Abstract
P-selectin (CD62P) is a member of the selectin family of adhesion molecules involved in the regulation of leukocyte traffic. P-selectin glycoprotein ligand-1 (PSGL-1) is a mucin-like molecule that is thought to be a primary ligand for P-selectin. The interaction of P-selectin with PSGL-1 results in leukocyte rolling and recruitment of leukocytes to sites of inflammation and tissue injury. However, expression of PSGL-1 protein alone is insufficient for binding to P-selectin. Several posttranslational modifications of PSGL-1, including sialylation, sulfation, and fucosylation by α1,3-fucosyltransferase(s) (FucT), are required for functional interaction with P-selectin. Recently, several groups have reported that PSGL-1 might also serve as a ligand for E-selectin. Differential posttranslational modifications of PSGL-1 may determine whether it can interact with either P- or E-selectin or both. To determine whether PSGL-1 is essential for adhesion to P- or E-selectin, we have constructed and analyzed a panel of stably transfected K562 cells. K562 cells express FucT-IV but not FucT-VII or PSGL-1, and do not bind to either E- or P-selectin. K562 cells transfected with PSGL-1 cDNA also did not bind to either P- or E-selectin. Binding to P-selectin occurred only when K562 cells were cotransfected with both FucT-VII and PSGL-1. In contrast, expression of FucT-VII alone was sufficient for E-selectin binding. These data demonstrate that expression of PSGL-1 is not required for adhesion of a stably transfected hematopoietic cell line to E-selectin, and suggest that FucT-IV alone cannot properly modify PSGL-1, expressed in transfected K562 cells, to bind P-selectin.
THE SELECTINS are a family of Ca2+-dependent lectins that mediate the initial adhesion of leukocytes to platelets or the lumenal surface of vessels during inflammation.1,2 L-selectin is expressed on leukocytes and interacts with constitutively expressed ligands on high-endothelial venules and inducible ligands on endothelium.3-5 E-selectin is expressed on cytokine-stimulated endothelium and mediates the rolling of myeloid cells and distinct subsets of lymphocytes.6,7 P-selectin is localized to the Weibel-Palade bodies of endothelial cells and the α-granules of platelets, and is translocated to the plasma membrane after induction by a variety of inflammatory mediators such as thrombin, histamine, and phorbol esters.8,9 Once displayed on the cell surface, P-selectin is able to interact with glycoprotein ligand(s) expressed by monocytes, neutrophils, and some T cells. P-selectin glycoprotein ligand-1 (PSGL-1) is a homodimeric sialoglycoprotein originally isolated from HL60 cells and neutrophils that has been shown to mediate the interaction of human neutrophils with P-selectin.10-12 However, expression of PSGL-1 protein alone is insufficient for binding to P-selectin. In addition to PSGL-1, cells must express α1,3-fucosyltransferase (FucT) activity.11,13-15 Binding of P-selectin to PSGL-1 also requires sialylated, O-linked glycans15,16 and tyrosine sulfation of a consensus region located at the amino terminal of PSGL-1.17-19
Several groups have reported that PSGL-1 also serves as a ligand for E-selectin, but the structural requirements for the binding of PSGL-1 to P- and E-selectin are believed to be different.14,15,18,20,21 Alternatively, P- and E-selectin may interact with different sites on PSGL-1.21 Adhesion of myeloid cells to P-selectin is abolished by O-sialyl-glycoprotease or chymotrypsin, whereas E-selectin binding is unaffected.16 22-24 Whether PSGL-1 is required for binding to E-selectin in vivo is unclear. In this report, evidence is presented that interactions between P-selectin and PSGL-1 require expression of both PSGL-1 and FucT-VII, whereas expression of FucT-VII alone is sufficient for binding to E-selectin. Thus, PSGL-1 expression is required for binding to P-selectin but not to E-selectin.
MATERIALS AND METHODS
Generation of K562 stable transfectants.cDNAs for PSGL-125 and FucT-VII26 were subcloned into a modified SRα vector27 containing either a neoR or hygroR cassette, and used to transfect human K562 cells by electroporation. Transfectants were selected in medium containing either 0.5 mg/mL Hygromycin B (Calbiochem, La Jolla, CA) or 1.0 mg/mL G418 (Geneticin; GIBCO-BRL, Grand Island, NY). Transfected cells expressing PSGL-1 were identified by flow cytometry with a new monoclonal antibody, designated KPL1 (K.R. Snapp, H. Ding, F.W. Luscinskas, G.S. Kansas, submitted). Expression of FucT-VII was detected by reverse transcriptase–polymerase chain reaction (RT-PCR) and confirmed by positive staining for HECA-452 and/or CSLEX-1 by flow cytometry. Positive transfectants were cloned by limiting dilution, and clones were tested as described for the parent population. Only clones that were 100% positive as compared with the untransfected parent cells were used. Cloned K562 transfectants were maintained in RPMI 1640 containing 10% fetal calf serum, 1% penicillin/streptomycin, and 2 mmol/L L-glutamine. For the experiments reported here, the K562/FucT-VII and K562/PSGL-1/FucT-VII cells were produced by transfection of K562 or cloned K562/PSGL-1 cells, respectively, with FucT-VII cDNA.
Flow cytometry.Expression of PSGL-1 was evaluated by direct immunofluorescence staining with fluorescein isothiocyanate (FITC) conjugated KPL1. Expression of specific carbohydrates was determined by staining with MMA,28 which recognizes Lex/CD15, or HECA-45229 or CSLEX-1,30 which recognize sLex/sCD15. Following primary incubation with the specific IgM anti-carbohydrate antibodies, cells were washed and stained with goat anti-mouse IgM-FITC (Biosource, Camarillo, CA). Data were collected on a total of 10,000 light-scatter–gated events, and fluorescence histograms are displayed on a four-decade logarithmic scale.
RT-PCR analysis.Southern blotting of RT-PCR products for detection of FucT-IV, FucT-VII, or pgk1 was performed exactly as previously described.31 Briefly, reverse transcription of 1 μg DNAse-treated total cellular RNA was performed in a final volume of 20 μL containing 10 mmol/L DTT (GIBCO-BRL), 137 pmol/L random hexamer (Promega, Madison, WI), 1 mmol/L each dATP, dCTP, dGTP, and dTTP (Perkin-Elmer, Branchburg, NJ), 20 U RNAsin (Promega), 1X first-strand buffer (GIBCO-BRL), and either 200 U Superscript II RT (GIBCO-BRL) or 1 μL DEPC H2O at 42°C for 1 hour. PCR reactions containing 5 μL of the appropriate RT reaction in a final reaction volume of 100 μL containing 1.5 mmol/L MgCl2 , 0.2 mmol/L each dNTP, 0.5 μmol/L specific sense and antisense primers,31 and 2.5 U Taq polymerase (Perkin-Elmer) were performed for 40 cycles of PCR with 1 minute at 94°C, 1 minute at 62°C, and 1 minute at 72°C. FucT-IV primers amplified a 480-bp product, FucT-VII a 500-bp product, and pgk1 a 374-bp product. DNA was transferred onto nitrocellulose in 10X SSC. Nitrocellulose filters were UV cross-linked, and prehybridization was performed at 42°C for 2 to 3 hours in a solution of 5X SSPE, 5X Denhardt solution, 1% sodium dodecyl sulfate (SDS), and 200 μg/mL denatured salmon sperm DNA. Radiolabeled probes were generated with the Random Primed DNA Labeling Kit (Boehringer-Mannheim, Indianapolis, IN) according to the manufacturer's protocol. Hybridization was performed at 42°C in a solution of 5X SSPE, 5X Denhardt solution, 50% formamide, 0.1% SDS, and 100 μg/mL denatured salmon sperm DNA containing 106 cpm/mL of probe. Following hybridization, blots were washed twice in 1X SSPE/0.1% SDS for 5 minutes at room temperature and twice in 0.1X SSPE/0.1% SDS for 15 minutes at 42°C. Bound radioactivity was detected by exposure to Hyperfilm (Amersham, Arlington Heights, IL).
Low–shear–force COS cell adhesion assay.Binding of leukocytes to COS cells transiently transfected with cDNA encoding either P- or E-selectin was as previously described.5,32 33 Briefly, COS cells were transfected by the DEAE-dextran method in 100-mm tissue-culture grade petri dishes, replated on 35-mm dishes (assay plates), and allowed to readhere overnight. The next day, HL60 cells or transfectants were washed twice in RPMI 1640, resuspended at 3.3 × 106 cells/mL, and placed on ice. Each petri dish was washed three times with unsupplemented RPMI 1640, followed by the addition of 2 × 106 cells in a total volume of 0.6 mL. Cells were incubated on a constantly rocking platform for 15 minutes at 4°C, and the plates were washed five times with RPMI 1640 followed by fixation with cold 0.37% formaldehyde/RPMI 1640. The mean number of cells bound per COS cell was determined by counting the number of cells bound per COS cell on approximately 100 COS cells in multiple 40× fields using a standard inverted light microscope.
Parallel-plate flow assay.The parallel-plate flow chamber and flow assay protocol have been described elsewhere.34 Rolling of cell lines and transfectants at 1.9 dynes/cm2 on monolayers of stably transfected Chinese hamster ovary (CHO) cells expressing either P- or E-selectin was analyzed. Levels of P- and E-selectin on stably transfected CHO cells were similar to the level of E-selectin expressed by tumor necrosis factor (TNF) stimulated human umbilical vein endothelial cells (HUVECs). The data were analyzed with Celltrak software developed with Compix for use on their C-Imaging 1280 Computerized Morphometry System (Compix Inc, Mars, PA). Celltrak tracks leukocytes in real time as they flow over transfected CHO cells. Sequential images of the tracked cells were digitized and matched on the basis of trajectories and morphology. Velocities were calculated using the time delay between captured images, statistics were compiled for the prescribed number of fields, and histograms were generated for the velocity distribution. Histograms were plotted using Stanford Graphics and consisted of 1,000 to 4,000 interactive “events” collected from 60 to 100 sequential fields during 2 to 6 minutes of observation.
RESULTS
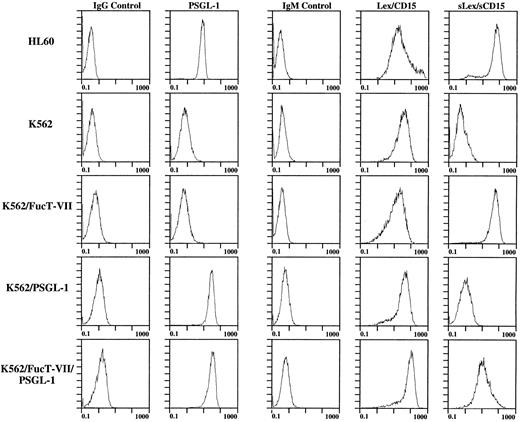
Expression of surface carbohydrates and PSGL-1, FucT-IV, and FucT-VII on untransfected and transfected K562 cells.The human K562 erythroleukemia cell line does not interact with either E- or P-selectin and constitutively expresses FucT-IV but not FucT-VII or PSGL-1. Therefore, K562 cells stably transfected with cDNA encoding PSGL-1,25 FucT-VII,26 or both were established to investigate the contributions of these gene products to the expression of functional selectin ligands on hematopoietic cells. K562 cells stably transfected with cDNA encoding either PSGL-1 alone or both PSGL-1 plus FucT-VII express levels of PSGL-1 similar to or slightly higher than those found on HL60 cells (Fig 1). The comparison with HL60 cells is important, since HL60 cells express PSGL-1 and bind with high affinity to both P- and E-selectin. Untransfected K562 cells, K562 transfectants, and HL60 cells were stained with anti-carbohydrate antibodies to confirm the presence of fucosylated structures corresponding to specific FucT activity (Fig 1). As expected, all cells stained with the MMA antibody, which recognizes Lex (CD15) and is associated with the endogenous FucT-IV activity found in both HL60 and K562 cells.34 35 Expression of FucT-VII cDNA induced expression of sialylated Lewis X epitopes. Thus, only HL60 cells and K562 cells transfected with FucT-VII cDNA were positive with both CSLEX1 (not shown) and HECA-452 (Fig 1). Untransfected K562 cells and K562 cells transfected with PSGL-1 cDNA alone were negative with CSLEX1 and HECA-452.
Flow cytometry analysis of expression of PSGL-1, Lex/CD15, and sLex/sCD15 on K562 cells stably transfected with cDNA encoding PSGL-1, FucT-VII, or both. Note that PSGL-1 levels on K562/PSGL-1 and K562/PSGL-1/FucT-VII cells are equivalent and slightly higher than the levels of HL60 cells, and that the level of sLex on K562/PSGL-1/FucT-VII cells is lower than on K562/FucT-VII cells, corresponding to lower levels of FucT-VII mRNA (see Fig 2).
Flow cytometry analysis of expression of PSGL-1, Lex/CD15, and sLex/sCD15 on K562 cells stably transfected with cDNA encoding PSGL-1, FucT-VII, or both. Note that PSGL-1 levels on K562/PSGL-1 and K562/PSGL-1/FucT-VII cells are equivalent and slightly higher than the levels of HL60 cells, and that the level of sLex on K562/PSGL-1/FucT-VII cells is lower than on K562/FucT-VII cells, corresponding to lower levels of FucT-VII mRNA (see Fig 2).
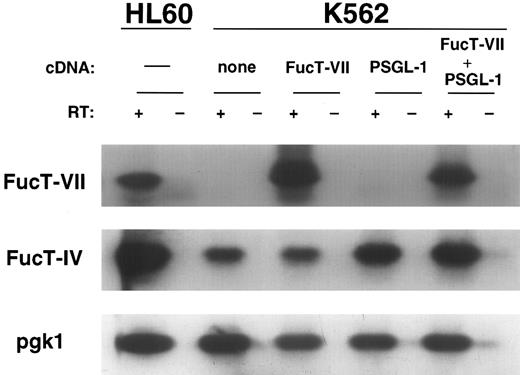
Expression of FucT mRNA was determined by Southern blotting of RT-PCR products, and correlated with surface expression of carbohydrates recognized by MMA, CSLEX1, and HECA-452. HL60 cells express FucT-IV, FucT-VII, and pgk1, and were included in each experiment as a positive control (Fig 2). All cells (both transfected and untransfected) expressed both FucT-IV and pgk1. K562 cells transfected with FucT-VII only or PSGL-1 plus FucT-VII expressed FucT-VII in addition to FucT-IV and pgk1 (Fig 2). K562 cells cotransfected with both PSGL-1 and FucT-VII cDNA expressed lower levels of sLex when compared with the FucT-VII–only transfectant (Fig 1), corresponding to lower levels of FucT-VII (Fig 2).
RT-PCR Southern blot analysis of FucT-IV and FucT-VII mRNA expression in K562 transfectants. Essentially equivalent FucT-IV levels are seen in the K562 parents and transfectants, which are lower than the FucT-IV level in HL60 cells. Note that the FucT-VII level in K562/FucT-VII cells is greater than in K562/PSGL-1/FucT-VII cells, corresponding to the levels of surface sLex (see Fig 1). Untransfected K562 cells and K562/PSGL-1 cells express no detectable FucT-VII.
RT-PCR Southern blot analysis of FucT-IV and FucT-VII mRNA expression in K562 transfectants. Essentially equivalent FucT-IV levels are seen in the K562 parents and transfectants, which are lower than the FucT-IV level in HL60 cells. Note that the FucT-VII level in K562/FucT-VII cells is greater than in K562/PSGL-1/FucT-VII cells, corresponding to the levels of surface sLex (see Fig 1). Untransfected K562 cells and K562/PSGL-1 cells express no detectable FucT-VII.
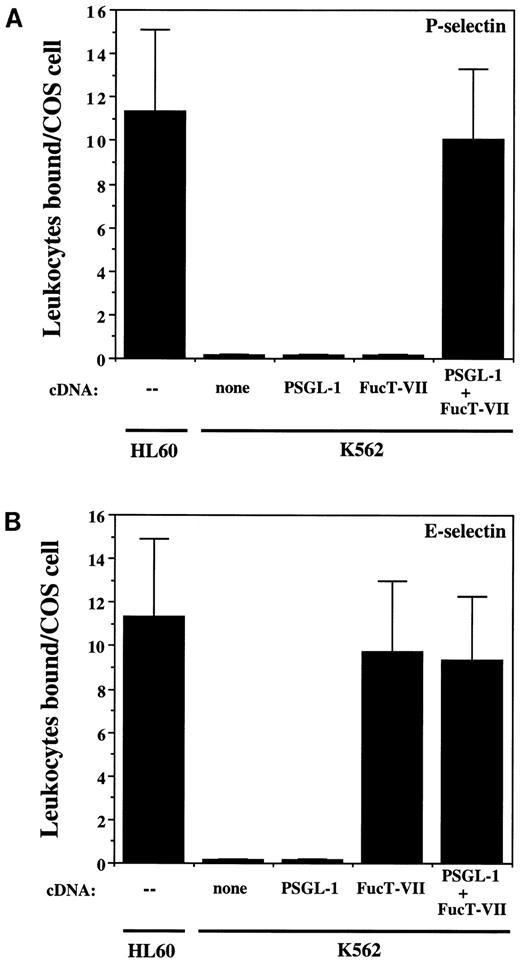
Adhesion to P- and E-selectin under low-shear conditions.To determine if expression of PSGL-1 alone, FucT-IV alone, FucT-VII alone, or a combination of these molecules was required for binding to P- and/or E-selectin, adhesion assays were performed. HL60 cells were included in each experiment as a positive control (Fig 3). Untransfected K562 cells or cells stably transfected with either FucT-VII cDNA only or PSGL-1 cDNA only did not bind to COS cells expressing P-selectin (Fig 3A). Similarly, untransfected K562 cells or cells transfected with PSGL-1 cDNA only were unable to bind to COS cells transfected with E-selectin (Fig 3B). Thus, expression of FucT-IV alone or FucT-IV plus PSGL-1 was not sufficient for binding to either P-selectin or E-selectin. However, K562 cells transfected with FucT-VII alone were able to bind to E-selectin–expressing COS cells, and this adhesion was similar to that observed with HL60 cells binding to E-selectin (Fig 3B). Expression of FucT-VII alone or in combination with FucT-IV is therefore sufficient for binding to E-selectin but not to P-selectin in transfected K562 cells, and this adhesion is not dependent on expression of PSGL-1. In contrast, binding of transfected K562 cells to P-selectin required coexpression of both PSGL-1 and FucT-VII (Fig 3A).
Binding of K562 transfectants to COS/P-selectin and COS/E-selectin. (A) Adhesion to P-selectin; (B) adhesion to E-selectin. K562/FucT-VII cells bind well to E-selectin but do not bind to P-selectin, whereas K562/PSGL-1/FucT-VII cells bind to both.
Binding of K562 transfectants to COS/P-selectin and COS/E-selectin. (A) Adhesion to P-selectin; (B) adhesion to E-selectin. K562/FucT-VII cells bind well to E-selectin but do not bind to P-selectin, whereas K562/PSGL-1/FucT-VII cells bind to both.
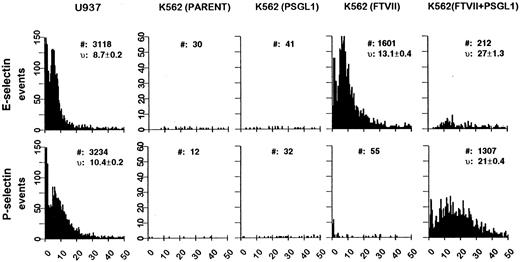
Adhesion of stably transfected K562 cells to P- and E-selectin under conditions of defined shear stress.These results were extended to an analysis of rolling under shear flow. This assay more closely models physiologic conditions by using a defined shear stress and monolayers of transfected CHO cells expressing levels of P- and E-selectin similar to E-selectin levels on TNF-stimulated HUVECs. U937 cells or K562 transfectants were incubated on monolayers of CHO cells stably transfected with either P- or E-selectin at a constant shear stress of 1.9 dynes/cm2, and the rolling velocity and number of interactions were determined for the different cell types (Fig 4). U937 cells, like HL60 cells, adhered well to both P- and E-selectin and demonstrated low rolling velocities (10.4 ± 0.2 μm/s on P-selectin and 8.7 ± 0.2 μm/s on E-selectin) and high levels of interaction with each selectin (3,234 events on P-selectin and 3,118 on E-selectin). Similar to the results in the COS cell assays, untransfected K562 cells or cells transfected with PSGL-1 only were unable to roll on either P- or E-selectin–expressing CHO cells (Fig 4). K562 cells transfected with FucT-VII only were able to roll well on E-selectin (velocity 13.1 ± 0.4 μm/s and 1,601 interactions), but were unable to interact with P-selectin (Fig 4). However, cells transfected with both PSGL-1 and FucT-VII were able to roll on both P- and E-selectin. These cells interacted significantly better with P-selectin (21 ± 0.4 μm/s and 1,307 events) than with E-selectin (27 ± 1.3 μm/s and 212 events). K562/FucT-VII transfectants also interacted significantly better with E-selectin than the PSGL-1/FucT-VII double transfectant (Fig 4). The basis for this observation is not known, but is likely to be related to lower levels of FucT-VII in the double transfectant as compared with cells transfected with FucT-VII only (Fig 2).
Analysis of K562 transfectant rolling on E- and P-selectin under defined shear flow. Velocity distributions of the different cell lines on CHO cells transfected with E- or P-selectin. Histograms depict the distribution of velocities from 0 to 50 μm/s at 1.9 dynes/cm2 of linear shear stress. #, total events between 0 and 50 μm/s; v, mean of velocity measurements ± SE.
Analysis of K562 transfectant rolling on E- and P-selectin under defined shear flow. Velocity distributions of the different cell lines on CHO cells transfected with E- or P-selectin. Histograms depict the distribution of velocities from 0 to 50 μm/s at 1.9 dynes/cm2 of linear shear stress. #, total events between 0 and 50 μm/s; v, mean of velocity measurements ± SE.
Taken together, these concordant data from both the COS cell and flow chamber assays demonstrate that in stably transfected K562 cells, coexpression of both FucT-VII and PSGL-1 is required for binding to P-selectin. In contrast, FucT-VII expression alone, in the absence of PSGL-1, is sufficient for adhesion of transfected K562 cells to E-selectin.
DISCUSSION
A series of stably transfected human hematopoietic cell lines were developed to study the interactions of PSGL-1 with P- and E-selectin. K562 cells express FucT-IV but not PSGL-1 or FucT-VII, and do not bind to either P- or E-selectin. Transfection of K562 cells with cDNA encoding PSGL-1 only did not result in binding to either P- or E-selectin as measured by two independent adhesion assays. Transfection with FucT-VII alone conferred binding to E-selectin but not to P-selectin. Adhesion to P-selectin occurred only when K562 cells were cotransfected with both FucT-VII and PSGL-1 cDNAs. Thus, PSGL-1 is essential for binding to P-selectin but is not required for binding to E-selectin in stably transfected K562 cells.
Two independent adhesion assays demonstrated an absolute requirement of PSGL-1 for binding to P-selectin by stably transfected K562 cells. Transfected COS cells used in the low-shear adhesion assay expressed P- and E-selectin at levels considerably above those in normal endothelium. Thus, this assay is extremely sensitive in measuring low-shear interactions between PSGL-1–expressing transfectants and P- and E-selectin. In this assay, binding to P-selectin occurred only when PSGL-1 was present and properly modified by FucT-VII. These results were confirmed in a parallel-plate assay performed under defined shear stress and using CHO cells expressing P- or E-selectin at levels similar to those of E-selectin on TNF-stimulated HUVECs. This is a higher stringency assay that more closely mimics physiologic conditions. In addition to confirming an absolute requirement of PSGL-1 for P-selectin binding, the flow assay showed greater E-selectin binding by K562 transfectants expressing FucT-VII only when compared with cells receiving cDNAs encoding both FucT-VII and PSGL-1. The FucT-VII–only transfectants had higher levels of surface sLex (Fig 1) and FucT-VII (Fig 2) when compared with K562 cells transfected with both FucT-VII and PSGL-1 cDNAs. Thus, rolling on E-selectin–expressing CHO cells by these two transfectants correlated closely with FucT-VII levels only, since addition of PSGL-1, even at levels adequate for P-selectin binding, did not enhance binding to E-selectin. Increased levels of FucT-VII, in the absence of PSGL-1, enhanced binding to E-selectin. Therefore, PSGL-1, even when expressed and functional for binding to P-selectin, does not appear to be an important ligand for E-selectin in stably transfected K562 cells.
Our data demonstrate that expression of FucT-VII alone was sufficient for E-selectin binding of transfected K562 cells, and that this adhesion occurs in the absence of PSGL-1. In contrast, other groups have reported an absolute requirement for PSGL-1 in interactions with E-selectin.14,20,21 Purified neutrophils have been reported to bind CHO cells transfected with E-selectin under flow conditions in a PSGL-1– and L-selectin–dependent manner.21 In contrast, K562 cells transfected with FucT-VII cDNA do not express L-selectin or PSGL-1 and were able to bind to E-selectin in two different adhesion assays. The observed differences regarding the lack of a requirement for PSGL-1 in binding to E-selectin may be due to differences in host cell type and/or adhesion assays. However, in both assays described in this report, we have observed strong binding to E-selectin in the absence of PSGL-1 expression with several other human hematopoietic cell lines and transfectants (A.J. Wagers, R. Craig, L.M. Stoolman, G.S. Kansas, submitted).
PSGL-1 requires sialylated and fucosylated O-linked glycans to bind P-selectin,11,13-16 but it is not clear which fucosyltransferases are required for functional O-linked glycan expression on PSGL-1 in leukocytes. Li et al,14 using transfected CHO cells, suggested that FucT-IV could effectively modify PSGL-1 for binding to both P- and E-selectin. However, in transfected K562 cells, FucT-IV could not efficiently modify PSGL-1 for binding to P-selectin, whereas FucT-VII could. These differences might be due to increased levels of FucT-IV in the transfected CHO cells as compared with the K562 hematopoietic cell line, or to the unique glycosylation phenotype36-38 of the CHO cells used in those studies. Whether FucT-IV can synthesize ligands for P-selectin when expressed at the higher levels found in myeloid cells remains to be determined. However, the HPB-ALL human T-cell line, which endogenously expresses both PSGL-1 and FucT-IV at the high levels present in HL60 cells, is unable to bind P-selectin, but will bind P-selectin after transfection of FucT-VII cDNA (A.J. Wagers, R. Craig, L.M. Stoolman, G.S. Kansas, submitted). Finally, mice rendered genetically deficient in FucT-VII lack functional ligands for P-selectin (and E- and L-selectin), since neutrophils from these animals are unable to bind P-selectin in vitro and have sharply impaired rolling in vivo.39 Taken together, these data strongly implicate FucT-VII as an essential enzyme for appropriate modification of PSGL-1 in normal leukocytes.
K562 cells provide an excellent model system to study interactions of hematopoietic cells with both P- and E-selectin, since they do not endogenously express L-selectin, PSGL-1, or FucT-VII. Thus, adhesion of transfected K562 cells to P-selectin was dependent on the presence of both PSGL-1 and FucT-VII, whereas adhesion to E-selectin by K562 cells transfected with FucT-VII only was independent of PSGL-1. Future studies will elucidate further the molecular requirements for leukocyte interaction with vascular selectins.
ACKNOWLEDGMENT
We thank John Lowe for providing FucT-IV and FucT-VII cDNA; Louis Picker for providing HECA-452; and AMGEN for providing PSGL-1 cDNA.
Supported by the Charles M. Bane Charitable Trust, a grant from the Illinois Division of the American Cancer Society, and a gift from an anonymous foundation (G.S.K.); and grants no. AI 33189 and HL 31963 (L.M.S.). G.S.K. is an Established Investigator of the American Heart Association. A.J.W. is a predoctoral fellow of the National Science Foundation.
Address reprint requests to Geoffrey S. Kansas, PhD, Department of Microbiology-Immunology, Northwestern Medical School/Morton 6-654, 303 E Chicago Ave, Chicago, IL 60611.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal