q21q25),del(2)(p23),+del(2) (p23),−4,−6,+7x2,−9,−11,−17[10]/46,XY[3] |
| 4/100 = 4% |
The number of interphase nuclei exhibiting paired or fused green and red signals (numerator), the total number of nuclei examined (denominator), and the percentage of nuclei apparently positive for the t(2; 5) are shown for those cases in which 150 or fewer nuclei were analyzed.
In initial cytogenetic characterization, case no. 921332 was thought to contain the t(2; 5). The nomenclature shown is a revision following reexamination of the sample subsequent to NPM-ALK FISH analysis.
As one means of rapidly detecting the t(2; 5) in clinical samples, we have devised a two-color fluorescent in situ hybridization (FISH) assay using DNA probes specific for the 5′ region of NPM and the 3′ region of ALK. This assay permits detection of the NPM-ALK fusion in both metaphase and interphase cells. In this report, we describe the application of this two-color FISH assay for the detection of NPM-ALK to a series of lymphoma biopsy specimens.
MATERIALS AND METHODS
Clinical samples and cell lines.Control cell lines, including the t(2; 5)-positive lymphoma lines SU-DHL-1, SUP-M2,33 and UCONN-L225 and the t(2; 5)-negative leukemia lines HL-60 and K562,34 were cultured in RPMI 1640 with glutamine supplemented with 15% fetal calf serum and antibiotics. Lymph node biopsy specimens from patients with pathologically confirmed NHL or Hodgkin's disease (HD) were mechanically dissociated, placed in short-term culture, then procured using routine cytogenetic techniques. Harvests were performed following a 2-hour incubation in colcemid by hypotonic exposure, followed by fixation in 3:1 methanol: acetic acid. Following three fixations, the cells were suspended in fixative and dropped onto slides for cytogenetic analysis. Leftover cell suspensions were initially stored at 4°C for 2 to 4 months, then transferred to −20°C for prolonged storage. Samples stored in this manner for as long as 10 years were analyzed successfully in this study.
The presence or absence of the t(2; 5) in the clinical samples analyzed in this study had been previously determined by cytogenetic analysis and was confirmed in most of the cases by NPM-ALK RT-PCR.9 35 Each sample was coded for subsequent identification and analyzed blindly by the individuals performing FISH.
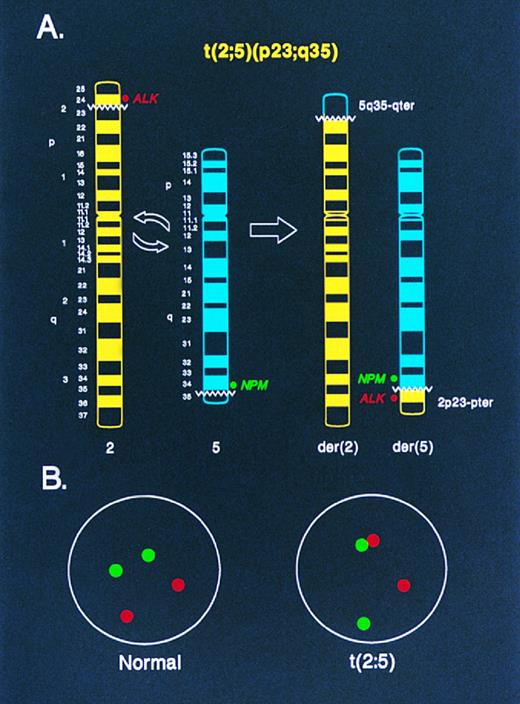
Diagrammatic representation of two-color NPM-ALK FISH in the analysis of (A) metaphase chromosomes and (B) interphase nuclei. In (A), an idiogram of normal chromosomes 2 and 5, and the derivative chromosomes 2 and 5 produced by the t(2; 5), is shown. The location of the ALK and NPM DNA probes used for FISH in this study relative to the t(2; 5) breakpoints on the normal chromosomes 2 and 5, respectively, is indicated. Juxtaposition of the green (NPM ) and red (ALK ) fluorescent signals in a metaphase preparation identifies the der(5) chromosome, which encodes the oncogenic NPM-ALK fusion protein. In (B), the random spacing of NPM (green) and ALK (red) fluorescent signals in a normal diploid interphase nucleus is shown. In nuclei from cells containing the t(2; 5), the chromatin strands representing the der(5)-encoded NPM-ALK fusion gene are apparent as paired green and red signals or as yellow “fusion” signals resulting from overlapping green and red fluorescence.
Diagrammatic representation of two-color NPM-ALK FISH in the analysis of (A) metaphase chromosomes and (B) interphase nuclei. In (A), an idiogram of normal chromosomes 2 and 5, and the derivative chromosomes 2 and 5 produced by the t(2; 5), is shown. The location of the ALK and NPM DNA probes used for FISH in this study relative to the t(2; 5) breakpoints on the normal chromosomes 2 and 5, respectively, is indicated. Juxtaposition of the green (NPM ) and red (ALK ) fluorescent signals in a metaphase preparation identifies the der(5) chromosome, which encodes the oncogenic NPM-ALK fusion protein. In (B), the random spacing of NPM (green) and ALK (red) fluorescent signals in a normal diploid interphase nucleus is shown. In nuclei from cells containing the t(2; 5), the chromatin strands representing the der(5)-encoded NPM-ALK fusion gene are apparent as paired green and red signals or as yellow “fusion” signals resulting from overlapping green and red fluorescence.
Probes.Chromosome 5 cosmid clones 13, 15-2, and 47C12 are located immediately centromeric to the NPM gene locus and are retained on the der(5) chromosome produced by the t(2; 5). These overlapping clones, which span a genomic distance of approximately 90 kilobases, were isolated from a chromosome 5-specific cosmid library (kindly provided by L. Deaven, Los Alamos, NM) during chromosome walking performed to identify the breakpoint of the t(2; 5).25 An ALK P1 clone (designated ALK-DMPC-HFF#1-1111H1) was isolated from the DuPont Merck Pharmaceutical Company human foreskin fibroblast P1 library #1 by PCR-based screening (Genome Systems, Inc, St Louis, MO) using the primers 5′-CTAGAGCCCTCTTCGCTGACT-3′ and 5′-CCACGGTCTTAGGGATCCCAA-3′ that are homologous to 3′ ALK coding sequence or reverse complementary to 3′ untranslated sequence, respectively. This P1 clone is homologous to a region of the ALK genomic locus that is translocated to the der(5) chromosome of the t(2; 5).
FISH.Cosmid clones 13, 15-2, and 47C12 were combined in equal amounts (∼300 ng each) and labeled with digoxigenin-11-dUTP (GIBCO/Bethesda Research Laboratories, Gaithersburg, MD) and the ALK P1 clone (1 μg) was labeled with biotin-dUTP (Boehringer Mannheim, Indianapolis, IN) by nick translation. The labeled probes (50 ng each) were lyophilized in a SpeedVac (Savant, Farmingdale, NY) together with 2 μg Cot-1 DNA (GIBCO-BRL) for blocking of repetitive sequences, then dissolved in 30 μL of 50% formamide, 12% dextran sulfate and 2× SSC. The slides were denatured for 30 seconds in 70% formamide at 70°C, dehydrated in 70%, 80%, and 95% ETOH in H2O at room temperature for 1 minute each, then placed on a warm (37°C) surface. The probe/Cot-1 mixture was denatured for 5 minutes at 70°C, then applied to the specimen slides, which were cover-slipped and hybridized overnight in a humidified chamber at 37°C. After hybridization, the slides were washed by gentle agitation for 5 minutes in 50% formamide at 37°C and for 1 minute in 2× SSC at room temperature. Specific hybridization signals were detected by incubating slides with fluorescein-conjugated sheep antibodies to digoxigenin (Boehringer Mannheim) and avidin-Texas red (Vector Laboratories, Inc, Burlingame, CA), followed by counterstaining with 4′, 6-diamidino-2-phenylindole (DAPI) in antifade. The slides were examined with a Zeiss Axiophot microscope equipped with a triple band pass filter set and CytoVision imaging system (Applied Imaging, Pittsburgh, PA). Analyses were performed by experienced molecular cytogenetic technologists at both the University of Nebraska Medical Center (Omaha, NE) and St Jude Children's Research Hospital (Memphis, TN).
Statistical methods.Initial studies of known t(2; 5)-negative cell lines identified random pairing or overlap of NPM and ALK signals in 2% to 7% of interphase nuclei (false positive rate); analysis of translocation-positive lines revealed a failure to visualize specific pairing of the signals in ≤10% of interphase nuclei (false negative rate). These observations were considered, together with the expected variability in tumor cell content of individual biopsy specimens, in the design of statistical guidelines for the determination of true positives and true negatives, as follows. An optimal sample size (number of interphase nuclei to be screened) was based on testing the hypothesis, H0 :π ≤0.07 versus Ha :π >0.07, where π denotes the true, unknown, proportion of cells in the sample that express the translocation. The value of 0.07 is an upper bound on the false positive rate of the in situ hybridization procedure. One can determine the false positive rate specific to a given laboratory and using appropriate confidence intervals (eg, 90% to 95%), determine a sufficient sample size corresponding to the worst case scenario and assuming the false positive rate to be the upper limit of the confidence interval. The in situ hybridization procedure described in this study was assumed to have a false negative rate of 0.10, and the statistical test was designed to have 90% power (α = .01) for correctly concluding that the t(2; 5)(p23; q35) and NPM-ALK fusion gene was present in a tissue sample containing at least 10% cells with the translocation and 90% normal cells. For example, based on the exact binomial distribution36 and the above parameters, under optimal conditions one would study 181 interphases, concluding that the translocation is present only if at least 23 nuclei contain paired red and green signals, or yellow fusion signals produced by overlapping red and green probes. The t(2; 5) status of the clinical specimens analyzed by FISH in this study was determined based on these statistical considerations, using the available patient material in the assays. In those cases in which fewer than 181 interphase nuclei were examined, the number of nuclei apparently positive for the t(2; 5) and the total number of nuclei analyzed are shown in Table 1.
RESULTS
Although the t(2; 5) is typically a balanced reciprocal translocation, the biologically important gene product resulting from the rearrangement, the NPM-ALK oncoprotein, is encoded on the derivative 5 chromosome whereas the der(2) chromosome resulting from the translocation appears to be transcriptionally silent.25 With this consideration in mind, we designed a two-color “come-together” FISH assay to detect the der(5) chromosome using genomic DNA clones located immediately adjacent to the 5′ portion of the NPM locus on chromosome 5, which lies centromeric to the t(2; 5) breakpoint, and from the extreme 3′ portion of the ALK locus, which lies telomeric to the t(2; 5) breakpoint on chromosome 2 (Fig 1A). The der(5) chromosome in metaphase preparations is identified in this assay by the juxtaposition of green (NPM ) and red (ALK) fluorescent signals that reside separately on chromosomes 5 and 2, respectively, in normal cells (Figs 1A, 2A and 2B). In interphase nuclei of t(2; 5)-positive cells, the der(5) is evident as pairing of the green and red signals or as a yellow “fusion” signal produced by overlapping green and red fluorescence (Figs 1B, 3B, 3C and 4A). By contrast, in normal cells the green and red signals are distributed randomly throughout the interphase nuclei (Figs 1B, 3A, and 4A).
Two-color NPM-ALK FISH assay of (A) a normal metaphase chromosome preparation illustrating the hybridization pattern of the ALK (red) and NPM (green) probes to normal chromosomes 2 and 5, respectively, and (B) a metaphase preparation from a t(2; 5)-positive LCL showing the paired red and green, or yellow fusion, signals present on the der(5) produced by the translocation, in addition to the nonpaired green or red signals present on the nonrearranged chromosomes.
Two-color NPM-ALK FISH assay of (A) a normal metaphase chromosome preparation illustrating the hybridization pattern of the ALK (red) and NPM (green) probes to normal chromosomes 2 and 5, respectively, and (B) a metaphase preparation from a t(2; 5)-positive LCL showing the paired red and green, or yellow fusion, signals present on the der(5) produced by the translocation, in addition to the nonpaired green or red signals present on the nonrearranged chromosomes.
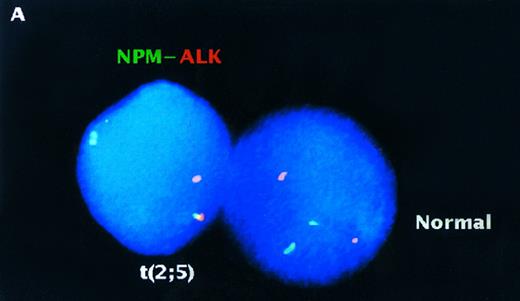
Analysis of interphase nuclei using two-color NPM-ALK FISH. (A) Normal PB lymphocyte nucleus showing nonpaired, randomly spaced green and red fluorescent signals. (B) Interphase nucleus from the t(2; 5)-positive cell line SUP-M2 illustrating a yellow fusion signal produced by overlapping NPM and ALK DNA probes (upper arrow), as well as paired green and red fluorescent signals (lower arrow) produced by juxtaposition of the probes on the der(5) chromatin strands. (C) A representative interphase nucleus from the analysis of a t(2; 5)-positive clinical sample is shown (case no. 932499). Overlapping (yellow) and paired (green and red) signals (arrows) indicating the der(5) of the t(2; 5) are seen in this translocation-positive NHL sample. The nuclei in (A) and (C) were counterstained with DAPI which produces a blue background; (B) was photographed from a preparation that was not counterstained.
Analysis of interphase nuclei using two-color NPM-ALK FISH. (A) Normal PB lymphocyte nucleus showing nonpaired, randomly spaced green and red fluorescent signals. (B) Interphase nucleus from the t(2; 5)-positive cell line SUP-M2 illustrating a yellow fusion signal produced by overlapping NPM and ALK DNA probes (upper arrow), as well as paired green and red fluorescent signals (lower arrow) produced by juxtaposition of the probes on the der(5) chromatin strands. (C) A representative interphase nucleus from the analysis of a t(2; 5)-positive clinical sample is shown (case no. 932499). Overlapping (yellow) and paired (green and red) signals (arrows) indicating the der(5) of the t(2; 5) are seen in this translocation-positive NHL sample. The nuclei in (A) and (C) were counterstained with DAPI which produces a blue background; (B) was photographed from a preparation that was not counterstained.
Two-color NPM-ALK FISH of t(2; 5)-positive (A) and -negative (B,C) NHL samples. (A) Interphase nuclei prepared from the bone marrow of a t(2; 5)-positive NHL case (accession number 931737) showing adjacent translocation-containing and normal nuclei. (B) Metaphase chromosome preparation from a t(2; 5)-negative NHL illustrating the lack of pairing of green and red signals in an aneuploid tumor. (C) Interphase nuclei prepared from the same tumor as shown in (B) showing the presence of multiple chromosomes 2 and 5 but the absence of the der(5) produced by the t(2; 5). This case (accession number 921332) was initially thought to be t(2; 5)-positive based on classical cytogenetic analysis that revealed an abnormality of distal 2p.
Two-color NPM-ALK FISH of t(2; 5)-positive (A) and -negative (B,C) NHL samples. (A) Interphase nuclei prepared from the bone marrow of a t(2; 5)-positive NHL case (accession number 931737) showing adjacent translocation-containing and normal nuclei. (B) Metaphase chromosome preparation from a t(2; 5)-negative NHL illustrating the lack of pairing of green and red signals in an aneuploid tumor. (C) Interphase nuclei prepared from the same tumor as shown in (B) showing the presence of multiple chromosomes 2 and 5 but the absence of the der(5) produced by the t(2; 5). This case (accession number 921332) was initially thought to be t(2; 5)-positive based on classical cytogenetic analysis that revealed an abnormality of distal 2p.
To validate the t(2; 5) FISH assay, we initially analyzed interphase nuclei and metaphase chromosomes prepared from the t(2; 5)-positive lymphoma cell lines SU-DHL-1, SUP-M2,33 and UCONN-L2.25 As expected, the NPM-ALK gene fusion was observed in metaphase preparations as juxtaposed red and green signals on the der(5), whereas the normal chromosomes 2 and 5 possessed either red or green signals only, respectively (data not shown). As illustrated in Fig 3B, in interphase nuclei the NPM-ALK fusion was readily identified as paired red and green or overlapping (yellow) fluorescent signals. Identical results were observed in the analysis of all three NPM-ALK–positive cell lines. By comparison, hybridization of interphase nuclei from normal peripheral blood (PB) lymphocytes (Fig 3A) or the t(2; 5)-negative leukemia cell lines HL-60 and K562 (not shown) revealed random, nonpaired spacing of the fluorescent signals.
To test the specificity and sensitivity of the two-color FISH assay for detection of the NPM-ALK gene fusion, we next examined a series of biopsy samples from both non-Hodgkin's and Hodgkin's lymphoma patients (see Table 1). These cases, all of which had previously undergone successful conventional cytogenetic characterization, were coded for subsequent identification and analyzed by two-color FISH in a blinded fashion. Our experience using the FISH assay to analyze interphase nuclei from cell lines known to be t(2; 5)-negative had indicated that a low frequency of nuclei (up to 7%) may exhibit random overlap of NPM and ALK fluorescent signals. Conversely, in translocation-positive cell lines, a population of nuclei (as high as 10%) failed to show NPM-ALK fusion even under optimal analysis conditions. Based on these observations, we used statistical methods to determine the optimal number of interphase nuclei to examine from each clinical sample to reliably detect even a small number (as low as 10%) of t(2; 5)-positive lymphoma cells within a given biopsy (see Materials and Methods). To be considered positive for the t(2; 5), samples had to conform to these statistically-derived parameters.
The pathologic diagnosis, tissue analyzed, results of conventional cytogenetics, and two-color FISH assay results for each of the clinical samples included in this study are shown in Table 1. As indicated, the seven cases that were negative for the t(2; 5) by conventional cytogenetic study were also found to be negative by FISH, including four cases of HD. Analysis by FISH of the eight t(2; 5)-positive cases also revealed complete concordance with conventional cytogenetic results. It should be noted that these FISH results are derived from the analysis of interphase nuclei almost exclusively, as only rare metaphase chromosomes were noted in a few of the samples tested.
The analysis of one of the samples tested in this study was especially instructive, pointing out a strength of the FISH assay as compared to conventional cytogenetic analysis. The initial cytogenetic analysis of case no. 921332 revealed an incomplete abnormal karyotype with poor morphology which was thought to include a typical t(2; 5). Two-color FISH of this case (Fig 4B and C) was clearly negative, however, and further conventional cytogenetic analysis identified a del(2)(p23) rather than the t(2; 5). The absence of NPM-ALK transcripts in this case was confirmed by RT-PCR analysis9 and immunostaining with an ALK-specific antibody was negative (data not shown).
DISCUSSION
We describe in this report a two-color “come-together” FISH assay for detection of the t(2; 5) (p23; q35) in NHL. This assay, which detects the 5′NPM-3′ALK gene fusion encoded on the der(5) chromosome produced by the translocation, can be used to detect the rearrangement using either metaphase chromosomes or interphase nuclei. Similar assays have been developed to detect the BCR-ABL fusion in chronic myelogenous leukemia and the PAX3-FKHR chimeric gene in alveolar rhabdomyosarcoma.37 38
The results described here indicate that the FISH assay, which we have developed is a rapid and highly specific means of detecting the NPM-ALK fusion gene produced by the t(2; 5) in clinical tissue specimens. The unequivocal detection of the t(2; 5) in LCL may be important to ensure correct clinical management given the frequent difficulty in the pathologic diagnosis of these lymphomas. For example, the pleomorphic appearance of the malignant cells in ALCL has led to the misdiagnosis of these cases as metastatic carcinoma, malignant histiocytosis, malignant fibrous histiocytoma, melanoma, and even viral infections. In one recent series of ALCL/Ki-1+ lymphoma nearly one-third of the patients were initially diagnosed incorrectly, with some receiving inappropriate therapy.17 In addition, because many of the NHLs that contain the t(2; 5) share a number of clinical and pathological features with HD, including a bimodal age distribution, expression of the CD30 antigen and the interleukin-2 receptor, and the presence of Reed-Sternberg–like cells,39 there may be diagnostic confusion in differentiating between these two types of lymphoma. The t(2; 5) has not been observed by cytogenetics in any non-hematopoietic malignancies. Furthermore, although not yet completely resolved, the bulk of data suggest that the translocation is found only rarely, if ever, in HD.24,35 40-46 Thus, detection of the NPM-ALK fusion gene by two-color FISH should permit the differentiation of NHL from these morphologically similar entities.
An obvious advantage of this FISH assay as compared with conventional cytogenetics is that it does not depend on the ability to obtain metaphase chromosomes from tumors. Clinically-based cytogenetic studies of lymphomas routinely report the unsuccessful characterization of a high percentage of samples (up to 50%) because of the lack of analyzable metaphases. As shown here, NPM-ALK FISH performed using the DNA probes described will also be beneficial for the analysis of the occasional cases of lymphoma that are difficult to characterize with respect to the t(2; 5) by routine cytogenetics. Given that the normal ALK protein is not expressed in hematopoietic cells, immunohistochemical staining using anti-ALK antibodies to analyze clinical biopsies has recently been suggested as a reliable means for the detection of NPM-ALK.24,32,44 46 It should be noted, however, that the spectrum of tumors that may express the normal ALK receptor is presently unknown and, therefore, that positive anti-ALK staining may not unequivocally differentiate between NPM-ALK expression in ALCL/Ki-1+ lymphoma and ALK expression in anaplastic tumors of uncertain lineage that could masquerade as NHL. The FISH assay detailed here, which specifically detects the NPM-ALK fusion, will readily discriminate between t(2; 5)-positive NHL and these other tumors and could be used accordingly.
A number of studies have recently described the use of formalin-fixed, paraffin-embedded tissues for diagnostic interphase FISH.47,48 Because formalin-fixed tissue is frequently the only material available for study in the clinical setting, the development of these techniques increases the possibility for analyzing large numbers of archival cases of even rare diseases. Although we have not tested our FISH assay on formalin-fixed samples, similar probe sets have been used successfully with no experimental alterations or with only minor technical differences, such as the direct labeling of probes with fluorochromes.47 Given that a number of the samples analyzed in this study were not ideal, some have been stored as cytogenetic preparations for close to 10 years, we believe that our assay is likely also to be applicable for the analysis of formalin-fixed tissue with no or only minor difficulty.
Rather than supplanting the other available means of detecting the t(2; 5), we believe that the NPM-ALK FISH assay complements these methods, which currently include Southern blot analysis, RT-PCR, DNA-PCR, and immunohistochemistry. An example of a potential use for this assay would be as a screening method to detect the t(2; 5) in morphologically diagnosed cases of NHL as well as in anaplastic tumors of uncertain derivation. Equivocal results, or results in conflict with clinical or pathologic suspicions, could then be corroborated using an alternative method of NPM-ALK detection.
ACKNOWLEDGMENT
The authors thank Dr James Boyett for helpful discussions regarding the biostatistical aspects of the use of two-color FISH for clinical diagnosis. The excellent secretarial support of Doris Dodson and Peggy Vandiveer is also gratefully acknowledged.
Supported by National Cancer Institute grants CA 01702 and CA 69129 (S.W.M.), by NCI Cancer Center CORE Grant CA 21765, and by the American Lebanese Syrian Associated Charities, St Jude Children's Research Hospital.
Address reprint requests to Stephan W. Morris, MD, Department of Experimental Oncology, Room 5025, Thomas Tower, St Jude Children's Research Hospital, 332 N Lauderdale, Memphis, TN 38105-2794.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal