Abstract
The CD2 glycoprotein has been implicated in both positive and negative regulation of T-cell mitogenesis. To study the involvement of CD2 in T-lymphocyte development and immune responses, we have analyzed two lines of CD2-null mice, each expressing a distinct class I major histocompatibility complex (MHC)-restricted T-cell receptor (TCR). In both situations, the absence of CD2 appeared to promote the positive selection of cells in a manner that is similar to that which occurs in the absence of CD5. Consistent with this, compound homozygotes that lacked both CD2 and CD5 showed evidence of enhanced positive selection even in the absence of a transgenic TCR. Despite the observed enhancement of positive selection, the lack of CD2 was associated with defects in proliferative responses and interferon-γ production when transgenic thymocytes and mature T lymphocytes were stimulated with the appropriate antigens. These findings raise the possibility that impaired sensitivity to selecting ligands in the thymus may provide a selective advantage that improves the efficiency of positive selection for certain TCRs. Furthermore, the results highlight the potential for a differential role for CD2 in thymocyte selection and T-cell immune responses.
THE CD2 CELL SURFACE glycoprotein was initially identified with monoclonal antibodies (MoAbs) that could either block or promote T-lymphocyte proliferation.1 It is a 40 to 60 kD member of the Ig superfamily that is expressed on T cells and natural killer (NK) cells in all species that have been studied and also found on rat and sheep splenic macrophages and mouse B cells.2-5 Ligands for CD2 include CD58, (lymphocyte function-associated antigen-3 [LFA-3]) CD48, and CD59,6-8 although binding between CD2 and CD59 may be of low or inconsequential affinity.9,10 CD48 and CD58 are found on endothelium and most hematopoietic cells in humans, but rodents may express only CD48.3 11
CD2 is expressed early in T-cell ontogeny after CD4−CD8− double negative thymocytes receive a signal to progress past the CD44− interleukin-2 receptor α-chain–positive (IL-2Rα+) stage on their way to expressing both CD4 and CD8.4,12-14 Mature single-positive (CD4+CD8− or CD4−CD8+) thymocytes express more CD2 than do their immediate CD4+CD8+ (double-positive) precursors.12 Interestingly, this pattern of expression is similar to that of CD5, a 68-kD cell surface glycoprotein that was recently shown to act as a negative regulator of thymocyte T-cell receptor (TCR) signal transduction.15 CD48 is also expressed in the thymus on cells of hematopoietic origin, whereas CD58 expression on thymic epithelium is controversial.16-18
CD2 has a relatively large cytoplasmic domain that is capable of interacting with signaling molecules such as p56lck and p59fyn.19-25 Immunoprecipitation experiments have provided evidence that CD2 can be closely associated with the antigen receptor (TCR) on the surface of T cells.21,26 Whereas γδ T cells can be activated with individual anti-CD2 MoAbs,27,28 a distinctive feature of αβ T-cell activation is the requirement for simultaneous addition of pairs of antibodies recognizing separate epitopes on the CD2 molecule.29 30 Neither the molecular basis for this last requirement nor the overall significance of CD2-mediated mitogenesis are well understood.
Inhibition of T-cell function with anti-CD2 MoAbs has been observed both in vitro and in vivo. In some instances, the effects of the antibodies cannot be adequately explained by simple blockade of ligand-receptor interactions. For example, T cells from mice that have been treated with a nondepleting anti-CD2 antibody show long-lasting hyporesponsiveness to stimulation with anti-CD3 MoAb, superantigens, or alloantigens, well after cell surface expression of CD2 is restored.31 Furthermore, anti-CD2 can prolong the survival of allografts when administered at the time of engraftment and this effect can be substantially enhanced by the coadministration of anti-CD48.32 A CD58/IgG fusion protein has also been used to block T-cell responses in a manner that further implicates CD2 in the induction of a nonresponsive state.33 In this case, the fusion protein blocked both xenogeneic and secondary allogeneic mixed lymphocyte reactions (MLRs), both of which are insensitive to CD2/CD58 blockade with MoAbs.
Two recent reports have suggested that CD2 signaling may be important for regulating the secretion or effectiveness of cytokines that drive the differentiation of Th1 cells. Thus, MoAbs directed against CD2 or CD58 can selectively inhibit IL-12–induced proliferation and interferon-γ (IFN-γ) production by phytohemagglutinin-activated T cells without affecting responsiveness to IL-2.34 Furthermore, mitogenic pairs of anti-CD2 MoAbs synergized with IL-12 in inducing both T-cell proliferation and IFN-γ production. In other experiments, interactions between CD2 on T cells and CD58 on monocytes have also been shown to be important for the synthesis of IFN-γ by T cells.35
Mice that lack expression of CD2 support the development of T and B cells that can mediate robust immune responses in several different settings.36,37 Despite this, we now find evidence that the development of T cells is atypical when CD2 is absent, suggesting that CD2 may act as a negative regulator of thymocyte sensitivity to positive selection signals. Consistent with this and the previously described phenotype of a CD5-null mouse,15 double null mice that lack both CD5 and CD2 showed increased proportions of single-positive cells in the thymus and a reduced representation of double-positive cells. Finally, peripheral CD8+ T cells from transgenic CD2-null mice showed impaired antigen-specific proliferative responses and IFN-γ production. In summary, the results support a subtle role for CD2 in T-lymphocyte development and the regulation of immune responses.
MATERIALS AND METHODS
Mice.C57BL/6, B10.BR, and DBA/2 mice were obtained from the Jackson Laboratories (Bar Harbor, ME). H-2b and H-2d H-Y,38 H-2b 2C,39 CD2 knockout,36 CD5 knockout,40 and β2-microglobulin knockout41 mice were produced as previously described. H-2k 2C mice were produced by backcrossing H-2b 2C mice to B10.BR (H-2k) mice and used after four to six generations of backcrossing. H-2b H-Y mice with the CD2 null mutation (CD2−/−) were produced by two generations of backcrossing of H-2b H-Y mice with H-2b CD2 null mice. Mice homozygous for the CD2 and CD5 null mutations were generated by mating mice heterozygous for these null mutations and selecting for progeny that were homozygous for these null mutations. Mice homozygous for the CD2 and/or CD5 null mutation were typed for the lack of CD2 and/or CD5 expression on peripheral blood lymphocytes. H-2b H-Y/CD2−/− mice were then backcrossed to CD2−/− mice for two more generations before being used for these studies. H-2b 2C/CD2−/− mice were produced and screened in a similar manner. H-2b H-Y and H-2b 2C mice homozygous for the β2m null mutation were produced by backcrossing the TCR transgenic mice with H-2b β2m knockout mice. Mice homozygous for the β2m null mutation were screened for the lack of class I major histocompatability complex (MHC) expression on peripheral blood lymphocytes. TCR transgenic mice was distinguished from nontrangenic littermates by staining with antibodies specific for the transgenic TCRs. Mice at 6 to 12 weeks of age were used for experimental studies.
Antibodies and flow cytometry.The following antibodies were used: anti-CD4 (GK1.5), anti-CD8 (53-6.7), anti-TCR Vβ8 (F23.1),42 anti-2C TCR idiotype (1B2),43 anti-TCR Vα3 (T3.70),44 anti-CD2 (RM2-5), anti-CD5 (53-7.3), anti-CD44 (Pgp-1), anti-CD69 (H1.2F3), anti-heat stable antigen (anti-HSA; M1/69),45 anti-KbDb (HB51). Biotinylated antibodies specific for CD2, CD5, or CD69 were obtained from Cedarlane (Hornby, Ontario, Canada). The hybridoma lines producing antibodies specific for KbDb or CD44 were obtained from the American Type Culture Collection (ATCC; Rockville, MD). Phycoerythrin-labeled GK1.5 MoAbs were obtained from Collaborative Biomedical Products (Bedford, MA). The Streptavidin-Tricolor reagent used to detect biotinylated antibodies was obtained from Cedarlane. Cell staining and flow cytometry were performed according to standard procedures. The Lysys II software program (Becton Dickinson, Mountain View, CA) was used for data acquisition and analysis. For 3-color analysis, a total of 30,000 events were acquired.
Proliferation assays.The indicated number of thymocytes or lymph node cells were stimulated with 5 × 105 irradiated (20 Gy) spleen cells in a volume of 0.20 mL in Iscove's Modified Dulbecco's Media supplemented with 5 × 10-5 mol/L 2-mercaptoethanol and antibiotics. All cultures were set up in triplicates. Where indicated, the cultures were supplemented with 20 U/mL of recombinant IL-2. The recombinant IL-2 was provided in the form of spent culture medium of IL-2 gene-transfected X63/0 cells,46 which typically contained ≈3,000 U IL-2/mL. For cultures involving 2C thymocytes, the proliferative response peaked at 72 hours and 1 μCi of 3H-thymidine (TdR) was added to each culture during the last 6 hours of the 72-hour culture period. The proliferative response for cultures involving H-Y thymocytes peaked at 84 hours and l μCi of 3H-TdR was added to each culture during the last 16 hours of the 84-hour culture period.
IFN-γ assay.This assay was performed using a 2-site MoAb enzyme-linked immunosorbent assay (ELISA) as previously described.47 The capture antibody used was R46A2 (obtained from ATCC), and the biotinylated anti–IFN-γ MoAb was XMG1.2,48 which was provided by Dr T. Mossman (University of Alberta, Edmonton, Canada). Recombinant IFN-γ used for calibrating the assay was obtained from Cedarlane.
RESULTS
Characteristics of TCR transgenic mice used in this study.Two different lines of TCR transgenic mice were selected to study the involvement of CD2 in thymocyte development. One line of mice expressed a TCR that is specific for the male (H-Y) antigen presented by H-2Db.38,49 This H-Y TCR is positively selected by H-2Db.49 The other TCR transgenic line expressed the 2C TCR, which is specific for the p2Ca peptide presented by H-2Ld.39,50 The p2Ca peptide is derived from a mitochondrial protein, 2-oxoglutarate dehydrogenase.51 The 2C TCR is positively selected by H-2Kb52 and has a very high affinity for the p2Ca/Ld ligand.53 There is evidence that the H-Y and the 2C TCRs may have different affinities for their respective positively-selecting ligands.54,55 Specifically, immature CD4+CD8+ thymocytes expressing the H-Y TCR in a positively-selecting environment can tolerate a high level of transgenic CD8 expression. By contrast, transgenic overexpression of CD8 causes the deletion of immature CD4+CD8+ thymocytes expressing the 2C TCR in a positively-selecting (H-2b) thymus.54 55 These two lines of transgenic mice therefore provided a means to evaluate the function of CD2 in the development of CD8+ T cells with apparently different affinity for their selecting ligands.
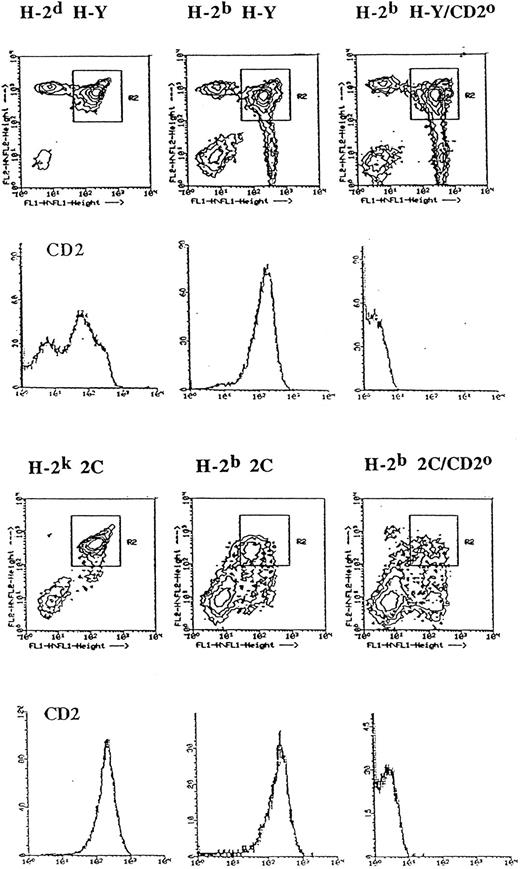
CD2 upregulation is a marker for positive selection. CD2 is expressed at higher levels on mature thymocytes compared with their immature precursors.12 To study the relationship between positive selection and CD2 upregulation in more detail, we have compared the level of CD2 expressed by CD4+CD8+ H-Y TCR+ thymocytes in H-2b or H-2d thymi, because positive selection of cells expressing the H-Y TCR occurs in the former, but not in the latter. As shown in Fig 1, CD4+CD8+ thymocytes from H-2d H-Y TCR transgenic mice expressed a heterogeneous level of CD2, segregating into populations that either expressed low or high levels of CD2. By contrast, in the positively-selecting H-2b thymus, the CD4+CD8+ thymocytes expressed a uniformly high level of CD2. Thus, for the H-Y TCR transgenic mice, the occurrence of positive selection is associated with a high level of CD2 expression on CD4+CD8+ thymocytes.
CD2 expression level on CD4+CD8+ thymocytes in TCR transgenic mice is influenced by the MHC of the thymus. Thymocytes from the indicated mice were stained with phycoerythrin-labeled anti-CD4, fluorescein isothiocyanate-labeled anti-CD8, and biotinylated MoAb specific for CD2 followed by Streptavidin Tricolor and analyzed in the FACScan flow cytometer. A total of 30,000 events were collected. The contour plots indicate CD4 (FL2) and CD8 (FL1) expression levels by thymocytes from the indicated TCR transgenic mice on different MHC backgrounds with or without the CD2 null mutation (CD2o). The histograms directly below the individual contour plots denote CD2 expression level (X-axis) of CD4+CD8+ thymocytes (R2 gate) from the indicated mice. The Y-axis of the histograms denote relative cell number.
CD2 expression level on CD4+CD8+ thymocytes in TCR transgenic mice is influenced by the MHC of the thymus. Thymocytes from the indicated mice were stained with phycoerythrin-labeled anti-CD4, fluorescein isothiocyanate-labeled anti-CD8, and biotinylated MoAb specific for CD2 followed by Streptavidin Tricolor and analyzed in the FACScan flow cytometer. A total of 30,000 events were collected. The contour plots indicate CD4 (FL2) and CD8 (FL1) expression levels by thymocytes from the indicated TCR transgenic mice on different MHC backgrounds with or without the CD2 null mutation (CD2o). The histograms directly below the individual contour plots denote CD2 expression level (X-axis) of CD4+CD8+ thymocytes (R2 gate) from the indicated mice. The Y-axis of the histograms denote relative cell number.
We also compared the level of CD2 that was expressed by CD4+CD8+ thymocytes in 2C mice of the H-2b and H-2k haplotypes. It has been previously shown that the 2C TCR is not positively selected in H-2s39 mice. We backcrossed the 2C TCR onto H-2k mice because we did not have H-2s mice in our animal colony, and we also wished to determine the fate of thymocytes expressing the 2C TCR in H-2k mice. As shown in Fig 1, antibodies specific for CD4 and CD8 showed dramatic differences in the relative proportions of thymocyte subsets in these two MHC backgrounds. The double-positive thymocytes from H-2k 2C mice constituted the largest fraction of thymocytes (61%) from these mice. The level of CD4 and CD8 expressed by H-2k double-positive thymocytes was also similar to that observed in H-2d H-Y mice. Furthermore, the yield of thymocytes from H-2k 2C mice was similar to that of H-2d H-Y mice (≈1 × 108) and was about fivefold higher than that recovered from H-2b 2C mice (≈2 × 107). These observations suggest that the 2C TCR is unlikely to be positively-selected in H-2k mice. However, despite these differences, CD4+CD8+ thymocytes from both types of mice expressed a uniformly high level of CD2, similar to that observed above for female H-Y TCR thymocytes from a H-2b thymus. This latter observation suggests that the 2C TCR may be positively-selected in H-2k mice and that the positive selection of the 2C TCR in H-2k mice may be distinct from that observed in H-2b 2C mice.
Positive selection of the above TCR transgenic thymocytes depends on the expression of MHC class I molecules and is abrogated by the absence of β2-microglobulin. To provide a truly nonselecting thymus for the 2C TCR, we backcrossed the β2-microglobulin null mutation onto H-2b 2C mice. Figure 2 shows TCR, CD2, and CD5 expression levels for CD4+CD8+ thymocytes from 2C TCR transgenic mice that were homozygous for the β2-microglobulin null (β2m°) mutation. Data from H-2d H-Y and H-2b H-Y/β2m° mice were included as the appropriate controls. The similar distribution of these molecules on the double-positive thymocytes of H-2b H-Y/β2m° and H-2d H-Y transgenic mice indicate that the H-2b β2m° mice did provide a nonselecting thymus for the H-Y TCR. Figure 2 also shows that the level of expression of all of these molecules on double-positive thymocytes was increased on cells from H-2k 2C TCR transgenic mice compared with that on cells from β2m° 2C TCR transgenic mice. Thus, upregulation of CD2 appears to accompany positive selection in a similar fashion to that previously described for upregulation of CD5.56 We also found that single CD8 positive thymocytes and lymph node cells from H-2k 2C mice can proliferate and become cytotoxic T cells in response to stimulation by the p2Ca/Ld ligand (our unpublished observations, November, 1995). These observations strengthened the conclusion that the 2C TCR is indeed positively selected in H-2k mice and that CD2 upregulation on double-positive thymocytes is a reliable marker for positive selection.
Positive selection of CD4+CD8+ thymocytes from H-2k 2C mice is associated with upregulation of CD2 expression. Thymocytes from the indicated mice were stained and analyzed as described in Fig 1. FL1 = CD8 log fluorescence; FL2 = CD4 log fluorescence; X-axis = log fluorescence; Y-axis = relative cell number. The fluorescence level of the indicated molecule on gated CD4+CD8+ thymocytes with (dotted line) and without (solid line) the β2-microglobulin null mutation (β2mo) is shown.
Positive selection of CD4+CD8+ thymocytes from H-2k 2C mice is associated with upregulation of CD2 expression. Thymocytes from the indicated mice were stained and analyzed as described in Fig 1. FL1 = CD8 log fluorescence; FL2 = CD4 log fluorescence; X-axis = log fluorescence; Y-axis = relative cell number. The fluorescence level of the indicated molecule on gated CD4+CD8+ thymocytes with (dotted line) and without (solid line) the β2-microglobulin null mutation (β2mo) is shown.
Positive selection in CD2-null TCR transgenic mice.The recovery of thymocytes and the proportions of CD4/CD8 thymocytes in H-2b H-Y and H-2b 2C TCR transgenic mice with or without the CD2 null mutation are summarized in Table 1. In female H-Y TCR transgenic mice, the most noticeable effect of the CD2 null mutation was an increase in the proportion of CD4−CD8+ thymocytes (from 12.9% to 27.6%) and a decrease in the proportion of CD4+CD8+ thymocytes (from 61.7% to 51.6%). By contrast, although 2C thymocytes with the CD2 null mutation had smaller proportions of CD4+CD8+ thymocytes, this did not lead to a corresponding increase in the proportion of CD4−CD8+ thymocytes in 2C TCR transgenic mice (Table 1). These data indicate that although the CD2 null mutation did not alter the cellularity of the thymus in both H-Y and 2C TCR transgenic mice, it differentially affected the proportions of CD4+CD8+ and CD4−CD8+ thymocytes in these TCR transgenic mice. Specifically, the CD2 null mutation caused a twofold increase in the proportion of CD4−CD8+ thymocytes with a compensatory decrease in the proportion of CD4+CD8+ thymocytes in H-Y TCR transgenic mice.
CD2 Differentially Affects the Proportions of Thymocyte Subsets in TCR Transgenic Mice
| Mutation . | No. Thymocytes × 10−7 (Mean ± SEM) . | Percent of Total Thymocytes (mean ± SEM) . | |||
|---|---|---|---|---|---|
| . | . | CD4−8− . | CD4+8+ . | CD4+8− . | CD4−8+ . |
| H-Y | 9.8 ± 0.3 | 15 ± 1.7 | 61 ± 3.0 | 8.5 ± 1.0 | 16 ± 1.2 |
| H-Y/CD2o | 9.5 ± 1.5 | 15 ± 1.6 | 46 ± 3.7 | 8.2 ± 0.2 | 31 ± 3.2 |
| 2C | 1.8 ± 0.5 | 51 ± 0.6 | 19 ± 2.0 | 9.4 ± 1.7 | 21 ± 1.8 |
| 2C/CD2o | 1.9 ± 0.4 | 56 ± 2.6 | 13 ± 2.3 | 9.7 ± 1.2 | 21 ± 1.3 |
| Mutation . | No. Thymocytes × 10−7 (Mean ± SEM) . | Percent of Total Thymocytes (mean ± SEM) . | |||
|---|---|---|---|---|---|
| . | . | CD4−8− . | CD4+8+ . | CD4+8− . | CD4−8+ . |
| H-Y | 9.8 ± 0.3 | 15 ± 1.7 | 61 ± 3.0 | 8.5 ± 1.0 | 16 ± 1.2 |
| H-Y/CD2o | 9.5 ± 1.5 | 15 ± 1.6 | 46 ± 3.7 | 8.2 ± 0.2 | 31 ± 3.2 |
| 2C | 1.8 ± 0.5 | 51 ± 0.6 | 19 ± 2.0 | 9.4 ± 1.7 | 21 ± 1.8 |
| 2C/CD2o | 1.9 ± 0.4 | 56 ± 2.6 | 13 ± 2.3 | 9.7 ± 1.2 | 21 ± 1.3 |
Five mice (6 to 12 weeks old) were analyzed per group. Thymocytes from the indicated TCR transgenic mice were stained with phycoerythrin-labeled anti-CD4 and fluorescein isothiocyanate-labeled anti-CD8 MoAbs and analyzed in the FACScan flow cytometer. The total number of viable thymocytes recovered from these transgenic lines are indicated.
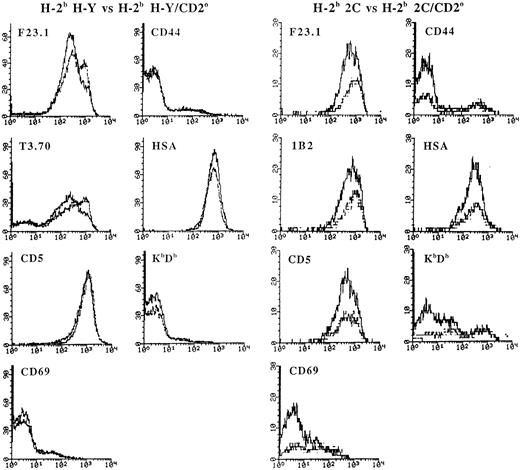
As shown above, the early stages of positive selection of immature CD4+CD8+ thymocytes are associated with an increase in the expression level of the TCR and CD5.38,56,57 Double-positive cells have also been shown to upregulate CD69 in response to positive selection signals.58 During the later stages of maturation, the more mature thymocytes exhibit a decrease in the expression of the HSA, but increase the expression of molecules such as CD44 and class I MHC.59 In H-Y TCR transgenic mice with the CD2 null mutation, there was a higher proportion of CD4+CD8+ thymocytes that expressed a very high level of the transgenic TCR β and α chains, although there was no significant alteration in the expression level of CD69, CD44, HSA, and class I MHC (Fig 3). In 2C TCR transgenic mice, the CD2 null mutation had no obvious effect on the expression level of the 2C TCR β chain (detected by the F23.1 MoAb), the 2C TCR idiotypic determinant (detected by the 1B2 MoAb),43 CD5, and HSA. However, there was a significant increase in the proportion of CD4+CD8+ thymocytes that expressed a high level of CD69, CD44, and class I MHC (Fig 3).
Phenotypic analysis of CD4+CD8+ thymocytes from H-2b H-Y and H-2b 2C mice with the CD2 null mutation. Thymocytes from H-2b H-Y or H-2b 2C mice with or without the CD2 null mutation were stained with PE-labeled anti-CD4, FITC-labeled anti-CD8, and biotinylated MoAb to the indicated marker followed by Streptavidin Tricolor and analyzed in the FACScan flow cytometer. A total of 30,000 events were collected. The fluorescence level of the indicated molecule on gated CD4+CD8+ thymocytes with (dotted line) and without (solid line) the β2-microglobulin null mutation (β2mo) is shown.
Phenotypic analysis of CD4+CD8+ thymocytes from H-2b H-Y and H-2b 2C mice with the CD2 null mutation. Thymocytes from H-2b H-Y or H-2b 2C mice with or without the CD2 null mutation were stained with PE-labeled anti-CD4, FITC-labeled anti-CD8, and biotinylated MoAb to the indicated marker followed by Streptavidin Tricolor and analyzed in the FACScan flow cytometer. A total of 30,000 events were collected. The fluorescence level of the indicated molecule on gated CD4+CD8+ thymocytes with (dotted line) and without (solid line) the β2-microglobulin null mutation (β2mo) is shown.
Taken together, the above data indicate that the absence of CD2 influenced the selection of CD4+CD8+ thymocytes expressing the H-Y and the 2C TCRs in different ways. In H-Y TCR transgenic mice, the absence of CD2 improved positive selection as shown by the twofold increase in the proportion of CD4−CD8+ thymocytes that expressed a uniformly high level of the transgenic TCR (data not shown). In 2C TCR transgenic mice, there was also evidence of altered selection as reflected by the increased proportion of CD4+CD8+ cells expressing high levels of the late maturation markers CD69, CD44, and class I MHC.
Positive selection in CD2, CD5 double-null mice.To a first approximation, the phenotype of mice that lack CD2 is somewhat similar to that of CD5-deficient mice in that both types of null mutations do not drastically interfere with T- or B-cell development.15,40 Nonetheless, stimulation of thymocytes from CD5-null mice with anti-CD3 MoAbs or concanavalin A results in increased proliferative responses. This hyperresponsiveness is correlated with increased phosphorylation of the TCR ζ chain, p95vav and phospholipase C (PLC)γ , as well as an increased mobilization of calcium after TCR ligation. Consistent with these findings, CD5-null mice show aberrant selection of thymocytes expressing transgenic TCRs. Overall, the interpretation of these results is that CD5 performs a negative regulatory function on thymocyte TCR-dependent signal transduction.15 In consideration of the similarities between the CD5 phenotype and the above results for CD2-null mice, we have intercrossed the two strains of mice to generate double mutant animals that lack expression of both CD2 and CD5. Table 2 shows that the combined loss of both molecules results in a distortion of the normal proportions of thymocyte subsets as shown by fluorescence-activated cell sorting (FACS) analysis. In particular, there is a highly reproducible decrease in the number of double-positive thymocytes and a concomitant increase in the number of mature single-positive cells. At the same time, the total number of thymocytes is apparently unaffected, suggesting that the rate or efficiency of positive selection may be improved in these mice. Despite the above alterations in thymic development, the relative representation of subsets of T and B cells in the peripheral lymph nodes was indistinguishable from normal mice by FACS analysis. Overall, the results suggest a synergistic effect of the two mutations on positive selection in a manner that is consistent with observations made on the TCR transgenic mice.
Enhancement of Positive Selection in CD2, CD5 Double Knockout Mice
| Mutation . | No. Thymocytes × 10−7 (mean ± SEM) . | Percent of Total Thymocytes (mean ± SEM) . | |||
|---|---|---|---|---|---|
| . | . | CD4−8− . | CD4+8+ . | CD4+8− . | CD4−8+ . |
| WT | 13.7 ± 1.6 | 3 ± 0.6 | 84 ± 1.7 | 10 ± 1.2 | 3 ± 1.2 |
| CD2 | 14.7 ± 2.7 | 3 ± 1.2 | 81 ± 2.4 | 13 ± 0.6 | 3 ± 0.6 |
| CD5 | 17.8 ± 2.6 | 3 ± 0.6 | 82 ± 1.7 | 11 ± 1.7 | 4 ± 1.2 |
| CD2, CD5 | 12.8 ± 1.8 | 3 ± 0.6 | 72 ± 2.9 | 18 ± 1.7 | 7 ± 1.7 |
| Mutation . | No. Thymocytes × 10−7 (mean ± SEM) . | Percent of Total Thymocytes (mean ± SEM) . | |||
|---|---|---|---|---|---|
| . | . | CD4−8− . | CD4+8+ . | CD4+8− . | CD4−8+ . |
| WT | 13.7 ± 1.6 | 3 ± 0.6 | 84 ± 1.7 | 10 ± 1.2 | 3 ± 1.2 |
| CD2 | 14.7 ± 2.7 | 3 ± 1.2 | 81 ± 2.4 | 13 ± 0.6 | 3 ± 0.6 |
| CD5 | 17.8 ± 2.6 | 3 ± 0.6 | 82 ± 1.7 | 11 ± 1.7 | 4 ± 1.2 |
| CD2, CD5 | 12.8 ± 1.8 | 3 ± 0.6 | 72 ± 2.9 | 18 ± 1.7 | 7 ± 1.7 |
Three mice (5 to 10 weeks old) were analyzed per group. The same trends were also observed in a total of five different experiments that included the above combinations of mice.
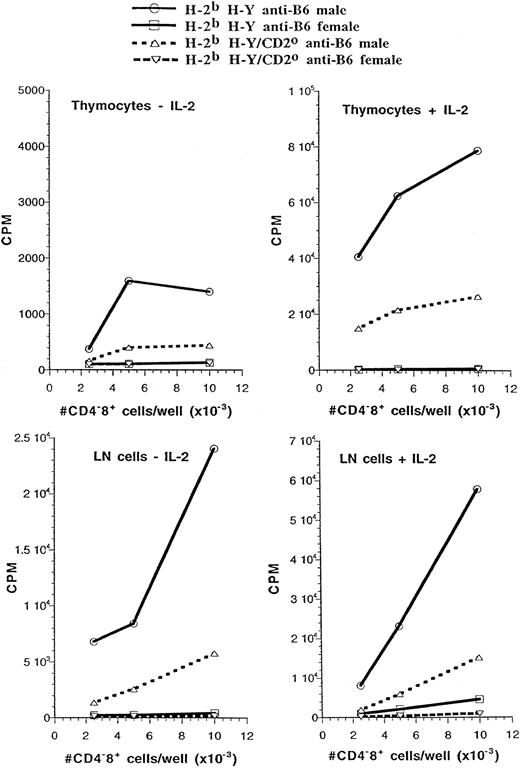
Antigen-specific CD4−CD8+ thymocytes and peripheral T cells display differential dependence on CD2 for their responses to antigen in different TCR transgenics.We next determined whether the lack of CD2 influenced the antigen-specific proliferative responses of cells from the two types of transgenic mice. CD4−CD8+ thymocytes and T cells from H-2b H-Y and H-2b 2C TCR transgenic mice expressed the same level of transgenic TCR regardless of whether they developed in the presence or absence of the CD2 gene (data not shown). In these experiments, each culture was adjusted to contain the same number of CD4−CD8+ cells that expressed the transgenic TCR. In one series of experiments, CD4−CD8+ thymocytes from H-2b H-Y TCR transgenic mice were tested for proliferation in response to stimulation with H-2b male spleen cells. As shown in Fig 4, cells from CD2-expressing mice mounted a weak, but specific, response to this stimulus that was greatly augmented by the addition of exogenous IL-2. By contrast, proliferation of CD2-null cells in this assay was barely above background in the absence of exogenous IL-2. The addition of IL-2 to the cultures improved the response, but did not restore it to the level observed with cells that expressed endogenous CD2. Peripheral CD4−CD8+ T cells from H-2b H-Y mice were less dependent on exogenous IL-2 for the male-specific proliferative response. Again, peripheral CD4−CD8+ T cells that lacked CD2 expression responded less well than those that expressed CD2, and the addition of exogenous IL-2 did not bring the response back up to the level observed in CD4−CD8+ T cells that expressed CD2.
CD2 enhances the proliferative response of CD4−CD8+ thymocytes and lymph node cells from H-2b H-Y mice to stimulation by the male antigen. An equivalent number of CD4−CD8+ thymocytes or lymph node cells from the indicated mice were cultured with male or female C57BL/6 (B6) spleen cells with or without added IL-2. The proliferative response was determined by assessing thymidine incorporation during the last 16 hours of an 84-hour culture period.
CD2 enhances the proliferative response of CD4−CD8+ thymocytes and lymph node cells from H-2b H-Y mice to stimulation by the male antigen. An equivalent number of CD4−CD8+ thymocytes or lymph node cells from the indicated mice were cultured with male or female C57BL/6 (B6) spleen cells with or without added IL-2. The proliferative response was determined by assessing thymidine incorporation during the last 16 hours of an 84-hour culture period.
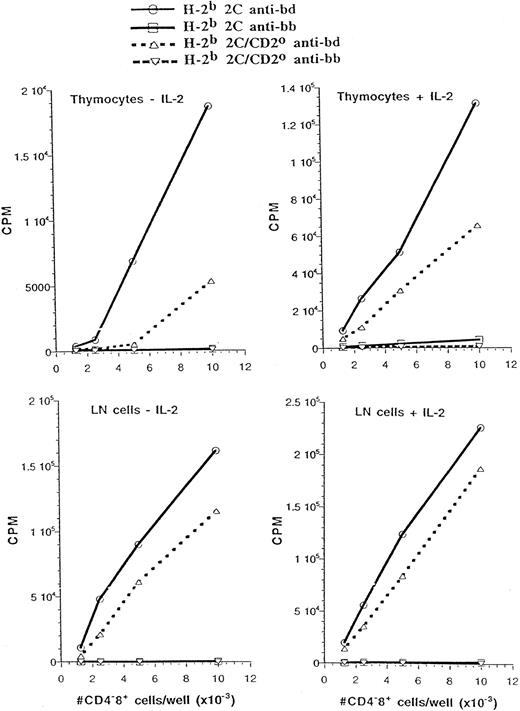
In a parallel series of experiments, we also determined whether the absence of CD2 influenced the H-2d–specific proliferative responses of CD4−CD8+ thymocytes and T cells from H-2b 2C TCR transgenic mice (Fig 5). In contrast to H-Y TCR transgenic mice, 2C CD4−CD8+ thymocytes gave a fairly strong response to stimulation by H-2d even in the absence of added IL-2. This response was reduced for CD2-null thymocytes, but was significantly augmented by the addition of exogenous IL-2, such that proliferation approached within 50% of the response by thymocytes that expressed CD2. Peripheral CD4−CD8+ T cells from 2C mice gave a very high proliferative response to stimulation by H-2d and this response was only marginally improved by the addition of exogenous IL-2. Interestingly, 2C peripheral CD4−CD8+ T cells that lacked CD2 responded almost as well as 2C CD4−CD8+ T cells that expressed CD2, regardless of whether exogenous IL-2 was added to these cultures. These results indicate that depending on their antigen specificity, CD4−CD8+ thymocytes and T cells exhibit different levels of dependence on CD2 in their proliferative response to antigenic stimulation.
CD2 marginally improves the proliferative response of H-2b 2C CD4−CD8+ thymocytes and lymph node cells to stimulation by H-2d antigens. Equivalent number of CD4−CD8+ thymocytes or lymph node cells from the indicated mice were cultured with the indicated spleen cells with or without added IL-2. The proliferative responses of the responder cells to stimulation by (C57BL/6 × DBA/2)F1 (bd) and C57BL/6 (bb) spleen cells are shown.
CD2 marginally improves the proliferative response of H-2b 2C CD4−CD8+ thymocytes and lymph node cells to stimulation by H-2d antigens. Equivalent number of CD4−CD8+ thymocytes or lymph node cells from the indicated mice were cultured with the indicated spleen cells with or without added IL-2. The proliferative responses of the responder cells to stimulation by (C57BL/6 × DBA/2)F1 (bd) and C57BL/6 (bb) spleen cells are shown.
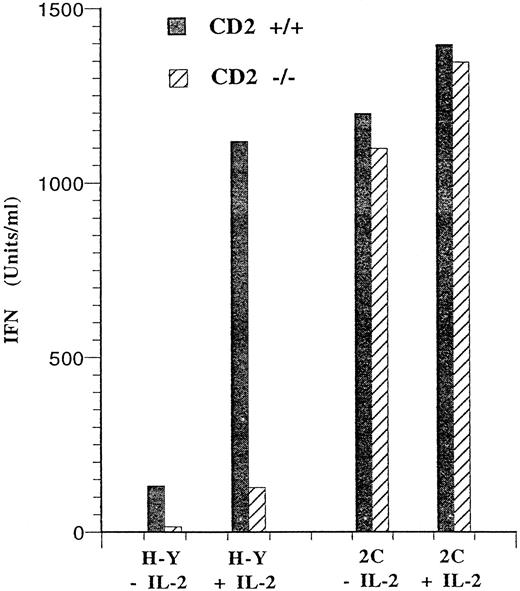
Because the CD2/LFA-3 (CD58) pathway has been implicated in the synthesis of IFN-γ by T cells,35 we determined whether IFN-γ production after stimulation of peripheral T cells by antigen was influenced by the absence of CD2 expression. The results in Fig 6 show that lymph node cells from H-Y and 2C mice differed in their ability to produce IFN-γ after antigen stimulation. T cells from 2C mice produced large amounts of IFN-γ regardless of whether exogenous IL-2 was added to the culture medium. Furthermore, 2C T cells that lacked CD2 produced just as much IFN-γ as those that expressed CD2. By contrast, antigen stimulation of T cells from H-Y mice in the absence of exogenous IL-2 led to the production of very low levels of IFN-γ. This low level of IFN-γ production was greatly augmented by the addition of exogenous IL-2. More importantly, antigen-stimulated T cells expressing the H-Y TCR were highly dependent on CD2 for IFN-γ production regardless of whether exogenous IL-2 was added to these cultures. Thus, similar to the proliferative responses above, T cells expressing the 2C or the H-Y TCR display a differential requirement for CD2 for IFN-γ production after antigen stimulation.
Differential requirement for CD2 in IFN-γ production by antigen-stimulated CD4−CD8+ T cells. A total of 1 × 105 CD4−CD8+ lymph node cells from H-2b H-Y mice were stimulated with 1 × 106 B6 male spleen cells in a volume of 0.2 mL with or without IL-2 (20 U/mL). The supernatants from these cultures were collected after 40 hours. The amount of IFN-γ present in the supernatants from H-Y cells with or without the CD2 null mutation are shown. The same experiment was performed for CD4−CD8+ thymocytes or lymph node cells from H-2b 2C mice except that these cells were stimulated with 1 × 106 (C57BL/6xDBA/2)F1 spleen cells. The amount of IFN-γ produced by lymph node cells from H-Y and 2C mice was less than 10 U/mL when these cells were stimulated with female B6 spleen cells (data not shown).
Differential requirement for CD2 in IFN-γ production by antigen-stimulated CD4−CD8+ T cells. A total of 1 × 105 CD4−CD8+ lymph node cells from H-2b H-Y mice were stimulated with 1 × 106 B6 male spleen cells in a volume of 0.2 mL with or without IL-2 (20 U/mL). The supernatants from these cultures were collected after 40 hours. The amount of IFN-γ present in the supernatants from H-Y cells with or without the CD2 null mutation are shown. The same experiment was performed for CD4−CD8+ thymocytes or lymph node cells from H-2b 2C mice except that these cells were stimulated with 1 × 106 (C57BL/6xDBA/2)F1 spleen cells. The amount of IFN-γ produced by lymph node cells from H-Y and 2C mice was less than 10 U/mL when these cells were stimulated with female B6 spleen cells (data not shown).
DISCUSSION
Although CD2 is not required for the positive selection of CD4−CD8+ T cells, we have found that its absence can affect the efficiency of the process in an unexpected fashion. In the H-Y TCR transgenic mice, there is an enhancement of positive selection in the absence of CD2, which is reflected in a twofold increase in the proportion of CD4−CD8+ thymocytes that expressed the H-Y TCR with a corresponding decrease in the proportion of CD4+CD8+ thymocytes. Furthermore, a larger proportion of CD4+CD8+ thymocytes in CD2 null animals expressed a higher level of the transgenic TCR. In the 2C mice, the lack of CD2 apparently did not cause either an increase the proportion of CD4−CD8+ thymocytes or an increase in the proportion of CD4+CD8+ thymocytes that expressed higher levels of the transgenic TCR. However, in these mice, the lack of CD2 led to an increase in the proportion of CD4+CD8+ thymocytes that expressed high levels of CD69, CD44, and class I MHC.
The differential effects of CD2 on the selection of the H-Y and 2C TCR are likely related to the difference in affinity of these TCRs for their respective selecting ligands. The following observations suggest that the 2C TCR has a higher affinity than the H-Y TCR for the positively-selecting ligand. First, CD4+CD8+ thymocytes expressing the H-Y TCR can tolerate a very high level of transgenic CD8 without being deleted. By contrast, CD4+CD8+ thymocytes expressing the 2C TCR are sensitive to elevated levels of CD8 expression; expression of a transgenic CD8 in the positively-selecting H-2b thymus led to the deletion of CD4+CD8+ thymocytes in these mice.54,55 Second, CD4+CD8+ thymocytes from H-2b 2C mice expressed a much lower level of the CD8 molecule than CD4+8+ thymocytes from H-2b H-Y mice (see Fig 1). The lower level of CD8 expression by 2C thymocytes is determined by the H-2 haplotype of the thymus because CD4+CD8+ thymocytes from H-2k 2C mice expressed the same level of CD8 as those from H-2b H-Y mice. Third, H-2b 2C mice have very small thymi (see Table 1). Finally, CD4+CD8− thymocytes are poorly developed in H-2b 2C mice (Fig 1 and Table 1). All of these observations are consistent with the conclusion that the 2C TCR has a very high affinity for the positively selecting ligand in the thymus of the H-2b haplotype such that CD4+CD8+ thymocytes in H-2b 2C mice that expressed higher levels of CD8 were deleted. This would account for the small size of the thymus observed in H-2b 2C mice. The poor development of CD4+CD8− thymocytes and peripheral T cells in H-2b 2C mice would also be consistent with the deletion of CD4+CD8+ thymocytes, because CD4+8− thymocytes and T cells are known to be derived from CD4+CD8+ precursors.60 An alternative explanation for the poor development of the double-positive and CD4 single-positive cells in H-2b 2C mice is that the 2C TCR is selected with very high efficiency in these mice such that there is a rapid transit of cells from the double-positive into the single-positive CD8 pool. Nevertheless, regardless of the mechanisms that contribute to the poor development of double-positive and single-positive CD4 cells in these mice, it is possible that the effects of the CD2 null mutation observed in H-2b 2C mice are typical of high affinity TCRs, whereas those observed in H-2b H-Y mice are typical of TCRs with much lower affinity for their positively selecting ligands.
Superficially, the observations on the H-Y TCR transgenic mouse presented in this report appear to be at odds with the initial description of the CD2-null phenotype that showed a more modest effect in the efficiency of positive selection when CD2 is absent.36 Perhaps the major difference between these two studies is the relative genetic heterogeneity of the mice under analysis. The initial analysis was conducted using mice of a mixed genetic background including contributions from 129/Sv, C57BL/6, and DBA/2. By contrast, the data presented in this report were collected using CD2-null mice derived from a 129/Sv chimera that had been back-crossed to the C57BL/6 background five times and then intercrossed more than five times. The H-Y TCR transgenic stock was also extensively back-crossed to C57BL/6. Thus, the effect of the CD2 mutation on positive selection was more obvious after purifying the genetic background. Nonetheless, it is apparent that the CD2 null mutation is not crippling for T-cell development and positive selection proceeds in all situations so far examined, albeit with variable efficiency.
The apparent similarity between the CD2 and CD5-null phenotypes is particularly striking.15 Both types of mice show enhanced positive selection of the H-Y TCR and they also show inappropriate negative selection of cells expressing putative high affinity TCRs (ie, the 2C and P14 TCRs for CD2 and CD5-null mice, respectively). The expression of both CD2 and CD5 also appears to be coordinately upregulated on double-positive cells that are undergoing positive selection to the CD8 lineage. In mice that lack both CD2 and CD5, we have shown that there is an increase in the number of mature single-positive cells suggestive of improved positive selection. Thus, the combined influence of both molecules appears to be important in regulating the efficiency of positive selection, most likely through a direct effect on the sensitivity of thymocytes to TCR ligation.15
The molecular mechanisms underlying the effect of either CD2 or CD5 on positive selection remains to be established. Indeed, despite the synergistic effect of the two null mutations on thymocyte positive selection, there is currently no evidence that the functions of the two molecules are either interchangeable or directly connected with a common signaling pathway. In this regard, it is perhaps paradoxical that the absence of CD5 should enhance the proliferative capacity of thymocytes, whereas the loss of CD2 appears to do the opposite. Both molecules have been found in immunoprecipitates that also include the TCR, raising the possibility that these glycoproteins may directly regulate TCR signaling in response to the binding of peptide ligands presented by the thymic epithelium. One possibility is that structures like CD2 or CD5 may limit the rate of aggregation of the TCR by peptide/MHC, such that the formation of signaling complexes could be potentiated by their absence. Alternatively, CD2 or CD5 may recruit negative regulators of signal transduction to sites of TCR engagement; such regulators may include tyrosine kinases like p50csk or phosphatases like PTP-1C. In this last regard, both CD2 and CD5 have large cytoplasmic domains that have previously been found to associate with signaling molecules including p59fyn and p56lck.
CD4−CD8+ thymocytes and T cells from H-Y TCR transgenic mice showed a greater dependence on CD2 for their proliferative response and production of IFN-γ in response to antigen stimulation than CD4−CD8+ thymocytes and T cells from 2C TCR transgenic mice. Although the affinity of the H-Y TCR for its ligand has not been determined, several observations indirectly support the conclusion that this TCR has a relatively low affinity for its ligand. First, the parental CD4−CD8+ T-cell clone from which the TCR transgenes were isolated for the construction of the H-Y TCR transgenic mice, as well as the male antigen-activated CD4−CD8+ T cells from the H-Y TCR transgenic mice, were inefficient killers. That this poor lytic capability is a property of the TCR is underscored by the observation that these cells lysed target cells efficiently in a redirected cytolytic assay that is mediated either by antibody to the TCR or by lectins (our unpublished observations, January 1990). Such a dissociation between lytic potential and proliferative capacity has been noted in other cytotoxic T lymphocyte clones and was attributed to low ligand affinity.61 62 Therefore, the data presented here are consistent with the hypothesis that, for CD4−CD8+ T cells expressing TCRs with low affinity for their antigenic ligands, signaling via the CD2 receptor may be required for efficient proliferation and IFN-γ synthesis. However, for CD4−CD8+ T cells expressing TCRs that bind their antigenic ligands with high affinity, the contribution of the CD2 signaling pathway may be less significant.
Finally, there is the paradoxical question of why the absence of CD2 appears to promote the positive selection of H-Y TCR transgenic thymocytes while interfering with their proliferative responses and IFN-γ production in the face of antigenic stimulation. It is possible that impaired TCR signaling may constitute a selective advantage for the H-Y TCR, although transgenic experiments involving overexpression of CD8 would suggest otherwise. Alternatively, it may be true that CD2 performs functions in the antigen-specific proliferative assays that are distinct from its activities during thymocyte development. A proper resolution to this paradox awaits a better understanding of the molecular details of how CD2 participates in thymocyte selection and T-cell activation.
ACKNOWLEDGMENT
We thank S. Ip for technical assistance. We also thank Drs D. Loh (Nippon Roche Research Center, Kamakura, Kanagawa, Japan) and O. Smithies (University of North Carolina, Chapel Hill, NC) for making available to us the 2C TCR transgenic mice and the β2-microglobulin knockout mice, respectively.
Supported by grants from the Arthritis Society and the Medical Research Council of Canada to H.S.T. and by a special Fellowship from the Leukemia Society to N.K. D.R.L. is supported by the Howard Hughes Medical Institute.
Address reprint requests to Hung-Sia Teh, PhD, Department of Microbiology and Immunology, University of British Columbia, 6174 University Blvd, Vancouver, British Columbia, Canada V6T 1Z3.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal