Abstract
To understand the molecular events for the proliferation of B cells, we studied the induction of telomerase activity in vitro after stimulation to B-cell antigen receptor (BCR) on human peripheral B cells. Although unstimulated purified B cells of tonsils and peripheral blood from healthy volunteers do not express detectable telomerase activity, anti-IgM beads induce telomerase activity in these B cells. Soluble anti-IgM antibody (Ab) alone does not induce telomerase activity, but the second signal, given by either one of the cytokines of interleukin-2 (IL-2), IL-4, and IL-13 or by anti-CD40 monoclonal Ab (MoAb), is effective as the costimulation for the induction of the activity. Stimulation with antiIgM Ab and anti-CD40 MoAb induces telomerase activity in most mature B cells of the tonsils and peripheral blood. The stimuli to both IgM and IgD receptors similarly induce the activity. Induction of telomerase activity is accompanied with the proliferation of B cells, but is not absolutely correlated with the extent of B-cell growth. Phorbol dibutylate (PDB) plus calcium (Ca) ionophore (PDB/Ca), which replace the activation through BCR and the costimulatory molecules, also induce telomerase activity. Moreover, it is suggested that phosphoinositide (PI) 3-kinase plays a role for the induction of telomerase activity in B cells stimulated with anti-IgM Ab and anti-CD40 MoAb. These results suggest that telomerase activity is induced in the B-cell activation of the antigen specific immune response.
ANTIGEN STIMULATION INDUCES the activation of lymphocytes for the proliferation and differentiation with the help of soluble cytokines and/or cell-cell interaction via surface molecules. The stimulation causes a rapid expansion and a long-term survival of antigen-specific lymphocytes. Antigen binding to specific receptors triggers signal transduction with rapid tyrosine kinase activation leading to several second messenger pathways, inducing the upregulation of transcription factors and the many kinds of molecular events for the proliferation of lymphocytes.1-3 The activation of protein kinase C (PKC) after the increase of phopspholipid metobolite, diacylglycerol (DG), and the induction of intracellular Ca2+ are well characterized as important molecular events for the signal transduction of B-cell proliferation.4,5 Recent studies have shown that other phosphatidylinositol phosphate production is also involved in the antigen receptor signal transduction.6 It is not clear how these multiple signal transduction pathways play roles in the activation and proliferation of B cells.
Maintenance of telomere lengths of the chromosomes is probably an essential function of dividing cells. Cells from primitive to the rodent animals possess telomerase activity that can maintain the telomere lengths during cell division.7-9 In humans, although telomerase activity is detectable in the early immature cells such as hematopoietic precursor cells in the bone marrow at infantile ages, it gradually disappears in somatic cells during aging and becomes undetectable in normal cells of adult humans.10,11 Malignant cells or transformed cells show telomerase activity in the adult; therefore, it is suggested that the existence of telomerase activity is a possible marker for the malignancy.12 The appearance of telomerase activity is suggested to be involved in Epstein-Barr virus (EBV) transformation of B-lineage cells.13
However, the results of recent studies showed that the similar level of telomerase activity can be induced in adult peripheral lymphocytes in vitro by the stimulation with mitogens11 and by the cross-linkage of T-cell antigen receptor (TCR).14 Stimulation with anti-CD3 monoclonal antibody (MoAb) or phorbol dibutylate plus calcium (PDB/Ca) induced telomerase activity in adult peripheral T cells, suggesting that TCR-mediated signaling is involved in the induction of telomerase activity. In contrast, the B-cell antigen receptor (BCR) cross-linkage with soluble anti-IgM Ab did not induce a demonstrable telomerase activity. It is not clear whether normal B cells can express inducible telomerase activity to the antigenic stimulation or whether the appearance of telomerase activity is closely related to the malignant transformation of B cells with EBV. We speculated that normal B cells might also express telomerase activity during the rapid proliferation in the peripheral lymphoid organs of adult life. To address these issues, we studied whether adult peripheral B cells express telomerase activity in vitro after stimulation with various reagents that are involved in B-cell proliferation of the physiological immune response. The results clearly demonstrate that antigenic stimulation induces telomerase activity in normal B cells from peripheral lymphoid organs.
MATERIALS AND METHODS
Preparation of cells.Tonsils were obtained from tonsillectomy specimens from Kumamoto Citizen Hospital, Asai Clinic, Ogata Clinic, and Toriya Clinic (Kumamoto, Japan). Tonsillar mononuclear cells were separated by the standard Ficoll/Conray gradient method. Cells were washed with the buffer containing amphotericin B (2.5 μg/mL; Wako Co, Osaka, Japan) and gentamicin (200 μg/mL; Wako). Peripheral blood lymphocytes (PBLs) were isolated from healthy donors by Ficoll/Conray density gradient centrifugation. B-cell enrichment with B-cell lympho-kwik and T cell with T-cell lympho-kwik were performed as described in the company's protocol (One Lambda Inc, Los Angeles, CA). The enrichment was tested by staining with anti-CD3 MoAb and anti-IgM Ab. B-cell fractions usually show purity of more than 95% of sIgM+ CD3−. Purified B or T cells were cultured in RPMI-1640 medium supplemented with 10% heat-inactivated fetal calf serum, 2 mmol/L L-glutamine, 50 mmol/L 2-mercaptoethanol, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in a 5% CO2 humidified incubator.
Reagents.Ca ionophore (A23187; Calbiochem, La Jolla, CA), PDB (Sigma, St Louis, MO), goat F(ab′)2 antihuman IgM Ab (Zymed Laboratories Inc, South San Francisco, CA), goat antihuman IgM sepharose-beads (EY Laboratories Inc, San Mateo, CA), biotin-labeled mouse antihuman IgM MoAb (Zymed), biotin-labeled mouse antihuman IgD MoAb (Southern Biotechnology Associates Inc, Birmingham, AL), streptavidin (Vector Laboratories Inc, Burlingame, CA), and Wortmannin (Sigma) were used. Anti-CD3 MoAb (OKT3, IgG2a) was purified as described previously.14 Anti-CD40 MoAb and anti-CD19 MoAb were kind gifts of Dr T.F. Tedder (Duke Medical Center, Durham, NC). Anti-HLA DR MoAb was a kind gift of Dr Y. Nishimura (Kumamoto University Graduate School of Medical Sciences, Kumamoto, Japan). The recombinant cytokines were obtained as interleukin (IL-2) from Ajinomoto Co (Tokyo, Japan), IL-4 from Onoyakuhin Co (Osaka, Japan), and IL-6 from Dr K. Yasukawa (Tosoh Co, Tokyo, Japan). IL-13 was purchased (Pepro Tech Inc, James Square, London, UK).
B-cell proliferation assay.Purified B cells were cultured in flat-bottomed microtiter plates at a concentration of 1 × 105 cells in the presence of various stimulatory reagents as described. Triplicate cultures were pulsed with 1 μCi/well of [3H]-thymidine (TdR; 25 Ci/mmol; Amersham Corp, Arlington Heights, IL) for the final 16 hours of culture. After harvesting cells, the incorporation of radioactivity was determined by a liquid scintillation counter (LSC-3050; Aloka Co, Tokyo, Japan).
Telomere repeat amplification protocol (TRAP) assay.Telomerase activity in the cell lysates was measured by the TRAP method described by Kim et al.12 Briefly, cultured cells were collected and washed twice with ice-cold wash buffer (10 mmol/L HEPES-KOH [pH, 7.5], 1.5 mmol/L MgCl2 , 10 mmol/L KCl, 1 mmol/L dithiothreitol). The viable cells (10 million) were lysed with 20 μL of the hypotonic detergent lysis buffer (10 mmol/L Tris-HCl [pH, 7.5], 1 mmol/L MgCl2 , 1 mmol/L EGTA, 0.1 mmol/L phenylmethylsulfonyl fluoride [PMSF], 5 mmol/L 2-mercaptoethanol, 0.5% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfate (CHAPS), 10% glycerol). The protein concentration was adjusted after measurement by the Bladford method (Protein Assay kit; Bio-Rad Laboratories, Richmond, CA). Before the polymerase chain reaction (PCR), 0.1 μg of CX primer (5′-CCCTTACCCTTACCCTTACCCTAA-3′) was lyophilized in the Hot-Start tube (Molecular Bio-Products, San Diego, CA). After sealing the CX primer by heating at 90°C for 30 seconds, 50 μL of TRAP reaction buffer containing 20 mmol/L Tris-HCl (pH, 8.3), 1.5 mmol/L MgCl2, 63 mmol/L KCl, 0.005% Tween 20 (Sigma), 1 mmol/L EGTA, 0.1 μg of TS oligonucleotide (5′-AATCCGTCGAGCAGAGTT-3′), 5 μmol/L of each deoxynucleotide triphosphate, 1 μg of T4-gene 32 protein (US Biochemical, Cleveland, OH), 2 U of Taq DNA polymerase (Ampli-Taq; Perkin Elmer-Cetus, Norwalk, CT), 2 μL of appropriately diluted CHAPS cell extract, and 5 μCi of [α-32P] deoxycytidine triphosphate (3,000 Ci/mmol; Amersham) was added onto a solidified WAX. Then, tubes were set in a thermal cycler (Perkin Elmer-Cetus), and telomere elongation was conducted for 10 minutes at 23°C. The PCR amplification was achieved with 27 cycles of incubation at 94°C for 30 seconds, 50°C for 30 seconds, and 72°C for 1.5 minutes. Aliquots (10 μL) of radiolabeled PCR products were separated on 15% polyacrylamide gel electrophoresis. The gels were dried and exposed for autoradiography. Densitometric measurements were made with the use of a BA100SF image analyzer (Fuji Co, Ltd, Tokyo, Japan).
RESULTS
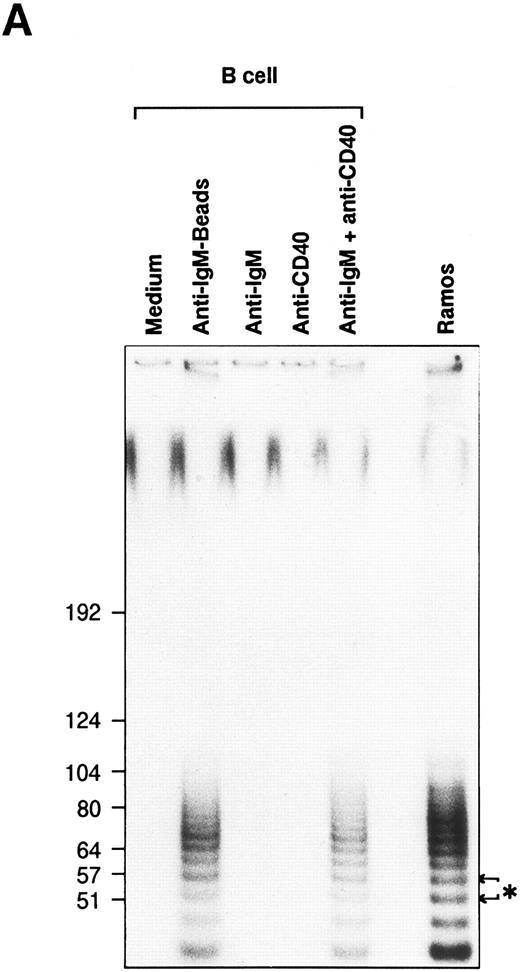
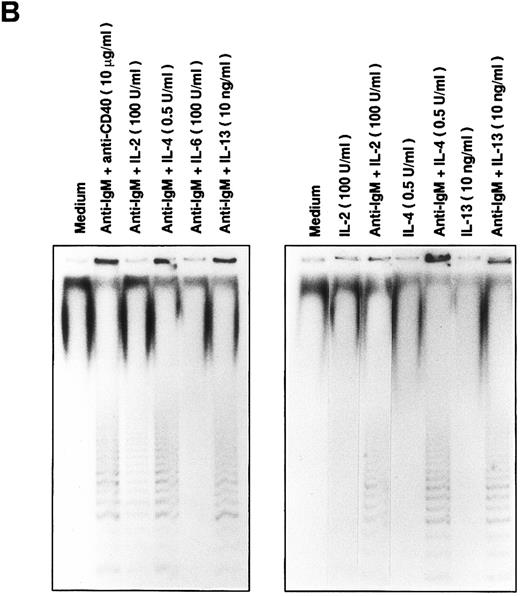
Telomerase activity is induced in normal B cells.To study the inducible expression of telomerase activity in normal human B cells, B cells were purified from tonsils as described in Materials and Methods. The purified B cells, more than 95% of sIgM+CD3− B cells, were stimulated with various stimulatory reagents that induce the proliferation of B cells in vitro. We used a sensitive TRAP method that measures the radioactive PCR products of oligonulceotide primarily catalyzed by the cellular telomerase activity. Primary telomeric repeats, synthesized onto the nontelomeric oligonucleotide (TS) derived from the sequence of the chromosome 16 break-point junction found in α-thalassaemia,15 are then amplified using a set of primers as described in Materials and Methods. Stimulation with anti-IgM beads induced telomerase activity in B cells from about half of the healthy volunteers tested, one of which is shown by the TRAP assay with 6-bp ladder bands (Fig 1A, the 6-bp distance is shown by an asterisk). Treatment by RNase or phenol extraction of the extracts abolished the ladder bands, confirming that the ladder bands were created by the induced-telomerase activity in B cells (data not shown). Soluble anti-IgM Ab or anti-CD40 MoAb alone did not induce detectable telomerase activity; however, the combination of these Abs induced telomerase activity in B cells (Fig 1A). Single addition of either anti-IgM Ab or anti-CD40 MoAb did not induce the activity at doses up to 100 μg/mL (data not shown). Telomerase activity induced in normal B cells is weaker than that of a Burkitt lymphoma cell line (Ramos), indicating that the BCR cross-linkage induced comparable telomerase activity in normal B cells. To test the additional molecules involved in the induction of telomerase activity, B cells were stimulated with soluble anti-IgM Ab and several cytokines (IL-2, IL-4, IL-6, and IL-13), which act synergistically on B-cell proliferation and differentiation. Telomerase activity is detected in B cells by the combinatory stimulation with soluble anti-IgM Ab and one of the cytokines of IL-2, IL-4, and IL-13, whereas IL-2, IL-4, or IL-13 alone does not induce comparable telomerase activity (Fig 1B). Parallel experiments were performed to test the proliferation of B cells by the same stimuli. To adjust the extent of B-cell proliferation, we first measured the [3H]-TdR incorporation and prepared the cell lysates from B cells stimulated with anti-IgM Ab and appropriate doses of cytokines. Using the above stimulatory conditions, B cells are mostly alive (viability around 90 %) after a 48 hour-culture in vitro. After learning what equivalent doses of cytokines induce similar proliferation of B cells, TRAP assay was performed at least three times using three sets of dilution of the cellular lysates, and the result of a typical experiment is shown in percent response of telomerase activity (Table 1). Cells are recovered in proportion to the amount of thymidine incorporation. IL-6 did not induce the similar proliferation of B cells or telomerase activity in combination with anti-IgM Ab. IL-4 and IL-13 induced comparable levels of the activity (IL-4, 84.3%; IL-13, 62.1%), but IL-2 induced a lower level of activity (32.7%), despite inducing a similar level of proliferation (6,228 cpm). The response appears as early as 12 hours and continues until 72 hours after the stimulation with soluble anti-IgM Ab and anti-CD40 MoAb (Fig 1C). Anti-class II MoAb (HLA DR) and anti-CD19 MoAb used as negative controls for anti-CD40 MoAb did not show any induction of the activity (data not shown). These results clearly showed that telomerase activity is induced in BCR-stimulated B cells.
Induction of telomerase activity in normal B cells. Intrinsic telomerase activity was measured by TRAP assay as described in Materials and Methods. (A) Purified B cells from tonsils were stimulated in vitro with soluble goat antihuman IgM Ab (10 μg/mL), goat antihuman IgM Ab bound to beads (10 μg/mL), anti-CD40 MoAb (10 μg/mL) or soluble anti-IgM Ab plus anti-CD40 MoAb for 2 days. (B) Tonsillar B cells were stimulated with various kinds of cytokines in the presence or absence of soluble anti-IgM Ab (10 μg/mL) for 2 days. TRAP assay was performed using the lysate from cells that showed equivalent proliferation. (C) Telomerase activity was measured at the points of 12, 24, 48, 72, 96, 120, 144, and 168 hours after the stimulation with soluble anti-IgM Ab plus anti-CD40 MoAb.
Induction of telomerase activity in normal B cells. Intrinsic telomerase activity was measured by TRAP assay as described in Materials and Methods. (A) Purified B cells from tonsils were stimulated in vitro with soluble goat antihuman IgM Ab (10 μg/mL), goat antihuman IgM Ab bound to beads (10 μg/mL), anti-CD40 MoAb (10 μg/mL) or soluble anti-IgM Ab plus anti-CD40 MoAb for 2 days. (B) Tonsillar B cells were stimulated with various kinds of cytokines in the presence or absence of soluble anti-IgM Ab (10 μg/mL) for 2 days. TRAP assay was performed using the lysate from cells that showed equivalent proliferation. (C) Telomerase activity was measured at the points of 12, 24, 48, 72, 96, 120, 144, and 168 hours after the stimulation with soluble anti-IgM Ab plus anti-CD40 MoAb.
B-Cell Proliferation Induced by Various Stimuli In Vitro
| . | [3H]-TdR Incorporation* (cpm) . | Response in Telomerase Activity† (%) . |
|---|---|---|
| Medium | 573 ± 68 | Not detectable |
| Anti-IgM (10 μg/mL) | 1,652 ± 473 | 0.2 |
| Anti-IgM-Beads (10 μg/mL) | 7,267 ± 77 | 115.5 |
| Anti-IgM + anti-CD40 (10 μg/mL) | 6,716 ± 290 | 100 |
| Anti-IgM + IL-2 (100 U/mL) | 6,228 ± 314 | 32.7 |
| Anti-IgM + IL-4 (0.5 U/mL) | 6,608 ± 793 | 84.3 |
| Anti-IgM + IL-6 (100 U/mL) | 990 ± 106 | 0.4 |
| Anti-IgM + IL-13 (10 ng/mL) | 6,398 ± 524 | 62.1 |
| . | [3H]-TdR Incorporation* (cpm) . | Response in Telomerase Activity† (%) . |
|---|---|---|
| Medium | 573 ± 68 | Not detectable |
| Anti-IgM (10 μg/mL) | 1,652 ± 473 | 0.2 |
| Anti-IgM-Beads (10 μg/mL) | 7,267 ± 77 | 115.5 |
| Anti-IgM + anti-CD40 (10 μg/mL) | 6,716 ± 290 | 100 |
| Anti-IgM + IL-2 (100 U/mL) | 6,228 ± 314 | 32.7 |
| Anti-IgM + IL-4 (0.5 U/mL) | 6,608 ± 793 | 84.3 |
| Anti-IgM + IL-6 (100 U/mL) | 990 ± 106 | 0.4 |
| Anti-IgM + IL-13 (10 ng/mL) | 6,398 ± 524 | 62.1 |
Mean cpm ± SD (n = 3).
Percentage response in telomerase activity was determined by densitometric measurement with image analyzer. Percentage activity was calculated as the relative intensity in comparison to the maximum response (anti-IgM Ab + anti-CD40 MoAb).
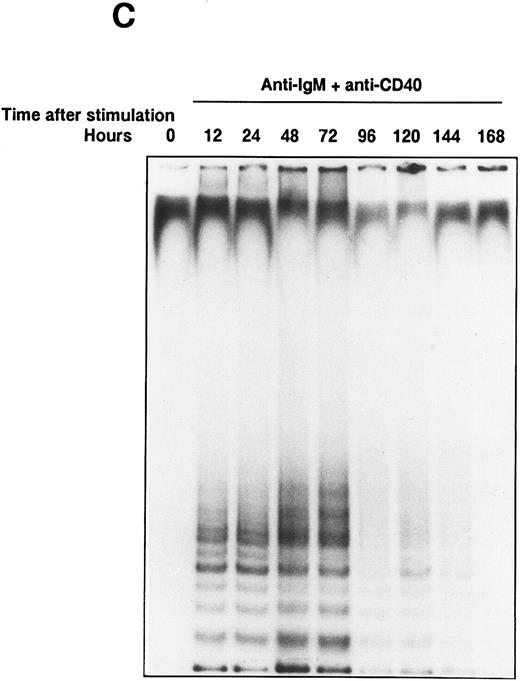
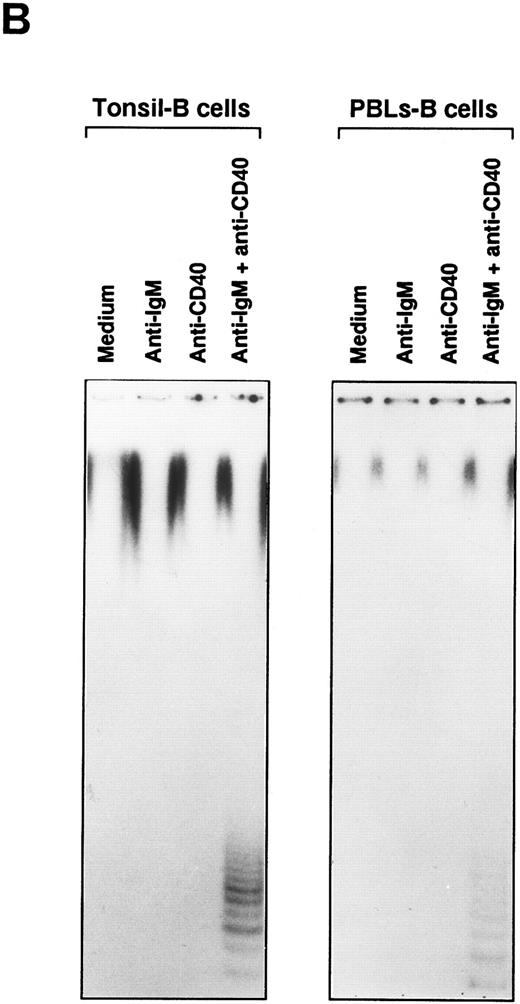
Telomerase activity is induced in B cells by anti-IgM and anti-IgD Abs.Peripheral B cells are composed of cells at heterogeneous stages of B-cell development. Surface IgD expression, as sIgM+sIgD− and sIgM+sIgD+, is a useful marker to differentiate B cells into immature and mature stages.16, 17 We studied whether there are differences in the potential of isotype-dependent signals to induce telomerase activity in B cells. Induction of telomerase activity was measured with biotinylated MoAb against IgM or IgD. Both MoAbs did not induce telomerase activity even after hypercross-linkage with streptavidin. Addition of anti-CD40 MoAb induced telomerase activity in both anti-IgM– and anti-IgD–stimulated B cells. Hypercross-linkage with streptavidin augmented the induction of the activity in both cases (Fig 2A). We could detect the inducible telomerase activity in B cells from both tonsils and PBLs by stimulation with anti-IgM Ab and anti-CD40 MoAb as shown in Fig 2B, although the level of activity is higher in tonsillar B cells. These results indicate that both sIgM and sIgD can mediate signals for the induction of telomerase activity.
Induction of telomerase activity in B cells of tonsils and PBLs. (A) Tonsillar B cells were stimulated with either biotin-labeled mouse antihuman IgM MoAb (10 μg/mL) or biotin-labeled mouse antihuman IgD MoAb (10 μg/mL) in combination with anti-CD40 MoAb (10 μg/mL). Streptavidin (20 μg/mL) was used for the hypercross-linkage of BCR. (B) B cells of tonsils and PBLs from the same donor were stimulated for 2 days with the various stimulatory reagents indicated. After stimulation, TRAP assay was conducted.
Induction of telomerase activity in B cells of tonsils and PBLs. (A) Tonsillar B cells were stimulated with either biotin-labeled mouse antihuman IgM MoAb (10 μg/mL) or biotin-labeled mouse antihuman IgD MoAb (10 μg/mL) in combination with anti-CD40 MoAb (10 μg/mL). Streptavidin (20 μg/mL) was used for the hypercross-linkage of BCR. (B) B cells of tonsils and PBLs from the same donor were stimulated for 2 days with the various stimulatory reagents indicated. After stimulation, TRAP assay was conducted.
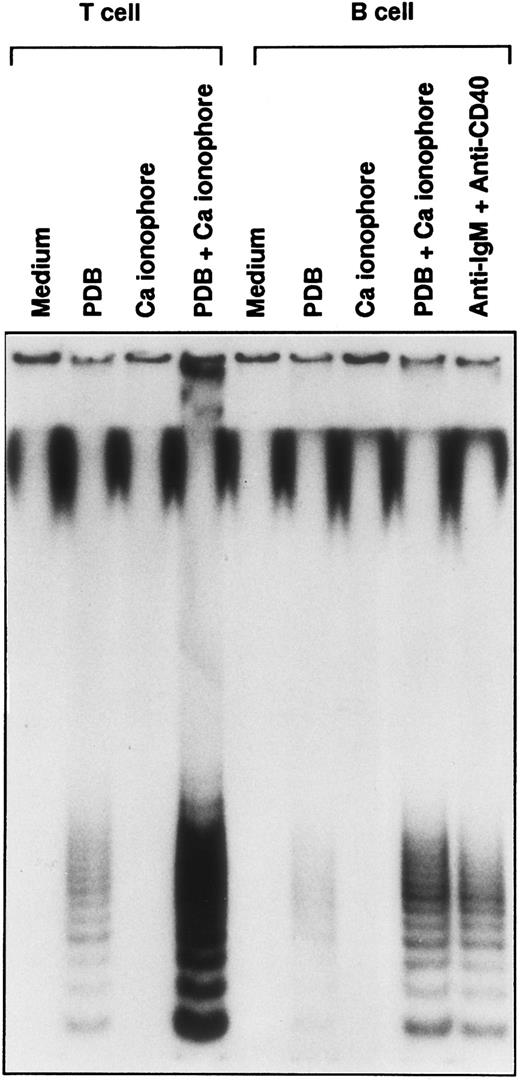
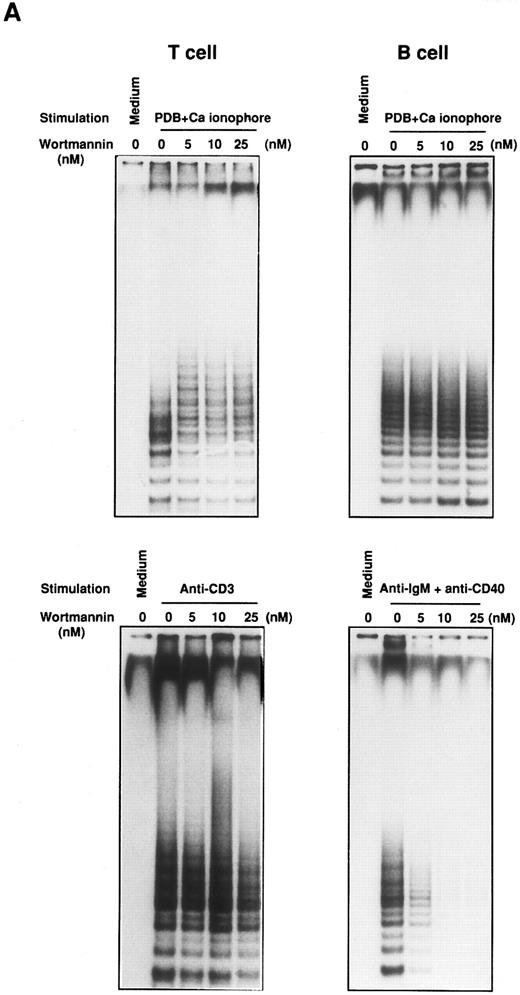
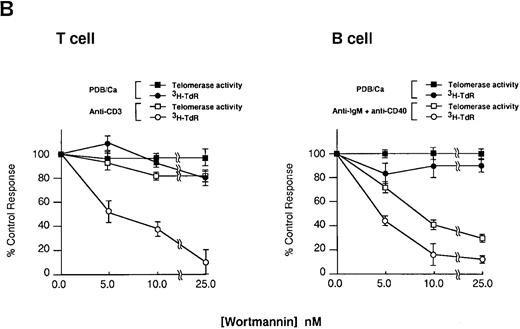
Telomerase activity is induced in B cells triggered through BCR-mediated signals.Because PDB/Ca could replace the stimulation of CD3 cross-linkage in the induction of telomerase activity,14 we studied the induction of telomerase activity in B cells by the same stimulation. The doses of PDB and Ca ionophore are predetermined by the proliferation assay (data not shown). As shown in Fig 3, PDB alone weakly induced telomerase activity in T and B cells, but Ca ionophore alone did not. PDB/Ca induced telomerase activity in B cells as similarly detected by the combination with soluble anti-IgM Ab and anti-CD40 MoAb, suggesting that PKC-mediated signal transduction and activation of Ca-dependent pathway transmit the signals resulting in the increase of telomerase activity in both T and B cells. Since PI-3 kinase is associated with BCR-complex and is activated by the cross-linkage with anti-IgM Ab,18 19 we studied whether PI3-kinase activity is involved in the induction of telomerase activity. Treatment of B cells with Wortmannin inhibited the induction of telomerase activity in B cells by anti-IgM Ab and anti-CD40 MoAb, whereas the same doses had less influence on T cells stimulated by anti-CD3 MoAb (Fig 4A lower panel and 4B; marked with -□-). The similar Wortmannin treatment inhibited the proliferation of both B and T cells (Fig 4B; marked with -○-). These results suggested that telomerase activity is induced in B cells by the signal through the PI-3 kinase-dependent pathway, which seemed to be different from the signal in T cells. On the contrary, Wortmannin treatment at the doses of 5 to 25 nmol/L did not influence the induction of telomerase activity and the proliferation of PDB/Ca-stimulated cells (Fig 4B; marked with -▪- and -•-). Both B and T cells stimulated by PDB/Ca showed the inducible telomerase activity over a wide range of Wortmannin treatment (Fig 4A; upper panel and Fig 4B; marked with -▪-). These results suggested that telomerase activity is induced by two kinds of signals mediated as Wortmannin-sensitive and insensitive pathways in B cells. The Wortmannin-sensitive (PI-3 kinase-dependent) pathway is functionally used in BCR-mediated signal transduction in B cells.
Involvement of PKC-mediated and Ca-dependent signals in the induction of telomerase activity. Purified T and B cells from the same donor were stimulated in vitro with PDB (20 ng/mL), Ca ionophore (0.1 μg/mL), or both of them for 2 days. The response of B cells, stimulated with soluble anti-IgM Ab plus anti-CD40 MoAb was compared parallel.
Involvement of PKC-mediated and Ca-dependent signals in the induction of telomerase activity. Purified T and B cells from the same donor were stimulated in vitro with PDB (20 ng/mL), Ca ionophore (0.1 μg/mL), or both of them for 2 days. The response of B cells, stimulated with soluble anti-IgM Ab plus anti-CD40 MoAb was compared parallel.
Effect of Wortmannin on the proliferation and telomerase activity in stimulated lymphocytes. Purified tonsillar B and T cells from the same donor were pretreated for 30 minutes in the presence of various concentrations of Wortmannin and were stimulated for 2 days with either one of the combination of PDB plus Ca ionophore or soluble anti-IgM Ab plus anti-CD40 MoAb for B cells or PDB plus Ca ionophore or anti-CD3 MoAb for T cells. After the culture, TRAP assay was performed (A).
Relative increase of telomerase activity was determined by densitometric scanning on the autoradiography film by focusing on the ladder bands. Relative intensity was calculated using the positive control with anti-IgM Ab and anti-CD40 MoAb stimulation as 100% and the negative control without any stimulation as 0%. The mean and standard deviation (SD) are given for extracts that were assayed twice. Cell proliferation assay was performed as described in Materials and Methods. The data are shown by the mean ± SD of triplicate assay performed in two separate experiments (B).
Effect of Wortmannin on the proliferation and telomerase activity in stimulated lymphocytes. Purified tonsillar B and T cells from the same donor were pretreated for 30 minutes in the presence of various concentrations of Wortmannin and were stimulated for 2 days with either one of the combination of PDB plus Ca ionophore or soluble anti-IgM Ab plus anti-CD40 MoAb for B cells or PDB plus Ca ionophore or anti-CD3 MoAb for T cells. After the culture, TRAP assay was performed (A).
Relative increase of telomerase activity was determined by densitometric scanning on the autoradiography film by focusing on the ladder bands. Relative intensity was calculated using the positive control with anti-IgM Ab and anti-CD40 MoAb stimulation as 100% and the negative control without any stimulation as 0%. The mean and standard deviation (SD) are given for extracts that were assayed twice. Cell proliferation assay was performed as described in Materials and Methods. The data are shown by the mean ± SD of triplicate assay performed in two separate experiments (B).
DISCUSSION
Normal human B cells are capable of inducing telomerase activity in vitro. Unstimulated B cells from adult PBL express low or undetectable activity as reported previously.11 20 Cross-linkage of B cells with anti-IgM beads induces telomerase activity, although the level is lower (about 60%) than those in a Burkitt B-cell lymphoma (Fig 1A) and B-leukemic cell lines (data not shown). Soluble anti-IgM Ab could not induce a detectable level of telomerase activity, whereas synergistic stimulation with soluble anti-IgM Ab and the cytokines IL-2, IL-4, and IL-13 induced demonstrable telomerase activity. Alternatively, the stimulation of two kinds of surface molecules, BCR and CD40, also induced the maximum level of telomerase activity in B cells. In addition, PDB/Ca, which probably replace the action of antigen receptor-mediated signal transduction, also induced telomerase activity in B cells. Therefore, it is suggested that antigen-driven B cells in the peripheral lymphoid organs express telomerase activity in the conventional antigen-specific immune response.
Cross-linkage of BCR with anti-IgM beads induces the proliferation and differentiation of B cells.21 Previous studies showed that anti-IgM beads effectively cross-link IgM receptors multivalently on B cells, which seems to be representative of T-cell–independent antigen (TI antigen) or the multivalent antigens. A soluble antigen with only a single antigenic determinant, referred to as T-cell–dependent antigen (TD antigen), less effectively stimulates B cells. To induce the activation of such B cells, costimulatory signals are necessary from activated T cells as cytokines or costimulatory molecules.22 Human B cells express the inducible telomerase activity by stimulation with multivalent cross-linkage of BCR with anti-IgM beads. However, stimulation with soluble anti-IgM Ab does not induce a detectable level of the activity, which probably represents the activation of B cells with TD antigen. Telomerase activity is induced in B cells that are effectively stimulated for the proliferation in vitro. The effect of B-cell stimulatory cytokine(s) is measured by the costimulatory assay cultured in vitro with anti-IgM Ab. IL-2, IL-4, and IL-13 augmented the proliferation of B cells in this assay.23-25 Telomerase activity is also induced with IL-2, IL-4, and IL-13 in B cells triggered with soluble anti-IgM Ab, although the levels of telomerase activity induced with anti-IgM Ab and cytokines are not parallel to the amounts of B-cell proliferation (Table 1). Particularly, IL-2 causing the high level proliferation of anti-IgM–stimulated B cells, do not augment the comparable amount of telomerase activity as similarly seen in T cells that were stimulated successively with anti-CD3 Ab and IL-2 as shown previously.14 Recent studies provided information concerning signal transduction pathways through cytokine receptors. Receptors for IL-2 and IL-4 share a receptor subunit of the common γ chain, which transmits a signal by the action of Jak1 and Jak3,26 whereas the IL-13 receptor transmits a signal mediated by Jak1 and Tyk2.27 Both types of signal transduction pathways might be involved in the induction of telomerase activity in B cells. Regardless of the common γ chain involvement, IL-2 and IL-4 cause different actions in B cells, which might be related to the difference of the α chains.28 29 Stimulation with anti-IgM Ab and anti-CD40 MoAb also induced telomerase activity in B cells. CD40 is a B-cell surface molecule that is considered to mediate the signal necessary for the survival and the long-term existence of antigen-driven B cells in the germinal center of the peripheral lymph nodes. The results presented here suggest that peripheral B cells would express telomerase activity during the active proliferation in vivo.
The physiological role of the inducible telomerase activity is still undetermined. Recent reports showed the expression of telomerase activity in hematopoietic precursor cells, especially in self-renewal stem cells of CD34+/lineage marker negative cell population.11 This evidence suggests that telomerase activity in normal hematopoietic cells is involved in the specific function to maintain the homeostasis of hematopoietic dynamics. Inducible telomerase activity in lymphocytes was observed in all healthy individuals tested from the age of 21 to 94 years old (data not shown). It might be one of the molecules that are upregulated by the signals through the antigen receptor-mediated activation of lymphocytes and do not have any specific function. We compared the levels of inducible telomerase activity in B cells of the tonsils and the PBLs. The purified B cells from both tissues showed inducible telomerase activity, suggesting that it appears in B cells at most developmental stages. The activity is always induced by the stimulation of BCR and CD40 molecules on B cells. Silvy et al30 suggested that anti-BCR and anti-CD40 Abs synergize for the growth of CD38− B cells while suppressing their capacity to differentiate along the plasmacytoid pathway and cause the clonal expansion of the memory pool. Once telomerase activity is induced in lymphocytes, it will elongate several hundred base pairs at the telomeres, which are probably sufficient lengths for the proliferation and expansion of B-cell clones.
It has been shown that pathways leading to both PKC activation and elevation of intracellular Ca2+ are critical for BCR-mediated G0 to G1 transition. Anti-BCR–induced signaling is bypassed by direct facilitating protein kinase C activation and Ca2+ mobilization using PDB and Ca ionophore in the majority of resting B cells, resulting in the transition into the G1 in the absence of additional signaling from accessory cells or cytokines.31 PDB/Ca induced the proliferation and telomerase activity of both B and T cells, which may suggest that BCR and TCR transmit the similar signals for the induction of telomerase activity. However, there is a peculiar difference in the sensitivity to PI3-kinase inhibitor Wortmannin between T and B cells.
Wortmannin treatment inhibited the induction of the proliferation and telomerase activity of B cells. Telomerase induction through BCR is sensitive to Wortmannin, although the induction through TCR is not. This difference could be explained by two kinds of models for the signal transduction pathways of telomerase induction in lymphocytes. One is that telomerase activity might be induced through two distinct signal transduction pathways of PI-3 kinase-dependent and -independent. In B cells, both types of pathways may transmit the signals leading to telomerase activity, as well as cell growth in vitro. PDB stimulation would cause the extensive activation of multiple PKC molecules32 including the BCR-associated and nonassociated PKCs, which probably induce telomerase activity in B cells. By stimulation with natural ligands or the Abs that trigger on the specific cell surface molecules, B cells express the telomerase activity through PI-3 kinase-dependent manner. The other possibility is that a specified PKC, whose activation is dependent on the upstream PI-3 kinase activity,33-35 might be involved in telomerase induction in B cells. Upstream regulation of novel PKC molecules such as PKCδ, ε, or η by the phosphatidylinositol-3, 4, 5-triphosphate (PIP3 ) produced with PI-3 kinase is reported both in vitro and in vivo. Among the members of PKCs, PKCε is the most sensitive to the second messenger, PIP3 , which is produced by PI-3 kinase activity.35 The PI-3 kinase inhibitor Wortmannin would block the PI-3 kinase-induced PKCε activation by anti-IgM and anti-CD40 stimulation, but would not block the induction by phorbol esters, which bypass PI-3 kinase.36 37
An important factor potentially controlling the expression of telomerase activity is the phase of the cell cycle. In tumor cell lines, telomerase activity is regulated in a cell cycle-dependent manner.38 Progression through the S phase was associated with increased telomerase activity. However, withdrawal from the cell cycle by serum deprivation did not cause a reduction of telomerase activity, suggesting that the expression of telomerase activity is not directly linked with the cell cycle progression. Our previous observation also showed that the induction of telomerase activity is not directly linked to the extent of the proliferation in anti-CD3–stimulated T cells.14 A number of studies analyzed the inhibition of telomerase activity by the cell cycle blockades commonly used for cancer therapy. Telomerase activity was inhibited by transforming growth factor-β1, methotrexate, and 5-fluorouracil, but not by hydroxyurea,38 suggesting that the expression of telomerase activity in tumor cells is also regulated by several complex mechanisms. Our results did not explain whether the increased telomerase activity is due to an increase in the specific activity of the enzyme or increased expression. We also do not know whether the inducible telomerase in B cells is identical to that of malignant cells. The antigen receptor signaling is potentially a useful system for the study of telomerase involvement in cell proliferation of both normal and malignant cells.
Recently, Greenwell et al39 isolated a cDNA clone, TEL1, that is involved in the telomere elongation in Saccharomyces cerevisiae. Tel1p is not a telomerase itself, but its mutation causes telomere shortening in the yeast. Amino acid sequence showed that tel1p is a member of PI-3 kinase-related kinase. A human homologue of such PI-3 kinase-related members could be involved in the regulation of telomerase activity in B cells. It is necessary to study the molecular mechanism for the induction of telomerase activity through the PI-3 kinase-related pathway in B cells. PI-3 kinase-dependent signal would play an important role in the BCR-mediated signal transduction for the proliferation and long-term survival of B cells.
Supported by research grants from the Ministry of Education, Science, Culture, and Sports of Japan and from The Agency in the Promotion of Science and Technology, Tokyo, Japan.
Address reprint requests to Nobuo Sakaguchi, MD, Department of Immunology, Kumamoto University School of Medicine, 2-2-1, Honjo, Kumamoto, 860, Japan.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal